Drug Delivery to the Posterior Segment of the Eye: Biopharmaceutic and Pharmacokinetic Considerations
Abstract
:1. Introduction
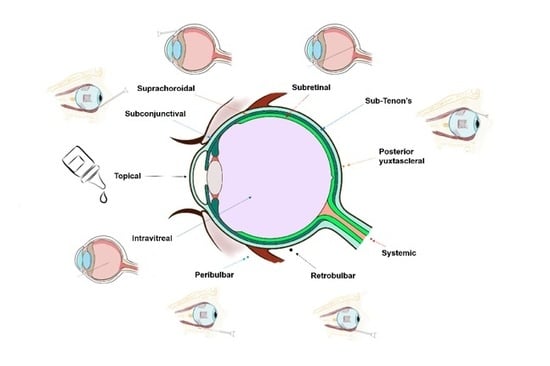
2. Routes of Drug Delivery to the Posterior Segment of the Eye
2.1. Intravitreal Administration
2.1.1. Vitreous and Posterior Compartment Barriers to Drug Delivery
Vitreous
Posterior Compartment Barriers to Drug Delivery
2.1.2. Advantages and Limitations
2.1.3. Pharmacokinetics
Drug Distribution in the Vitreous Humor
Drug Distribution to Surrounding Tissues
2.1.4. Drug Delivery Systems
2.2. Topical Administration
2.2.1. Ocular Barriers for the Entry of Drugs: Precorneal Factors
2.2.2. Corneal and Anterior Compartment Barriers
Cornea
Conjunctiva
2.2.3. Advantages and Limitations
2.2.4. Pharmacokinetics
2.2.5. Drug Delivery Systems
2.3. Systemic Administration
2.3.1. Advantages and Limitations
2.3.2. Pharmacokinetics
Absorption to Ocular Tissues
Distribution to Ocular Tissues
Drug Elimination
2.3.3. Drug Delivery Systems
2.3.4. Systemic Drugs for Posterior Segment Eye Diseases
2.4. Periocular Administration
2.4.1. Advantages and Limitations
2.4.2. Pharmacokinetics
Absorption
Elimination
2.4.3. Subconjunctival Route
Advantages and Limitations
Pharmacokinetics
Drug Delivery Systems
2.4.4. SubTenon’s Route
Advantages and Limitations
Pharmacokinetics
2.4.5. Retrobulbar Route
Advantages and Limitations
2.4.6. Peribulbar Route
Advantages and Limitations
2.4.7. Posterior Juxtascleral Route
2.4.8. Suprachoroidal Route
Advantages and Limitations
Pharmacokinetics
Drug Delivery Systems
2.4.9. Subretinal Route
Advantages and Limitations
Drug Delivery Systems
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO Team. World Report on Vision; WHO: Geneva, Switzerland, 2019; p. 180. ISBN 978-92-4-151657-0. [Google Scholar]
- Peynshaert, K.; Devoldere, J.; De Smedt, S.C.; Remaut, K. In vitro and ex vivo models to study drug delivery barriers in the posterior segment of the eye. Adv. Drug Deliv. Rev. 2018, 126, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Del Amo, E.M.; Rimpelä, A.-K.; Heikkinen, E.; Kari, O.K.; Ramsay, E.; Lajunen, T.; Schmitt, M.; Pelkonen, L.; Bhattacharya, M.; Richardson, D.; et al. Pharmacokinetic aspects of retinal drug delivery. Prog. Retin. Eye Res. 2017, 57, 134–185. [Google Scholar] [CrossRef]
- Hosoya, K.; Tachikawa, M. Inner Blood-Retinal Barrier Transporters: Role of Retinal Drug Delivery. Pharm. Res. 2009, 26, 2055–2065. [Google Scholar] [CrossRef] [PubMed]
- Gaudana, R.; Ananthula, H.K.; Parenky, A.; Mitra, A.K. Ocular drug delivery. AAPS J. 2010, 12, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Ranta, V.-P.; Urtti, A. Transscleral drug delivery to the posterior eye: Prospects of pharmacokinetic modeling. Adv. Drug Deliv. Rev. 2006, 58, 1164–1181. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.G.; Tan, L.E.; Mains, J. Principles of Retinal Drug Delivery from Within the Vitreous. In Drug Product Development for the Back of the Eye; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011; pp. 125–158. ISBN 978-1-4419-9920-7. [Google Scholar]
- Cunha-Vaz, J.G. The blood–retinal barriers system. Basic concept sand clinical evaluation. Exp. Eye Res. 2004, 78, 715–721. [Google Scholar] [CrossRef]
- Gunda, S.; Hariharan, S.; Mitra, A.K. Corneal absorption and anterior chamber pharmacokinetics of dipeptide monoester prodrugs of ganciclovir (GCV): In vivo comparative evaluation of these prodrugs with Val-GCV and GCV in rabbits. J. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. Ther. 2006, 22, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Zambito, Y.; Zaino, C.; Di Colo, G. Effects of N-trimethylchitosan on transcellular and paracellular transcorneal drug transport. Eur. J. Pharm. Biopharm. 2006, 64, 16–25. [Google Scholar] [CrossRef]
- Dey, S.; Mitra, A.K. Transporters and receptors in ocular drug delivery: Opportunities and challenges. Expert Opin. Drug Deliv. 2005, 2, 201–204. [Google Scholar] [CrossRef]
- Huang, D.; Chen, Y.-S.; Rupenthal, I.D. Overcoming ocular drug delivery barriers through the use of physical forces. Adv. Drug Deliv. Rev. 2018, 126, 96–112. [Google Scholar] [CrossRef]
- Hussain, A.A.; Starita, C.; Hodgetts, A.; Marshall, J. Macromolecular diffusion characteristics of ageing human Bruch’s membrane: Implications for age-related macular degeneration (AMD). Exp. Eye Res. 2010, 90, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Cruysberg, L.P.J.; Nuijts, R.M.M.A.; Geroski, D.H.; Koole, L.H.; Hendrikse, F.; Edelhauser, H.F. In vitro human scleral permeability of fluorescein, dexamethasone-fluorescein, methotrexate-fluorescein and rhodamine 6G and the use of a coated coil as a new drug delivery system. J. Ocul. Pharmacol. Ther. 2002, 18, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.G.; Young, R.D. Scleral structure, organisation and disease: A review. Exp. Eye Res. 2004, 78, 609–623. [Google Scholar] [CrossRef]
- Jaffe, G.J.; Ashton, P.; Andrew, P. Intraocular Drug Delivery, 1st ed.; CRC Press: Boca Raton, FL, USA, 2006; ISBN 978-0-8247-2860-1. [Google Scholar]
- Ambati, J.; Canakis, C.S.; Miller, J.W.; Gragoudas, E.S.; Edwards, A.; Weissgold, D.J.; Kim, I.; Delori, F.C.; Adamis, A.P. Diffusion of high molecular weight compounds through sclera. Invest. Ophthalmol. Vis. Sci. 2000, 41, 1181–1185. [Google Scholar]
- Cheruvu, N.P.S.; Kompella, U.B. Bovine and Porcine Transscleral Solute Transport: Influence of Lipophilicity and the Choroid–Bruch’s Layer. Invest. Ophthalmol. Vis. Sci. 2006, 47, 4513–4522. [Google Scholar] [CrossRef]
- Marsh, D.A. Selection of Drug Delivery Approaches for the Back of the Eye: Opportunities and Unmet Needs. In Drug Product Development for the Back of the Eye; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011; pp. 125–158. ISBN 978-1-4419-9920-7. [Google Scholar]
- Ashton, P. Retinal Drug Delivery. In Intraocular Drug Delivery; Taylor & Francis Group: New York, NY, USA, 2006; pp. 1–25. ISBN 978-1-4200-1650-5. [Google Scholar]
- Maroñas, O.; García-Quintanilla, L.; Luaces-Rodríguez, A.; Fernández-Ferreiro, A.; Latorre-Pellicer, A.; Abraldes, M.J.; Lamas, M.J.; Carracedo, Á. Anti-VEGF treatment and response in Age-related Macular Degeneration: Disease´s susceptibility, pharmacogenetics and pharmacokinetics. Curr. Med. Chem. 2019. [Google Scholar] [CrossRef]
- García-Quintanilla, L.; Luaces-Rodríguez, A.; Gil-Martínez, M.; Mondelo-García, C.; Maroñas, O.; Mangas-Sanjuan, V.; González-Barcia, M.; Zarra-Ferro, I.; Aguiar, P.; Otero-Espinar, F.J.; et al. Pharmacokinetics of Intravitreal Anti-VEGF Drugs in Age-Related Macular Degeneration. Pharmaceutics 2019, 11, 365. [Google Scholar] [CrossRef] [Green Version]
