Model-Based Scale-up Methodologies for Pharmaceutical Granulation
Abstract
:1. Introduction
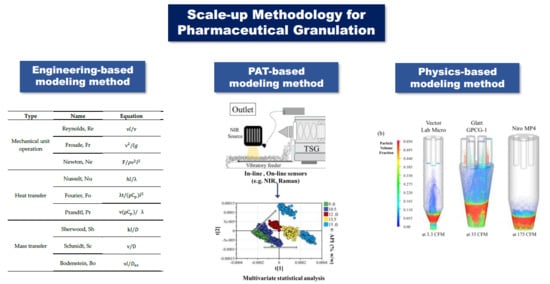
2. Engineering-Based Modeling Method
3. PAT-Based Modeling Method
4. Physics-Based Modeling Method
4.1. DEM
4.2. CFD
4.3. FEM
4.4. Coupled Physics-Based Modeling
5. Scale-Up Strategy for Wet Granulation
5.1. Scale-Up Studies on Wet Granulation Using an Engineering-Based Modeling Method
5.2. Scale-Up Studies on Wet Granulation Using a PAT-Based Modeling Method
5.3. Scale-Up Studies on Wet Granulation Using a Physics-Based Modeling Method
6. Scale-Up Strategy for Dry Granulation
6.1. Scale-Up Studies on Dry Granulation Using an Engineering-Based Modeling Method
6.2. Scale-Up Studies on Dry Granulation Using a PAT-Based Modeling Method
6.3. Scale-Up Studies on Dry Granulation using a Physics-Based Modeling Method
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pathi, S.; Kumar, R.; Surasani, V.K. Investigation on agglomeration kinetics of acetaminophen using fluidized bed wet granulation. Asia Pac. J. Chem. Eng. 2020, 15, e2416. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, B.C.-Y.; Zhou, W.; Xu, B.; Gao, X.; Qiao, Y.; Luo, G. Improved Understanding of the High Shear Wet Granulation Process under the Paradigm of Quality by Design Using Salvia miltiorrhiza Granules. Pharmaceutics 2019, 11, 519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmelzle, S.; Nirschl, H. DEM simulations: Mixing of dry and wet granular material with different contact angles. Granul. Matter. 2018, 20, 19. [Google Scholar] [CrossRef]
- Narang, A.S.; Tao, L.; Zhao, J.; Keluskar, R.; Gour, S.; Stevens, T.; Macias, K.; Remy, B.; Pandey, P.; LaRoche, R.D. Chapter 10—Effect of Binder Attributes on Granule Growth and Densification. In Handbook of Pharmaceutical Wet Granulation; Narang, A.S., Badawy, S.I.F., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 351–386. [Google Scholar]
- Badawy, S.; Pandey, P. Design, Development, and Scale-Up of the High-Shear Wet Granulation Process. In Developing Solid Oral Dosage Form; Elsevier: Amsterdam, The Netherlands, 2017; pp. 749–776. [Google Scholar]
- Börner, M.; Michaelis, M.; Siegmann, E.; Radeke, C.; Schmidt, U. Impact of impeller design on high-shear wet granulation. Powder Technol. 2016, 295, 261–271. [Google Scholar] [CrossRef]
- De Simone, V.; Caccavo, D.; Lamberti, G.; d’Amore, M.; Barba, A.A. Wet-granulation process: Phenomenological analysis and process parameters optimization. Powder Technol. 2018, 340, 411–419. [Google Scholar] [CrossRef]
- Faure, A.; Grimsey, I.; Rowe, R.; York, P.; Cliff, M. Importance of wet mass consistency in the control of wet granulation by mechanical agitation: A demonstration. J. Pharm. Pharm. 1998, 50, 1431–1432. [Google Scholar] [CrossRef] [PubMed]
- Hibare, S.; Acharya, K. Scale-up of detergent granules in a high shear mixer. Powder Technol. 2014, 254, 265–273. [Google Scholar] [CrossRef]
- Levin, M. How to Scale-up a Wet Granulation End Point Scientifically; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Mahmah, O.; Adams, M.; Omar, C.S.; Gururajan, B.; Salman, A.D. Roller compaction: Ribbon splitting and sticking. Int. J. Pharm. 2019, 559, 156–172. [Google Scholar] [CrossRef] [Green Version]
- Grote, S.; Kleinebudde, P. A comparative study of the influence of alpha-lactose monohydrate particle morphology on granule and tablet properties after roll compaction/dry granulation. Pharm. Dev. Technol. 2019, 24, 314–322. [Google Scholar] [CrossRef]
- Pishnamazi, M.; Casilagan, S.; Clancy, C.; Shirazian, S.; Iqbal, J.; Egan, D.; Edlin, C.; Croker, D.M.; Walker, G.M.; Collins, M.N. Microcrystalline cellulose, lactose and lignin blends: Process mapping of dry granulation via roll compaction. Powder Technol. 2019, 341, 38–50. [Google Scholar] [CrossRef]