- Macha, S.; Mitra, A.K. Ocular pharmacokinetics in rabbits using a novel dual probe microdialysis technique. Exp. Eye Res. 2001, 72, 289–299. [Google Scholar] [CrossRef]
- Castro-Balado, A.; Mondelo-García, C.; González-Barcia, M.; Zarra-Ferro, I.; Otero-Espinar, F.J.; Ruibal-Morell, Á.; Aguiar-Fernández, P.; Fernández-Ferreiro, A. Ocular Biodistribution Studies using Molecular Imaging. Pharmaceutics 2019, 11, 237. [Google Scholar] [CrossRef] [Green Version]
- Maurice, D.M. The Regurgitation of Large Vitreous Injections. J. Ocul. Pharmacol. Ther. 1997, 13, 461–463. [Google Scholar] [CrossRef]
- Stay, M.S.; Xu, J.; Randolph, T.W.; Barocas, V.H. Computer simulation of convective and diffusive transport of controlled-release drugs in the vitreous humor. Pharm. Res. 2003, 20, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Heys, J.J.; Barocas, V.H.; Randolph, T.W. Permeability and diffusion in vitreous humor: Implications for drug delivery. Pharm. Res. 2000, 17, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Bungay, P.M.; Lutz, R.J.; Augsburger, J.J.; Millard, R.W.; Sinha Roy, A.; Banerjee, R.K. Evaluation of coupled convective-diffusive transport of drugs administered by intravitreal injection and controlled release implant. J. Control. Release 2005, 105, 279–295. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, M.K.; Park, J.; Augsburger, J.J.; Banerjee, R.K. Effect of retinal permeability, diffusivity, and aqueous humor hydrodynamics on pharmacokinetics of drugs in the eye. J. Ocul. Pharmacol. Ther. 2008, 24, 255–267. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.; Boylan, N.J.; Suk, J.S.; Wang, Y.-Y.; Nance, E.A.; Yang, J.-C.; McDonnell, P.J.; Cone, R.A.; Duh, E.J.; Hanes, J. Nanoparticle diffusion in, and microrheology of, the bovine vitreous ex vivo. J. Control. Release 2013, 167, 76–84. [Google Scholar] [CrossRef] [Green Version]
- Cunha-Vaz, J.; Maurice, D. Fluorescein dynamics in the eye. Doc. Ophthalmol. 1969, 26, 61–72. [Google Scholar] [CrossRef]
- Thornit, D.N.; Vinten, C.M.; Sander, B.; Lund-Andersen, H.; la Cour, M. Blood-retinal barrier glycerol permeability in diabetic macular edema and healthy eyes: Estimations from macular volume changes after peroral glycerol. Invest. Ophthalmol. Vis. Sci. 2010, 51, 2827–2834. [Google Scholar] [CrossRef] [Green Version]
- Pitkänen, L.; Ranta, V.-P.; Moilanen, H.; Urtti, A. Permeability of Retinal Pigment Epithelium: Effects of Permeant Molecular Weight and Lipophilicity. Investig. Opthalmology Vis. Sci. 2005, 46, 641. [Google Scholar] [CrossRef] [Green Version]
- Käsdorf, B.T.; Arends, F.; Lieleg, O. Diffusion Regulation in the Vitreous Humor. Biophys. J. 2015, 109, 2171–2181. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.; Litt, M.; Buchsbaum, G. Rheology of the vitreous body: Part 3. Concentration of electrolytes, collagen and hyaluronic acid. Biorheology 1994, 31, 339–351. [Google Scholar] [CrossRef]
- Loukovaara, S.; Nurkkala, H.; Tamene, F.; Gucciardo, E.; Liu, X.; Repo, P.; Lehti, K.; Varjosalo, M. Quantitative Proteomics Analysis of Vitreous Humor from Diabetic Retinopathy Patients. J. Proteome Res. 2015, 14, 5131–5143. [Google Scholar] [CrossRef] [PubMed]
- Angi, M.; Kalirai, H.; Coupland, S.E.; Damato, B.E.; Semeraro, F.; Romano, M.R. Proteomic Analyses of the Vitreous Humour. Mediat. Inflamm. 2012, 2012, e148039. [Google Scholar] [CrossRef] [PubMed]
- Murthy, K.R.; Goel, R.; Subbannayya, Y.; Jacob, H.K.; Murthy, P.R.; Manda, S.S.; Patil, A.H.; Sharma, R.; Sahasrabuddhe, N.A.; Parashar, A.; et al. Proteomic analysis of human vitreous humor. Clin. Proteom. 2014, 11, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chirila, T.V.; Hong, Y. Chapter C2 The Vitreous Humor. In Handbook of Biomaterial Properties; Springer Nature: Basel, Switzerland, 2016; pp. 125–134. [Google Scholar]
- Maurice, D.M. Mishima Ocular Pharmacokinetics. In Pharmacology of the Eye; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; pp. 19–116. ISBN 978-3-642-69222-2. [Google Scholar]
- Del Amo, E.M.; Urtti, A. Rabbit as an animal model for intravitreal pharmacokinetics: Clinical predictability and quality of the published data. Exp. Eye Res. 2015, 137, 111–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maurice, D. Review: Practical issues in intravitreal drug delivery. J. Ocul. Pharmacol. Ther. 2001, 17, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, S.; Cheng, Y.L.; Saville, B. Drug distribution in the vitreous humor of the human eye: The effects of intravitreal injection position and volume. Curr. Eye Res. 1997, 16, 663–669. [Google Scholar] [CrossRef]
- Mandell, B.A.; Meredith, T.A.; Aguilar, E.; el-Massry, A.; Sawant, A.; Gardner, S. Effects of inflammation and surgery on amikacin levels in the vitreous cavity. Am. J. Ophthalmol. 1993, 115, 770–774. [Google Scholar] [CrossRef]
- Doft, B.H.; Weiskopf, J.; Nilsson-Ehle, I.; Wingard, L.B. Amphotericin clearance in vitrectomized versus nonvitrectomized eyes. Ophthalmology 1985, 92, 1601–1605. [Google Scholar] [CrossRef]
- Wingard, L.B.; Zuravleff, J.J.; Doft, B.H.; Berk, L.; Rinkoff, J. Intraocular distribution of intravitreally administered amphotericin B in normal and vitrectomized eyes. Invest. Ophthalmol. Vis. Sci. 1989, 30, 2184–2189. [Google Scholar]
- Ficker, L.; Meredith, T.A.; Gardner, S.; Wilson, L.A. Cefazolin levels after intravitreal injection. Effects of inflammation and surgery. Invest. Ophthalmol. Vis. Sci. 1990, 31, 502–505. [Google Scholar]
- Shaarawy, A.; Meredith, T.A.; Kincaid, M.; Dick, J.; Aguilar, E.; Ritchie, D.J.; Reichley, R.M. Intraocular injection of ceftazidime. Effects of inflammation and surgery. Retina 1995, 15, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Pearson, P.A.; Hainsworth, D.P.; Ashton, P. Clearance and distribution of ciprofloxacin after intravitreal injection. Retina 1993, 13, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, H.E.; Meredith, T.A.; el-Massry, A.; Shaarawy, A.; Kincaid, M.; Dick, J.; Ritchie, D.J.; Reichley, R.M.; Neisman, M.K. Vancomycin levels after intravitreal injection. Effects of inflammation and surgery. Retina 1995, 15, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Christoforidis, J.B.; Williams, M.M.; Wang, J.; Jiang, A.; Pratt, C.; Abdel-Rasoul, M.; Hinkle, G.H.; Knopp, M.V. Anatomic and pharmacokinetic properties of intravitreal bevacizumab and ranibizumab after vitrectomy and lensectomy. Retina 2013, 33, 946–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakinoki, M.; Sawada, O.; Sawada, T.; Saishin, Y.; Kawamura, H.; Ohji, M. Effect of vitrectomy on aqueous VEGF concentration and pharmacokinetics of bevacizumab in macaque monkeys. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5877–5880. [Google Scholar] [CrossRef] [Green Version]
- Niwa, Y.; Kakinoki, M.; Sawada, T.; Wang, X.; Ohji, M. Ranibizumab and Aflibercept: Intraocular Pharmacokinetics and Their Effects on Aqueous VEGF Level in Vitrectomized and Nonvitrectomized Macaque Eyes. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6501–6505. [Google Scholar] [CrossRef] [Green Version]
- Edington, M.; Connolly, J.; Chong, N.V. Pharmacokinetics of intravitreal anti-VEGF drugs in vitrectomized versus non-vitrectomized eyes. Expert Opin. Drug Metab. Toxicol. 2017, 13, 1217–1224. [Google Scholar] [CrossRef]
- Peyman, G.A.; Vastine, D.W.; Raichand, M. Experimental Aspects and Their Clinical Application. Ophthalmology 1978, 85, 374–385. [Google Scholar] [CrossRef]
- Campochiaro, P.A.; Lim, J.I. Aminoglycoside toxicity in the treatment of endophthalmitis. Arch. Ophthalmol. 1994, 112, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Da, M.; Li, K.K.W.; Chan, K.C.; Wu, E.X.; Wong, D.S.H. Distribution of Triamcinolone Acetonide after Intravitreal Injection into Silicone Oil-Filled Eye. BioMed Res. Int. 2016, 2016, 5485467. [Google Scholar] [CrossRef]
- Spitzer, M.S.; Kaczmarek, R.T.; Yoeruek, E.; Petermeier, K.; Wong, D.; Heimann, H.; Jaissle, G.B.; Bartz-Schmidt, K.U.; Szurman, P. The distribution, release kinetics, and biocompatibility of triamcinolone injected and dispersed in silicone oil. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2337–2343. [Google Scholar] [CrossRef]
- Aras, C.; Ozdamar, A.; Karacorlu, M.; Ozkan, S. Silicone oil in the surgical treatment of endophthalmitis associated with retinal detachment. Int. Ophthalmol. 2001, 24, 147–150. [Google Scholar] [CrossRef]
- Mannermaa, E.; Vellonen, K.-S.; Urtti, A. Drug transport in corneal epithelium and blood-retina barrier: Emerging role of transporters in ocular pharmacokinetics. Adv. Drug Deliv. Rev. 2006, 58, 1136–1163. [Google Scholar] [CrossRef] [PubMed]
- Vellonen, K.-S.; Hellinen, L.; Mannermaa, E.; Ruponen, M.; Urtti, A.; Kidron, H. Expression, activity and pharmacokinetic impact of ocular transporters. Adv. Drug Deliv. Rev. 2018, 126, 3–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias, C.S.; Anand, B.S.; Mitra, A.K. Effect of mono- and di-acylation on the ocular disposition of ganciclovir: Physicochemical properties, ocular bioreversion, and antiviral activity of short chain ester prodrugs. J. Pharm. Sci. 2002, 91, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Duvvuri, S.; Majumdar, S.; Mitra, A.K. Role of metabolism in ocular drug delivery. Curr. Drug Metab. 2004, 5, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Dias, C.S.; Mitra, A.K. Vitreal elimination kinetics of large molecular weight FITC-labeled dextrans in albino rabbits using a novel microsampling technique. J. Pharm. Sci. 2000, 89, 572–578. [Google Scholar] [CrossRef]
- Durairaj, C. Ocular Pharmacokinetics. In Pharmacologic Therapy of Ocular Disease; Handbook of Experimental Pharmacology; Springer: Cham, Switzerland, 2016; pp. 31–55. ISBN 978-3-319-58288-7. [Google Scholar]
- Kwak, H.W.; D’Amico, D.J. Evaluation of the retinal toxicity and pharmacokinetics of dexamethasone after intravitreal injection. Arch. Ophthalmol. 1992, 110, 259–266. [Google Scholar] [CrossRef]
- Barza, M.; Lynch, E.; Baum, J.L. Pharmacokinetics of newer cephalosporins after subconjunctival and intravitreal injection in rabbits. Arch. Ophthalmol. 1993, 111, 121–125. [Google Scholar] [CrossRef]
- Robertson, J.E.; Westra, I.; Woltering, E.A.; Winthrop, K.L.; Barrie, R.; O’Dorisio, T.M.; Holmes, D. Intravitreal injection of octreotide acetate. J. Ocul. Pharmacol. Ther. 1997, 13, 171–177. [Google Scholar] [CrossRef]
- Leeds, J.M.; Henry, S.P.; Truong, L.; Zutshi, A.; Levin, A.A.; Kornbrust, D. Pharmacokinetics of a potential human cytomegalovirus therapeutic, a phosphorothioate oligonucleotide, after intravitreal injection in the rabbit. Drug Metab. Dispos. Biol. Fate Chem. 1997, 25, 921–926. [Google Scholar]
- Durairaj, C.; Shah, J.C.; Senapati, S.; Kompella, U.B. Prediction of vitreal half-life based on drug physicochemical properties: Quantitative structure-pharmacokinetic relationships (QSPKR). Pharm. Res. 2009, 26, 1236–1260. [Google Scholar] [CrossRef] [PubMed]
- Christoforidis, J.B.; Chang, S.; Jiang, A.; Wang, J.; Cebulla, C.M. Intravitreal devices for the treatment of vitreous inflammation. Mediat. Inflamm. 2012, 2012, 126463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sides Media, www sidesmedia com Retina Today—Ocular Drug Delivery Systems for the Posterior Segment: A Review. Available online: http://retinatoday.com/2012/05/ocular-drug-delivery-systems-for-the-posterior-segment-a-review/ (accessed on 23 April 2019).
- Shikari, H.; Samant, P.M. Intravitreal injections: A review of pharmacological agents and techniques. J. Clin. Ophthalmol. Res. 2016, 4, 51. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, A.; Joshi, M.; Christoforidis, J. Drug Delivery Implants in the Treatment of Vitreous Inflammation. Mediators Inflamm. 2013, 2013, 780634. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.S.; Hughes, P.; Ross, A.D.; Robinson, M.R. Biodegradable implants for sustained drug release in the eye. Pharm. Res. 2010, 27, 2043–2053. [Google Scholar] [CrossRef]
- Smith, T.J.; Pearson, P.A.; Blandford, D.L.; Brown, J.D.; Goins, K.A.; Hollins, J.L.; Schmeisser, E.T.; Glavinos, P.; Baldwin, L.B.; Ashton, P. Intravitreal sustained-release ganciclovir. Arch. Ophthalmol. 1992, 110, 255–258. [Google Scholar] [CrossRef]
- Abrishami, M.; Abrishami, M.; Mahmoudi, A.; Mosallaei, N.; Vakili Ahrari Roodi, M.; Malaekeh-Nikouei, B. Solid Lipid Nanoparticles Improve the Diclofenac Availability in Vitreous after Intraocular Injection. J. Drug Deliv. 2016, 2016, 1368481. [Google Scholar] [CrossRef] [Green Version]
- Kambhampati, S.P.; Clunies-Ross, A.J.M.; Bhutto, I.; Mishra, M.K.; Edwards, M.; McLeod, D.S.; Kannan, R.M.; Lutty, G. Systemic and Intravitreal Delivery of Dendrimers to Activated Microglia/Macrophage in Ischemia/Reperfusion Mouse Retina. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4413–4424. [Google Scholar] [CrossRef] [Green Version]
- Pachis, K.; Blazaki, S.; Tzatzarakis, M.; Klepetsanis, P.; Naoumidi, E.; Tsilimbaris, M.; Antimisiaris, S.G. Sustained release of intravitreal flurbiprofen from a novel drug-in-liposome-in-hydrogel formulation. Eur. J. Pharm. Sci. 2017, 109, 324–333. [Google Scholar] [CrossRef]
- Wang, C.; Seo, S.-J.; Kim, J.-S.; Lee, S.-H.; Jeon, J.-K.; Kim, J.-W.; Kim, K.-H.; Kim, J.-K.; Park, J. Intravitreal implantable magnetic micropump for on-demand VEGFR-targeted drug delivery. J. Control. Release 2018, 283, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Genentech: Press Releases. 2018. Available online: https://www.gene.com/media/press-releases/14739/2018-07-25/genentech-unveils-positive-phase-ii-resu (accessed on 22 April 2019).