- Nesarikar, V.V.; Patel, C.; Early, W.; Vatsaraj, N.; Sprockel, O.; Jerzweski, R. Roller compaction process development and scale up using Johanson model calibrated with instrumented roll data. Int. J. Pharm. 2012, 436, 486–507. [Google Scholar] [CrossRef] [PubMed]
- Reynaud, P.; Dubois, J.; Rouby, D.; Fantozzi, G.; Weynant, E. Acoustic emission monitoring of uniaxial pressing of ceramic powders. Ceram. Int. 1992, 18, 391–397. [Google Scholar] [CrossRef]
- Kleinebudde, P. Roll compaction/dry granulation: Pharmaceutical applications. Eur. J. Pharm. Biopharm. 2004, 58, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Gago, A.P.; Reynolds, G.; Kleinebudde, P. Impact of roll compactor scale on ribbon density. Powder Technol. 2018, 337, 92–103. [Google Scholar] [CrossRef]
- Liu, Y.; Wassgren, C. Modifications to Johanson’s roll compaction model for improved relative density predictions. Powder Technol. 2016, 297, 294–302. [Google Scholar] [CrossRef] [Green Version]
- McAuliffe, M.; O’Mahony, G.; Blackshields, C.; Collins, J.; Egan, D.; Kiernan, L.; O’Neill, E.; Lenihan, S.; Walker, G.; Crean, A. The use of PAT and off-line methods for monitoring of roller compacted ribbon and granule properties with a view to continuous processing. Org. Process Res. Dev. 2014, 19, 158–166. [Google Scholar] [CrossRef]
- Raval, N.; Tambe, V.; Maheshwari, R.; Deb, P.K.; Tekade, R.K. Scale-Up Studies in Pharmaceutical Products Development. In Dosage Form Design Considerations; Academic Press: Cambridge, MA, USA, 2018; pp. 669–700. [Google Scholar]
- Amirkia, V.; Heinrich, M. Natural products and drug discovery: A survey of stakeholders in industry and academia. Front. Pharm. 2015, 6, 237. [Google Scholar] [CrossRef] [Green Version]
- Allesø, M.; Holm, R.; Holm, P. Roller compaction scale-up using roll width as scale factor and laser-based determined ribbon porosity as critical material attribute. Eur. J. Pharm. Sci. 2016, 87, 69–78. [Google Scholar] [CrossRef]
- Mazor, A.; Orefice, L.; Michrafy, A.; De Ryck, A.; Khinast, J.G. A combined DEM & FEM approach for modelling roll compaction process. Powder Technol. 2018, 337, 3–16. [Google Scholar] [CrossRef] [Green Version]
- Vladisavljević, G.T.; Khalid, N.; Neves, M.A.; Kuroiwa, T.; Nakajima, M.; Uemura, K.; Ichikawa, S.; Kobayashi, I. Industrial lab-on-a-chip: Design, applications and scale-up for drug discovery and delivery. Adv. Drug Deliv. Rev. 2013, 65, 1626–1663. [Google Scholar] [CrossRef] [Green Version]
- U.S: Food and Drug Administration. Pharmaceutical cGMPS for the 21st Century—A Risk-Based Approach: Second Progress Report and Implementation Plan. FDA website. Drugs section. 2003. Available online: www.fda.gov (accessed on 12 September 2004).
- Food, U.; Administration, D. Guidance for Industry: Q8 (R2) Pharmaceutical Development; Center for Drug Evaluation and Research: Silver Spring, MD, USA, 2009. [Google Scholar]
- Mahoney, J.F.; Yeralan, S. Dimensional Analysis. Procedia Manuf. 2019, 38, 694–701. [Google Scholar] [CrossRef]
- Nakamura, H.; Fujii, H.; Watano, S. Scale-up of high shear mixer-granulator based on discrete element analysis. Powder Technol. 2013, 236, 149–156. [Google Scholar] [CrossRef]
- Levin, M. Pharmaceutical Process Scale-Up, 2nd ed.; Taylor & Francis: Oxfordshire, UK, 2005; ISBN 9781574448764. [Google Scholar]
- Yu, M.; Omar, C.; Weidemann, M.; Schmidt, A.; Litster, J.D.; Salman, A.D. Roller compaction: Infrared thermography as a PAT for monitoring powder flow from feeding to compaction zone. Int. J. Pharm. 2020, 578, 119114. [Google Scholar] [CrossRef] [PubMed]
- Scott, B.; Wilcock, A. Process Analytical Technology in the Pharmaceutical Industry: A Toolkit for Continuous Improvement. PDA J. Pharm. Sci. Technol. 2006, 60, 17–53. [Google Scholar]
- U.S: Food and Drug Administration. Guidance for industry, PAT-A Framework for Innovative Pharmaceutical Development, Manufacturing and Quality Assurance. 2004. Available online: http://www.fda.gov/cder/guidance/published.html (accessed on 12 September 2004).