- Luaces-Rodríguez, A.; Mondelo-García, C.; Zarra-Ferro, I.; González-Barcia, M.; Aguiar, P.; Fernández-Ferreiro, A.; Otero-Espinar, F.J. Intravitreal anti-VEGF drug delivery systems for age-related macular degeneration. Int. J. Pharm. 2020, 573, 118767. [Google Scholar] [CrossRef] [PubMed]
- Barañano, D.E.; Kim, S.J.; Edelhauser, H.F.; Durairaj, C.; Kompella, U.B.; Handa, J.T. Efficacy and pharmacokinetics of intravitreal non-steroidal anti-inflammatory drugs for intraocular inflammation. Br. J. Ophthalmol. 2009, 93, 1387–1390. [Google Scholar] [CrossRef] [PubMed]
- Von Sallmann, L.; Meyer, K.; Grandi, J.D. Experimental study on penicillin treatment of ectogenous infection of vitreous. Arch. Ophthalmol. 1944, 32, 179–189. [Google Scholar] [CrossRef]
- Radhika, M.; Mithal, K.; Bawdekar, A.; Dave, V.; Jindal, A.; Relhan, N.; Albini, T.; Pathengay, A.; Flynn, H.W. Pharmacokinetics of intravitreal antibiotics in endophthalmitis. J. Ophthalmic Inflamm. Infect. 2014, 4, 22. [Google Scholar] [CrossRef]
- Barza, M.; Kane, A.; Baum, J. Pharmacokinetics of intravitreal carbenicillin, cefazolin, and gentamicin in rhesus monkeys. Investig. Ophthalmol. Vis. Sci. 1983, 24, 1602–1606. [Google Scholar]
- Doft, B.H.; Barza, M. Ceftazidime or Amikacin: Choice of Intravitreal Antimicrobials in the Treatment of Postoperative Endophthalmitis. Arch. Ophthalmol. 1994, 112, 17–18. [Google Scholar]
- Aydin, E.; Kazi, A.A.; Peyman, G.A.; Esfahani, M.R.; Muñoz-Morales, A.; Kivilcim, M.; Caro-Magdaleno, M. Retinal toxicity of intravitreal doxycycline. A pilot study. Arch. Soc. Espanola Oftalmol. 2007, 82, 223–228. [Google Scholar]
- Iyer, M.N.; He, F.; Wensel, T.G.; Mieler, W.F.; Benz, M.S.; Holz, E.R. Intravitreal clearance of moxifloxacin. Trans. Am. Ophthalmol. Soc. 2005, 103, 76–83. [Google Scholar] [CrossRef] [Green Version]
- Oztürk, F.; Kortunay, S.; Kurt, E.; Ilker, S.S.; Basci, N.E.; Bozkurt, A. Penetration of topical and oral ciprofloxacin into the aqueous and vitreous humor in inflamed eyes. Retina 1999, 19, 218–222. [Google Scholar] [CrossRef]
- Barza, M.; McCue, M. Pharmacokinetics of aztreonam in rabbit eyes. Antimicrob. Agents Chemother. 1983, 24, 468–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ay, G.M.; Akhan, S.C.; Erturk, S.; Aktas, E.S.; Ozkara, S.K.; Caglar, Y. Comparison of Intravitreal Ceftazidime and Meropenem in Treatment of Experimental Pseudomonal Posttraumatic Endophthalmitis in a Rabbit Model. J. Appl. Res. 2004, 4, 10. [Google Scholar]
- Loewenstein, A.; Zemel, E.; Lazar, M.; Perlman, I. Drug-induced retinal toxicity in albino rabbits: The effects of imipenem and aztreonam. Investig. Ophthalmol. Vis. Sci. 1993, 34, 3466–3476. [Google Scholar] [PubMed]
- Conway, B.P.; Campochiaro, P.A. Macular Infarction After Endophthalmitis Treated With Vitrectomy and Intravitreal Gentamicin. Arch. Ophthalmol. 1986, 104, 367–371. [Google Scholar] [CrossRef]
- Zachary, I.G.; Forster, R.K. Experimental Intravitreal Gentamicin. Am. J. Ophthalmol. 1976, 82, 604–611. [Google Scholar] [CrossRef]
- Bakri, S.J.; Snyder, M.R.; Reid, J.M.; Pulido, J.S.; Singh, R.J. Pharmacokinetics of Intravitreal Bevacizumab (Avastin). Ophthalmology 2007, 114, 855–859. [Google Scholar] [CrossRef]
- Bakri, S.J.; Snyder, M.R.; Reid, J.M.; Pulido, J.S.; Ezzat, M.K.; Singh, R.J. Pharmacokinetics of Intravitreal Ranibizumab (Lucentis). Ophthalmology 2007, 114, 2179–2182. [Google Scholar] [CrossRef]
- Drug Product Development for the Back of the Eye by Uday B. Kompella, Henry F. Edelhauser | 9781441999191 | Reviews, Description and More @ BetterWorldBooks.com. Available online: https://www.betterworldbooks.com/product/detail/drug-product-development-for-the-back-of-the-eye-1441999191 (accessed on 6 April 2018).
- Schopf, L.R.; Popov, A.M.; Enlow, E.M.; Bourassa, J.L.; Ong, W.Z.; Nowak, P.; Chen, H. Topical Ocular Drug Delivery to the Back of the Eye by Mucus-Penetrating Particles. Transl. Vis. Sci. Technol. 2015, 4. [Google Scholar] [CrossRef] [Green Version]
- Madni, A.; Rahem, M.A.; Tahir, N.; Sarfraz, M.; Jabar, A.; Rehman, M.; Kashif, P.M.; Badshah, S.F.; Khan, K.U.; Santos, H.A. Non-invasive strategies for targeting the posterior segment of eye. Int. J. Pharm. 2017, 530, 326–345. [Google Scholar] [CrossRef]
- Ruponen, M.; Urtti, A. Undefined role of mucus as a barrier in ocular drug delivery. Eur. J. Pharm. Biopharm. 2015, 96, 442–446. [Google Scholar] [CrossRef]
- Jünemann, A.G.M.; Choragiewicz, T.; Ozimek, M.; Grieb, P.; Rejdak, R. Drug bioavailability from topically applied ocular drops. Does drop size matter? Ophthalmol. J. 2016, 1, 29–35. [Google Scholar] [CrossRef] [Green Version]
- Vadlapudi, A.D.; CholKAr, K.; Dasari, S.R.; Mitra, A.K. Ocular Drug Delivery; Jones Bartlett Learn: Burlington, MA, USA, 2015; pp. 219–263. [Google Scholar]
- Watsky, M.A.; Jablonski, M.M.; Edelhauser, H.F. Comparison of conjunctival and corneal surface areas in rabbit and human. Curr. Eye Res. 1988, 7, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Coca-Prados, M. The blood-aqueous barrier in health and disease. J. Glaucoma 2014, 23, S36–S38. [Google Scholar] [CrossRef] [PubMed]
- Barar, J.; Javadzadeh, A.R.; Omidi, Y. Ocular novel drug delivery: Impacts of membranes and barriers. Expert Opin. Drug Deliv. 2008, 5, 567–581. [Google Scholar] [CrossRef] [PubMed]
- Kaur, I.P.; Kakkar, S. Nanotherapy for posterior eye diseases. J. Control. Release 2014, 193, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Boddu, S.H.S.; Gunda, S.; Earla, R.; Mitra, A.K. Ocular microdialysis: A continuous sampling technique to study pharmacokinetics and pharmacodynamics in the eye. Bioanalysis 2010, 2, 487–507. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Osuna, I.; Andrés-Guerrero, V.; Pastoriza Abal, P.; Molina-Martínez, I.T.; Herrero-Vanrell, R. Pharmaceutical microscale and nanoscale approaches for efficient treatment of ocular diseases. Drug Deliv. Transl. Res. 2016, 6, 686–707. [Google Scholar] [CrossRef]
- Quantitative and Qualitative Prediction of Corneal Permeability for Drug-Like Compounds—ScienceDirect. Available online: https://0-www-sciencedirect-com.brum.beds.ac.uk/science/article/pii/S003991401100779X?via%3Dihub (accessed on 18 April 2018).
- Shen, J.; Deng, Y.; Jin, X.; Ping, Q.; Su, Z.; Li, L. Thiolated nanostructured lipid carriers as a potential ocular drug delivery system for cyclosporine A: Improving in vivo ocular distribution. Int. J. Pharm. 2010, 402, 248–253. [Google Scholar] [CrossRef]
- Sociedad Española de Oftalmología. Available online: http://www.oftalmo.com/seo/archivos/articulo.php?idSolicitud=905&numR=9&mesR=9&anioR=2001&idR=50 (accessed on 20 April 2018).
- Ramsay, E.; Del Amo, E.M.; Toropainen, E.; Tengvall-Unadike, U.; Ranta, V.-P.; Urtti, A.; Ruponen, M. Corneal and conjunctival drug permeability: Systematic comparison and pharmacokinetic impact in the eye. Eur. J. Pharm. Sci. 2018, 119, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Stjernschantz, J.; Selén, G.; Astin, M.; Karlsson, M.; Resul, B. Effect of latanoprost on regional blood flow and capillary permeability in the monkey eye. Arch. Ophthalmol. 1999, 117, 1363–1367. [Google Scholar] [CrossRef] [Green Version]
- Utility of Transporter/Receptor(s) in Drug Delivery to the Eye. Available online: https://www.researchgate.net/publication/236974311_Utility_of_transporterreceptors_in_drug_delivery_to_the_eye (accessed on 6 April 2018).
- Thrimawithana, T.R.; Young, S.; Bunt, C.R.; Green, C.; Alany, R.G. Drug delivery to the posterior segment of the eye. Drug Discov. Today 2011, 16, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Kent, A.R.; Nussdorf, J.D.; David, R.; Tyson, F.; Small, D.; Fellows, D. Vitreous concentration of topically applied brimonidine tartrate 0.2%. Ophthalmology 2001, 108, 784–787. [Google Scholar] [CrossRef]
- Balguri, S.P.; Adelli, G.R.; Majumdar, S. Topical ophthalmic lipid nanoparticle formulations (SLN, NLC) of indomethacin for delivery to the posterior segment ocular tissues. Eur. J. Pharm. Biopharm. 2016, 109, 224–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ying, L.; Tahara, K.; Takeuchi, H. Drug delivery to the ocular posterior segment using lipid emulsion via eye drop administration: Effect of emulsion formulations and surface modification. Int. J. Pharm. 2013, 453, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Wang, J.; Jiang, M.; Bartlett, H.; Ouyang, D.; Eperjesi, F.; Liu, J.; Gan, Y. Recent advances in topical ophthalmic drug delivery with lipid-based nanocarriers. Drug Discov. Today 2013, 18, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.M.; Normando, E.M.; Guo, L.; Turner, L.A.; Nizari, S.; O’Shea, P.; Moss, S.E.; Somavarapu, S.; Cordeiro, M.F. Topical delivery of Avastin to the posterior segment of the eye in vivo using annexin A5-associated liposomes. Small Weinh. Bergstr. Ger. 2014, 10, 1575–1584. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, Y.; Zhang, L.; Wang, Q.; Zhang, D. Stability of nanosuspensions in drug delivery. J. Control. Release 2013, 172, 1126–1141. [Google Scholar] [CrossRef]
- Koeberle, M.J.; Hughes, P.M.; Skellern, G.G.; Wilson, C.G. Pharmacokinetics and disposition of memantine in the arterially perfused bovine eye. Pharm. Res. 2006, 23, 2781–2798. [Google Scholar] [CrossRef]
- Acheampong, A.A.; Shackleton, M.; John, B.; Burke, J.; Wheeler, L.; Tang-Liu, D. Distribution of brimonidine into anterior and posterior tissues of monkey, rabbit, and rat eyes. Drug Metab. Dispos. Biol. Fate Chem. 2002, 30, 421–429. [Google Scholar] [CrossRef] [Green Version]
- Loftsson, T.; Hreinsdóttir, D.; Stefánsson, E. Cyclodextrin microparticles for drug delivery to the posterior segment of the eye: Aqueous dexamethasone eye drops. J. Pharm. Pharmacol. 2007, 59, 629–635. [Google Scholar] [CrossRef]
- Sigurdsson, H.H.; Konráethsdóttir, F.; Loftsson, T.; Stefánsson, E. Topical and systemic absorption in delivery of dexamethasone to the anterior and posterior segments of the eye. Acta Ophthalmol. Scand. 2007, 85, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Díez, J.E.B.; Pujol, M.M. Farmacología Ocular; Univ. Politèc. de Catalunya: Catalunya, Spain, 2002; ISBN 978-84-8301-647-3. [Google Scholar]
- Del Amo Páez, E.M. Ocular and Systemic Pharmacokinetic Models for Drug Discovery and Development. Ph.D. Dissertation, University of Helsinki, Helsinki, Finland, 2015. [Google Scholar]
- Mitra, A.K.; Anand, B.S.; Duvvuri, S. Drug delivery to the eye. In The Biology of the Eye; Fischbarg, J., Ed.; Academic Press: New York, NY, USA, 2006; Volume 10, pp. 307–351. [Google Scholar]
- Janoria, K.G.; Gunda, S.; Boddu, S.H.S.; Mitra, A.K. Novel approaches to retinal drug delivery. Expert Opin. Drug Deliv. 2007, 4, 371–388. [Google Scholar] [CrossRef] [PubMed]
- Hughes, P.; Olejnik, O.; Changlin, J.; Wilson, C. Topical and systemic drug delivery to the posterior segments. Adv. Drug Deliv. Rev. 2005, 57, 2010–2032. [Google Scholar] [CrossRef] [PubMed]
- Kwan, A.S.L.; Barry, C.; McAllister, I.L.; Constable, I. Fluorescein angiography and adverse drug reactions revisited: The Lions Eye experience. Clin. Exp. Ophthalmol. 2006, 34, 33–38. [Google Scholar] [CrossRef]
- Vellonen, K.-S.; Soini, E.-M.; del Amo, E.M.; Urtti, A. Prediction of Ocular Drug Distribution from Systemic Blood Circulation. Mol. Pharm. 2016, 13, 2906–2911. [Google Scholar] [CrossRef]
- Hosoya, K.; Tomi, M. Advances in the cell biology of transport via the inner blood-retinal barrier: Establishment of cell lines and transport functions. Biol. Pharm. Bull. 2005, 28, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Routes of Administration for Ocular Medications—Pharmacology. Available online: https://www.msdvetmanual.com/pharmacology/systemic-pharmacotherapeutics-of-the-eye/routes-of-administration-for-ocular-medications (accessed on 14 May 2018).
- Gkretsi, V.; Zacharia, L.C.; Stylianopoulos, T. Targeting inflammation to improve tumor drug delivery. Trends Cancer 2017, 3, 621–630. [Google Scholar] [CrossRef] [Green Version]
- Urtti, A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1131–1135. [Google Scholar] [CrossRef]
- Toda, R.; Kawazu, K.; Oyabu, M.; Miyazaki, T.; Kiuchi, Y. Comparison of Drug Permeabilities across the Blood–Retinal Barrier, Blood–Aqueous Humor Barrier, and Blood–Brain Barrier. J. Pharm. Sci. 2011, 100, 3904–3911. [Google Scholar] [CrossRef]
- Gaudana, R.; Jwala, J.; Boddu, S.H.S.; Mitra, A.K. Recent Perspectives in Ocular Drug Delivery. Pharm. Res. 2009, 26, 1197–1216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiye, Y.; Jianting, Z. Multidrug resistance-associated protein 1 (MRP1/ABCC1) polymorphism: From discovery to clinical application. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2011, 36, 927–938. [Google Scholar]
- ABCC1 Gene—GeneCards | MRP1 Protein | MRP1 Antibody. Available online: https://www.genecards.org/cgi-bin/carddisp.pl?gene=ABCC1 (accessed on 16 May 2018).
- Pitkänen, L.; Ranta, V.-P.; Moilanen, H.; Urtti, A. Binding of Betaxolol, Metoprolol and Oligonucleotides to Synthetic and Bovine Ocular Melanin, and Prediction of Drug Binding to Melanin in Human Choroid-Retinal Pigment Epithelium. Pharm. Res. 2007, 24, 2063–2070. [Google Scholar] [CrossRef] [PubMed]
- Demetriades, A.M.; Deering, T.; Liu, H.; Lu, L.; Gehlbach, P.; Packer, J.D.; Gabhann, F.M.; Popel, A.S.; Wei, L.L.; Campochiaro, P.A. Trans-scleral Delivery of Antiangiogenic Proteins. J. Ocul. Pharmacol. Ther. 2008, 24, 70–79. [Google Scholar] [CrossRef] [Green Version]
- Menon, I.A.; Wakeham, D.C.; Persad, S.D.; Avaria, M.; Trope, G.E.; Basu, P.K. Quantitative determination of the melanin contents in ocular tissues from human blue and brown eyes. J. Ocul. Pharmacol. Ther. 1992, 8, 35–42. [Google Scholar] [CrossRef]
- Schoenwald, R.D.; Tandon, V.; Wurster, D.E.; Barfknecht, C.F. Significance of melanin binding and metabolism in the activity of 5-acetoxyacetylimino-4-methyl-Δ2-1, 3, 4,-thiadiazoline-2-sulfonamide1. Eur. J. Pharm. Biopharm. 1998, 46, 39–50. [Google Scholar] [CrossRef]
- Leblanc, B.; Jezequel, S.; Davies, T.; Hanton, G.; Taradach, C. Binding of drugs to eye melanin is not predictive of ocular toxicity. Regul. Toxicol. Pharmacol. 1998, 28, 124–132. [Google Scholar] [CrossRef]
- Larsson, B.S. Interaction between chemicals and melanin. Pigment Cell Res. 1993, 6, 127–133. [Google Scholar] [CrossRef]
- Bill, A.; Törnquist, P.; Alm, A. Permeability of the intraocular blood vessels. Trans. Ophthalmol. Soc. 1980, 100, 332–336. [Google Scholar]
- Guymer, R.H.; Bird, A.C.; Hageman, G.S. Cytoarchitecture of Choroidal Capillary Endothelial Cells. Investig. Opthalmol. Vis. Sci. 2004, 45, 1660. [Google Scholar] [CrossRef] [Green Version]
- Ranta, V.-P.; Mannermaa, E.; Lummepuro, K.; Subrizi, A.; Laukkanen, A.; Antopolsky, M.; Murtomäki, L.; Hornof, M.; Urtti, A. Barrier analysis of periocular drug delivery to the posterior segment. J. Control. Release 2010, 148, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Patel, A. Ocular drug delivery systems: An overview. World J. Pharmacol. 2013, 2, 47. [Google Scholar] [CrossRef] [PubMed]
- Constable, P.A.; Lawrenson, J.G.; Dolman, D.E.M.; Arden, G.B.; Abbott, N.J. P-Glycoprotein expression in human retinal pigment epithelium cell lines. Exp. Eye Res. 2006, 83, 24–30. [Google Scholar] [CrossRef]
- Eye Drugs—Prescribing and Administering. Patient. Available online: https://patient.info/doctor/eye-drugs-prescribing-and-administering (accessed on 14 May 2018).
- Samtani, S.; Amaral, J.; Campos, M.M.; Fariss, R.N.; Becerra, S.P. Doxycycline-Mediated Inhibition of Choroidal Neovascularization. Investig. Opthalmol. Vis. Sci. 2009, 50, 5098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kampougeris, G. Penetration of moxifloxacin into the human aqueous humour after oral administration. Br. J. Ophthalmol. 2005, 89, 628–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García, D.A.R. Chapter 5 Quimioterapia inmunosupresora en uveítis. In Oftalmología en la Opinión de los Expertos; Temas Selectos en Uveítis; Santos Garcia, A., Ed.; Publisher Garaitia Editores S.A. de C.V.: México D.F., México, 2011; Volume 7. [Google Scholar]
- Kaur, I.P.; Smitha, R.; Aggarwal, D.; Kapil, M. Acetazolamide: Future perspective in topical glaucoma therapeutics. Int. J. Pharm. 2002, 248, 1–14. [Google Scholar] [CrossRef]
- Shirasaki, Y. Molecular Design for Enhancement of Ocular Penetration. J. Pharm. Sci. 2008, 97, 2462–2496. [Google Scholar] [CrossRef]
- Pérez-Blázquez, E.; Redondo, M.I.; Gracia, T. Sida y oftalmología: Una visión actual. An. Sist. Sanit. Navar. 2008, 31, 69–81. [Google Scholar] [CrossRef]
- Benatar-Haserfaty, J.; Flores, J.A.P. Anestesia locorregional en oftalmología: Una puesta al día. Oculoplastia 2003, 50, 11. [Google Scholar]
- García, E.; Mensa, J.; Martínez, J.A. Diffusion and pharmacokinetics of antibiotics in the ocular globus. Therapeutic implications. Rev. Esp. Quim. 2001, 14, 331–339. [Google Scholar]
- Lin, P.; Suhler, E.B.; Rosenbaum, J.T. The Future of Uveitis Treatment. Ophthalmology 2014, 121, 365–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duica, I.; Voinea, L.-M.; Mitulescu, C.; Istrate, S.; Coman, I.-C.; Ciuluvica, R. The use of biologic therapies in uveitis. Rom. J. Ophthalmol. 2018, 62, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Kahn, M. Bioavailability of vitamin B using a small-volume nebulizer ophthalmic drug delivery system. Clin. Exp. Ophthalmol. 2005, 33, 402–407. [Google Scholar] [CrossRef]
- Yoo, W.S.; Kim, C.R.; Kim, B.J.; Ahn, S.K.; Seo, S.W.; Yoo, J.M.; Kim, S.J. Successful Treatment of Infectious Scleritis by Pseudomonas aeruginosa with Autologous Perichondrium Graft of Conchal Cartilage. Yonsei Med. J. 2015, 56, 1738–1741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, S.G.; Flynn, H.W. Update on the prevention and treatment of endophthalmitis. Expert Rev. Ophthalmol. 2014, 9, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Sallam, A.B.; Kirkland, K.A.; Barry, R.; Soliman, M.K.; Ali, T.K.; Lightman, S. A Review of Antimicrobial Therapy for Infectious Uveitis of the Posterior Segment. Med. Hypothesis Discov. Innov. Ophthalmol. 2018, 7, 140–155. [Google Scholar] [PubMed]
- Unidad de Enfermedades Vitreorretinianas—FISABIO. Available online: http://fisabio.san.gva.es/unidad-de-enfermedades-vitreorretinianas1 (accessed on 14 May 2018).
- Andrés, S.; Higueras, M.I.; Mozaz, T. Efectos Adversos Oculares Asociados a Medicamentos y Productos Oftálmicos. Colegio Oficial de Farmacéuticos de Zaragoza. Vocalía de Optica. 2008. Available online: https://www.academiadefarmaciadearagon.es/docs/Documentos/Documento24.pdf (accessed on 14 March 2020).
- Kim, H.; Robinson, M.R.; Lizak, M.J.; Tansey, G.; Lutz, R.J.; Yuan, P.; Wang, N.S.; Csaky, K.G. Controlled Drug Release from an Ocular Implant: An Evaluation Using Dynamic Three-Dimensional Magnetic Resonance Imaging. Investig. Opthalmology Vis. Sci. 2004, 45, 2722. [Google Scholar] [CrossRef] [Green Version]
- Geroski, D.H.; Edelhauser, H.F. Drug delivery for posterior segment eye disease. Investig. Ophthalmol. Vis. Sci. 2000, 41, 961–964. [Google Scholar]
- Prausnitz, M.R.; Noonan, J.S. Permeability of cornea, sclera, and conjunctiva: A literature analysis for drug delivery to the eye. J. Pharm. Sci. 1998, 87, 1479–1488. [Google Scholar] [CrossRef]
- Conrad, J.M.; Robinson, J.R. Mechanisms of anterior segment absorption of pilocarpine following subconjunctival injection in albino rabbits. J. Pharm. Sci. 1980, 69, 875–884. [Google Scholar] [CrossRef]
- Kaiser, P.K.; Goldberg, M.F.; Davis, A.A. Posterior Juxtascleral Depot Administration of Anecortave Acetate. Surv. Ophthalmol. 2007, 52, S62–S69. [Google Scholar] [CrossRef] [PubMed]
- Ambati, J.; Adamis, A.P. Transscleral drug delivery to the retina and choroid. Prog. Retin. Eye Res. 2002, 21, 145–151. [Google Scholar] [CrossRef]
- Ghate, D.; Edelhauser, H.F. Ocular drug delivery. Expert Opin. Drug Deliv. 2006, 3, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-B.; Geroski, D.H.; Prausnitz, M.R.; Edelhauser, H.F. Drug delivery through the sclera: Effects of thickness, hydration, and sustained release systems. Exp. Eye Res. 2004, 78, 599–607. [Google Scholar] [CrossRef]
- Bourges, J.L.; Bloquel, C.; Thomas, A.; Froussart, F.; Bochot, A.; Azan, F.; Gurny, R.; BenEzra, D.; Behar-Cohen, F. Intraocular implants for extended drug delivery: Therapeutic applications. Adv. Drug Deliv. Rev. 2006, 58, 1182–1202. [Google Scholar] [CrossRef] [PubMed]
- Raghava, S.; Hammond, M.; Kompella, U.B. Periocular routes for retinal drug delivery. Expert Opin. Drug Deliv. 2004, 1, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Olsen, T.W.; Edelhauser, H.F.; Lim, J.I.; Geroski, D.H. Human scleral permeability. Effects of age, cryotherapy, transscleral diode laser, and surgical thinning. Investig. Ophthalmol. Vis. Sci. 1995, 36, 1893–1903. [Google Scholar]
- Ambati, J.; Gragoudas, E.S.; Miller, J.W.; You, T.T.; Miyamoto, K.; Delori, F.C.; Adamis, A.P. Transscleral delivery of bioactive protein to the choroid and retina. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1186–1191. [Google Scholar]
- Marmor, M.F.; Negi, A.; Maurice, D.M. Kinetics of macromolecules injected into the subretinal space. Exp. Eye Res. 1985, 40, 687–696. [Google Scholar] [CrossRef]
- Geroski, D.H.; Edelhauser, H.F. Transscleral drug delivery for posterior segment disease. Adv. Drug Deliv. Rev. 2001, 52, 37–48. [Google Scholar] [CrossRef]
- Kim, S.H.; Csaky, K.G.; Wang, N.S.; Lutz, R.J. Drug elimination kinetics following subconjunctival injection using dynamic contrast-enhanced magnetic resonance imaging. Pharm. Res. 2008, 25, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Zhu, Y.; Yu, P.K.; Yu, X.; Sun, X.; Cringle, S.J.; Su, E.-N.; Yu, D.-Y. Quantitative study of the topographic distribution of conjunctival lymphatic vessels in the monkey. Exp. Eye Res. 2012, 94, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Myles, M.E.; Neumann, D.M.; Hill, J.M. Recent progress in ocular drug delivery for posterior segment disease: Emphasis on transscleral iontophoresis. Adv. Drug Deliv. Rev. 2005, 57, 2063–2079. [Google Scholar] [CrossRef]
- Shah, S.S.; Denham, L.V.; Elison, J.R.; Bhattacharjee, P.S.; Clement, C.; Huq, T.; Hill, J.M. Drug delivery to the posterior segment of the eye for pharmacologic therapy. Expert Rev. Ophthalmol. 2010, 5, 75–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kompella, U.B.; Bandi, N.; Ayalasomayajula, S.P. Subconjunctival nano- and microparticles sustain retinal delivery of budesonide, a corticosteroid capable of inhibiting VEGF expression. Investig. Ophthalmol. Vis. Sci. 2003, 44, 1192–1201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayalasomayajula, S.P.; Kompella, U.B. Celecoxib, a selective cyclooxygenase-2 inhibitor, inhibits retinal vascular endothelial growth factor expression and vascular leakage in a streptozotocin-induced diabetic rat model. Eur. J. Pharmacol. 2003, 458, 283–289. [Google Scholar] [CrossRef]
- Ayalasomayajula, S.P.; Kompella, U.B. Retinal delivery of celecoxib is several-fold higher following subconjunctival administration compared to systemic administration. Pharm. Res. 2004, 21, 1797–1804. [Google Scholar] [CrossRef]
- Ayalasomayajula, S.P.; Kompella, U.B. Subconjunctivally administered celecoxib-PLGA microparticles sustain retinal drug levels and alleviate diabetes-induced oxidative stress in a rat model. Eur. J. Pharmacol. 2005, 511, 191–198. [Google Scholar] [CrossRef]
- Amrite, A.C.; Ayalasomayajula, S.P.; Cheruvu, N.P.S.; Kompella, U.B. Single periocular injection of celecoxib-PLGA microparticles inhibits diabetes-induced elevations in retinal PGE2, VEGF, and vascular leakage. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1149–1160. [Google Scholar] [CrossRef] [Green Version]
- Misra, G.P.; Singh, R.S.J.; Aleman, T.S.; Jacobson, S.G.; Gardner, T.W.; Lowe, T.L. Subconjunctivally implantable hydrogels with degradable and thermoresponsive properties for sustained release of insulin to the retina. Biomaterials 2009, 30, 6541–6547. [Google Scholar] [CrossRef] [Green Version]
- Tsui, J.Y.; Dalgard, C.; Van Quill, K.R.; Lee, L.; Grossniklaus, H.E.; Edelhauser, H.F.; O’Brien, J.M. Subconjunctival topotecan in fibrin sealant in the treatment of transgenic murine retinoblastoma. Investig. Ophthalmol. Vis. Sci. 2008, 49, 490–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gangaputra, S.; Newcomb, C.W.; Liesegang, T.L.; Kaçmaz, R.O.; Jabs, D.A.; Levy-Clarke, G.A.; Nussenblatt, R.B.; Rosenbaum, J.T.; Suhler, E.B.; Thorne, J.E.; et al. Methotrexate for Ocular Inflammatory Diseases. Ophthalmology 2009, 116, 2188–2198.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, C.W.; Czarny, B.; Metselaar, J.M.; Ho, C.; Ng, S.R.; Barathi, A.V.; Storm, G.; Wong, T.T. Evaluation of subconjunctival liposomal steroids for the treatment of experimental uveitis. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rowe-Rendleman, C.L.; Durazo, S.A.; Kompella, U.B.; Rittenhouse, K.D.; Di Polo, A.; Weiner, A.L.; Grossniklaus, H.E.; Naash, M.I.; Lewin, A.S.; Horsager, A.; et al. Drug and Gene Delivery to the Back of the Eye: From Bench to Bedside. Investig. Ophthalmol. Vis. Sci. 2014, 55, 2714–2730. [Google Scholar] [CrossRef] [Green Version]
- Imai, H.; Misra, G.P.; Wu, L.; Janagam, D.R.; Gardner, T.W.; Lowe, T.L. Subconjunctivally Implanted Hydrogels for Sustained Insulin Release to Reduce Retinal Cell Apoptosis in Diabetic Rats. Investig. Ophthalmol. Vis. Sci. 2015, 56, 7839–7846. [Google Scholar] [CrossRef] [Green Version]
- Ghate, D.; Brooks, W.; McCarey, B.E.; Edelhauser, H.F. Pharmacokinetics of intraocular drug delivery by periocular injections using ocular fluorophotometry. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2230–2237. [Google Scholar] [CrossRef]
- Roper-Hall, M.J. Anesthesia and Akinesia for Eye Operations; Wright & Sons Ltd.: Bristol, UK, 1989. [Google Scholar]
- Canavan, K.S.; Dark, A.; Garrioch, M.A. Sub-Tenon’s administration of local anaesthetic: A review of the technique. Br. J. Anaesth. 2003, 90, 787–793. [Google Scholar] [CrossRef] [Green Version]
- JJ, K.; Bowling, B. Clinical Ophthalmology: A Systematic Approach, 7th ed.; Saunders/Elsevier: Philadelphia, PA, USA, 2011. [Google Scholar]
- Ehlers, J.P.; Gregory, L.F. The Wills Eye Manual: Office and Emergency Room Diagnosis and Treatment of Eye Disease, 5th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008. [Google Scholar]
- Lafranco Dafflon, M.; Tran, V.T.; Guex-Crosier, Y.; Herbort, C.P. Posterior sub-Tenon’s steroid injections for the treatment of posterior ocular inflammation: Indications, efficacy and side effects. Graefes Arch. Clin. Exp. Ophthalmol. 1999, 237, 289–295. [Google Scholar] [CrossRef]
- Tanner, V.; Kanski, J.J.; Frith, P.A. Posterior sub-Tenon’s triamcinolone injections in the treatment of uveitis. Eye Lond. Engl. 1998, 12 Pt 4, 679–685. [Google Scholar] [CrossRef]
- Choi, Y.J.; Oh, I.K.; Oh, J.R.; Huh, K. Intravitreal versus posterior subtenon injection of triamcinolone acetonide for diabetic macular edema. Korean J. Ophthalmol. KJO 2006, 20, 205–209. [Google Scholar] [CrossRef] [Green Version]
- Cardillo, J.A.; Melo, L.A.S.; Costa, R.A.; Skaf, M.; Belfort, R.; Souza-Filho, A.A.; Farah, M.E.; Kuppermann, B.D. Comparison of intravitreal versus posterior sub-Tenon’s capsule injection of triamcinolone acetonide for diffuse diabetic macular edema. Ophthalmology 2005, 112, 1557–1563. [Google Scholar] [CrossRef] [PubMed]
- Ozkiriş, A.; Erkiliç, K. Complications of intravitreal injection of triamcinolone acetonide. Can. J. Ophthalmol. 2005, 40, 63–68. [Google Scholar] [CrossRef]
- Shen, L.; You, Y.; Sun, S.; Chen, Y.; Qu, J.; Cheng, L. Intraocular and systemic pharmacokinetics of triamcinolone acetonide after a single 40-mg posterior subtenon application. Ophthalmology 2010, 117, 2365–2371. [Google Scholar] [CrossRef] [PubMed]
- Accola, P.J.; Bentley, E.; Smith, L.J.; Forrest, L.J.; Baumel, C.A.; Murphy, C.J. Development of a retrobulbar injection technique for ocular surgery and analgesia in dogs. J. Am. Vet. Med. Assoc. 2006, 229, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Kazancioglu, L.; Batcik, S.; Kazdal, H.; Sen, A.; Sekeryapan Gediz, B.; Erdivanli, B. Complication of Peribulbar Block: Brainstem Anaesthesia. Turk. J. Anesth. Reanim. 2017, 45, 231–233. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.; Laird, P.; Debiec, M.; Hwang, C.; Zhang, R.; Yan, J.; Hendrick, A.; Hubbard, G.B.; Bergstrom, C.S.; Yeh, S.; et al. Formulation of a Peribulbar Block for Prolonged Postoperative Pain Management in Vitreoretinal Surgery. Ophthalmol. Retina 2018, 2, 268–275. [Google Scholar] [CrossRef]
- Iriyama, A.; Obata, R.; Inoue, Y.; Takahashi, H.; Tamaki, Y.; Yanagi, Y. Effect of posterior juxtascleral triamcinolone acetonide on the efficacy and choriocapillaris hypoperfusion of photodynamic therapy. Graefes Arch. Clin. Exp. Ophthalmol. 2008, 246, 339–344. [Google Scholar] [CrossRef]
- Hayek, S.; Scherrer, M.; Barthelmes, D.; Fleischhauer, J.; Kurz-Levin, M.; Menghini, M.; Helbig, H.; Sutter, F. First Clinical Experience with Anecortave Acetate (Retaane®). Klin. Monatsblätter Für Augenheilkd. 2007, 224, 279–281. [Google Scholar] [CrossRef]
- Patel, S.R.; Lin, A.S.P.; Edelhauser, H.F.; Prausnitz, M.R. Suprachoroidal Drug Delivery to the Back of the Eye Using Hollow Microneedles. Pharm. Res. 2011, 28, 166–176. [Google Scholar] [CrossRef]
- Krohn, J.; Bertelsen, T. Corrosion casts of the suprachoroidal space and uveoscleral drainage routes in the human eye. Acta Ophthalmol. Scand. 2009, 75, 32–35. [Google Scholar] [CrossRef]
- Krohn, J.; Bertelsen, T. Light microscopy of uveoscleral drainage routes after gelatine injections into the suprachoroidal space. Acta Ophthalmol. Scand. 1998, 76, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Einmahl, S.; Savoldelli, M.; D’Hermies, F.; Tabatabay, C.; Gurny, R.; Behar-Cohen, F. Evaluation of a Novel Biomaterial in the Suprachoroidal Space of the Rabbit Eye. Retina 2002, 43, 7. [Google Scholar]
- Olsen, T.W.; Feng, X.; Wabner, K.; Conston, S.R.; Sierra, D.H.; Folden, D.V.; Smith, M.E.; Cameron, J.D. Cannulation of the Suprachoroidal Space: A Novel Drug Delivery Methodology to the Posterior Segment. Am. J. Ophthalmol. 2006, 142, 777–787.e2. [Google Scholar] [CrossRef] [PubMed]
- Kompella, U.B.; Edelhauser, H.F. Drug Product Development for the Back of the Eye; American Association of Pharmaceutical Scientists, Ed.; AAPS advances in the pharmaceutical sciences series; AAPS Press: New York, NY, USA, 2011; ISBN 978-1-4419-9919-1. [Google Scholar]
- Liu Suprachoroidal Injection of Ketorolac Tromethamine does not Cause Retinal Damage. Available online: http://www.nrronline.org/article.asp?issn=1673-5374;year=2012;volume=7;issue=35;spage=2770;epage=2777;aulast=Liu (accessed on 21 July 2018).
- Patel, S.R.; Berezovsky, D.E.; McCarey, B.E.; Zarnitsyn, V.; Edelhauser, H.F.; Prausnitz, M.R. Targeted Administration into the Suprachoroidal Space Using a Microneedle for Drug Delivery to the Posterior Segment of the Eye. Investig. Opthalmol. Vis. Sci. 2012, 53, 4433. [Google Scholar] [CrossRef]
- Falavarjani, K.G.; Nguyen, Q.D. Adverse events and complications associated with intravitreal injection of anti-VEGF agents: A review of literature. Eye 2013, 27, 787. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Tang, L.; Zhou, Y. Subretinal Injection: A Review on the Novel Route of Therapeutic Delivery for Vitreoretinal Diseases. Ophthalmic Res. 2017, 58, 217–226. [Google Scholar] [CrossRef]
- Johnson, C.J.; Berglin, L.; Chrenek, M.A.; Redmond, T.M.; Boatright, J.H.; Nickerson, J.M. Technical brief: Subretinal injection and electroporation into adult mouse eyes. Mol. Vis. 2008, 14, 2211. [Google Scholar]
- Timmers, A.M.; Zhang, H.; Squitieri, A.; Gonzalez-Pola, C. Subretinal injections in rodent eyes: Effects on electrophysiology and histology of rat retina. Mol. Vis. 2001, 7, 131–137. [Google Scholar]
- Qi, Y.; Dai, X.; Zhang, H.; He, Y.; Zhang, Y.; Han, J.; Zhu, P.; Zhang, Y.; Zheng, Q.; Li, X.; et al. Trans-Corneal Subretinal Injection in Mice and Its Effect on the Function and Morphology of the Retina. PLoS ONE 2015, 10, e0136523. [Google Scholar] [CrossRef]







| Pharmacologic Group | Drug | Characteristics | Half-Life Time (h) | Ref. |
|---|---|---|---|---|
| Corticosteroids | Dexamethasone | Low molecular weight Water insoluble | 3.48 | [66] |
| Antibiotics | Ceftizoxime | Low molecular weight Water soluble | 5.70 | [67] |
| Somatostatin analogues | Octreotide acetate | High molecular weight Water soluble | 16.00 | [68] |
| Antiviral | ISIS 2922 | High molecular weight Water soluble | 62.00 | [69] |
| Features | Anterior Route | Posterior Route |
|---|---|---|
| Tissue involved | BAB | BRB |
| Elimination pathway | Aqueous humor outflow | Choroidal flow |
| Molecule characteristics | Hydrophilic High molecular weight | Lipophilic Small molecular weight |
| Pharmacologic Group | Subgroup/Drug | Half-Life Time (h) | MRT (h) | Cmax (µg/mL) | Ref. |
|---|---|---|---|---|---|
| Nonsteroidal anti-inflammatory drugs | Ketorolac | 4.3 | 6.16 | 175 | [83] |
| Diclofenac | 2.05 | 2.95 | 65 | [83] | |
| Antibiotics | Penicillines | 10–20 | 5–25 | 1000–5000 | [84,85,86] |
| Cephalosporines | 5–15 | 5–30 | 1000–2250 | [48,85,87] | |
| Tetracyclines | 10–20 | NA | 125–400 | [86,88] | |
| Fluoroquinolones | 3.5–5.5 | 0.25–5 | 100–500 | [49,89,90] | |
| Monobactams | 7.5 | NA | 1000 | [91] | |
| Carbapenems | 2.5–10 | NA | 50–100 | [92,93] | |
| Macrolides | 40–60 | NA | 100–200 | [85,94,95] | |
| Antibodies | Bevacizumab | 4.32 | 5.92 | 400 | [96] |
| Ranibizumab | 2.88 | 4.03 | 162 | [97] |
| Pharmacologic Group | Drug | Pathology | Administration Route | Reference |
|---|---|---|---|---|
| Analgesics | Paracetamol | Ocular trauma treatment-associated pain | Oral | [153] |
| NSAIDs (Flurbiprofen, Ketorolac, Diclofenac, Bromfenac and Nepafenac) | Ocular trauma treatment-associated pain | Oral | [153] | |
| Antibiotics | Doxycycline | Neovascularization | Oral | [110,154] |
| Tetracycline | Ocular rosacea | Oral | [111,155] | |
| Erythromycin | Orbital cellulitis | Oral | [111] | |
| Minomycline | Ocular rosacea | Oral | [110] | |
| Corticosteroids | Dexamethasone | Giant cell arteritis | Oral | [153] |
| Immunosuppressants | Cyclosporine | Idiopathy or related-to-Behçet’s-disease uveitis | Oral | [156] |
| Carbonic anhydrase inhibitors | Acetazolamide (Diamox sequel®) | Glaucoma | Oral | [113,157] |
| Etoxolamide | Glaucoma | Oral | [114,158] |
| Pharmacologic Group | Drug | Pathology | Target | Route | Reference |
|---|---|---|---|---|---|
| Antibodies | Secukinumab Tocilizumab | Uveitis | Inflammatory cytokines | Intravenous | [162,163] |
| Ustekinumab | Subcutaneous | ||||
| Abatacept | T-cell activation | ||||
| Rituximab | B-cell targeting | Subcutaneous | |||
| Vitamins | B12 | Vitamin B12 Deficiency Optic Neuropathy | Folate receptor | Intramuscular | [164] |
| Antibiotics | Penicillin Gentamicin Ceftazidime Amikacin | Uveitis Scleritis Pseudoscleritis Endophtalmitis | Bacteria | Intravenous | [165,166,167] |
| Pharmacologic Group | Drug | Pathology | Reference |
|---|---|---|---|
| Antidiabetics | Insulin | Diabetic retinopathy | [193] |
| Chemotherapeutics | Carboplatin | Retinoblastoma | [194] |
| Topotecan | |||
| Folic acid analogues | Methotrexate | Granulomatous panuveitis | [195] |
| Corticosteroids | Dexamethasone | Uveitis | [196] |
| Pharmacologic Group | Drug | Half-Life Time (days) | MRT (days) | Cmax (ng/mL) | Tmax (h) | Reference |
|---|---|---|---|---|---|---|
| Corticosteroids | Triamcinolone acetonide | 17.1 | 23.1 | 22 | 24 | [209] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varela-Fernández, R.; Díaz-Tomé, V.; Luaces-Rodríguez, A.; Conde-Penedo, A.; García-Otero, X.; Luzardo-Álvarez, A.; Fernández-Ferreiro, A.; Otero-Espinar, F.J. Drug Delivery to the Posterior Segment of the Eye: Biopharmaceutic and Pharmacokinetic Considerations. Pharmaceutics 2020, 12, 269. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12030269
Varela-Fernández R, Díaz-Tomé V, Luaces-Rodríguez A, Conde-Penedo A, García-Otero X, Luzardo-Álvarez A, Fernández-Ferreiro A, Otero-Espinar FJ. Drug Delivery to the Posterior Segment of the Eye: Biopharmaceutic and Pharmacokinetic Considerations. Pharmaceutics. 2020; 12(3):269. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12030269
Chicago/Turabian StyleVarela-Fernández, Rubén, Victoria Díaz-Tomé, Andrea Luaces-Rodríguez, Andrea Conde-Penedo, Xurxo García-Otero, Asteria Luzardo-Álvarez, Anxo Fernández-Ferreiro, and Francisco J. Otero-Espinar. 2020. "Drug Delivery to the Posterior Segment of the Eye: Biopharmaceutic and Pharmacokinetic Considerations" Pharmaceutics 12, no. 3: 269. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12030269