- Kruisz, J.; Rehrl, J.; Sacher, S.; Aigner, I.; Horn, M.; Khinast, J.G. RTD modeling of a continuous dry granulation process for process control and materials diversion. Int. J. Pharm. 2017, 528, 334–344. [Google Scholar] [CrossRef]
- Román-Ospino, A.D.; Tamrakar, A.; Igne, B.; Towns Dimaso, E.; Airiau, C.; Clancy, D.J.; Pereira, G.; Muzzio, F.J.; Singh, R.; Ramachandran, R. Characterization of NIR interfaces for the feeding and in-line monitoring of a continuous granulation process. Int. J. Pharm. 2020, 574, 118848. [Google Scholar] [CrossRef]
- Khorasani, M.; Amigo, J.M.; Bertelsen, P.; Sun, C.C.; Rantanen, J. Process optimization of dry granulation based tableting line: Extracting physical material characteristics from granules, ribbons and tablets using near-IR (NIR) spectroscopic measurement. Powder Technol. 2016, 300, 120–125. [Google Scholar] [CrossRef]
- Chen, X.; Wang, L.G.; Ooi, J.Y. A DEM-PBM multiscale coupling approach for the prediction of an impact pin mill. Powder Technol. 2020, 366, 408–419. [Google Scholar] [CrossRef]
- Nagy, B.; Farkas, A.; Gyürkés, M.; Komaromy-Hiller, S.; Démuth, B.; Szabó, B.; Nusser, D.; Borbás, E.; Marosi, G.; Nagy, Z.K. In-line Raman spectroscopic monitoring and feedback control of a continuous twin-screw pharmaceutical powder blending and tableting process. Int. J. Pharm. 2017, 530, 21–29. [Google Scholar] [CrossRef]
- Šašić, S.; Loranger, S.I.; Johnson, B.A. Characterizing the structure of pharmaceutical granules obtained by wet granulation with varying amounts of water via Raman chemical imaging. Appl. Spectrosc. 2011, 65, 1291–1299. [Google Scholar] [CrossRef]
- Watano, S. Direct control of wet granulation processes by image processing system. Powder Technol. 2001, 117, 163–172. [Google Scholar] [CrossRef]
- Watano, S.; Numa, T.; Miyanami, K.; OSAKO, Y. On-line monitoring of granule growth in high shear granulation by an image processing system. Chem. Pharm. Bull. 2000, 48, 1154–1159. [Google Scholar] [CrossRef] [PubMed]
- Narang, A.S.; Stevens, T.; Paruchuri, S.; Macias, K.; Gao, Z.; Badawy, S.I.F.; Bindra, D.; Hubert, M. Chapter 14—Inline Focused Beam Reflectance Measurement During Wet Granulation. In Handbook of Pharmaceutical Wet Granulation; Narang, A.S., Badawy, S.I.F., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 471–512. [Google Scholar]
- Narang, A.S.; Stevens, T.; Macias, K.; Paruchuri, S.; Gao, Z.; Badawy, S. Application of in-line focused beam reflectance measurement to Brivanib alaninate wet granulation process to enable scale-up and attribute-based monitoring and control strategies. J. Pharm. Sci. 2017, 106, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Taylor, M.K.; Mehrotra, A.; Stagner, W.C. Real-time particle size analysis using focused beam reflectance measurement as a process analytical technology tool for a continuous granulation–drying–milling process. Aaps Pharmscitech 2013, 14, 523–530. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Kaul, G.; Utz, J.; Hernandez, P.; Wong, V.; Bradley, D.; Nagi, A.; O’Grady, D. A PAT approach to improve process understanding of high shear wet granulation through in-line particle measurement using FBRM C35. J. Pharm. Sci. 2010, 99, 3205–3212. [Google Scholar] [CrossRef]
- Burggraeve, A.; Van Den Kerkhof, T.; Hellings, M.; Remon, J.P.; Vervaet, C.; De Beer, T. Evaluation of in-line spatial filter velocimetry as PAT monitoring tool for particle growth during fluid bed granulation. Eur. J. Pharm. Biopharm. 2010, 76, 138–146. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, A.F.T. Process analytical technology for batch and continuous pharmaceutical process supervision. PhD Thesis, Ghent university, Ghent, Belgium, 2017. [Google Scholar]
- Barkhudarov, M.R. Lagrangian VOF Advection Method for FLOW-3D; Flow Science Inc.: Santa Fe, NM, USA, 2004. [Google Scholar]
- Tsujimoto, H.; Yokoyama, T.; Huang, C.; Sekiguchi, I. Monitoring particle fluidization in a fluidized bed granulator with an acoustic emission sensor. Powder Technol. 2000, 113, 88–96. [Google Scholar] [CrossRef]
- Lu, Z.; Hickey, C.J.; Sabatier, J.M. Effects of compaction on the acoustic velocity in soils. Soil Sci. Soc. Am. J. 2004, 68, 7–16. [Google Scholar] [CrossRef]
- Medendorp, J.; Lodder, R.A. Acoustic-resonance spectrometry as a process analytical technology for rapid and accurate tablet identification. Aaps Pharmscitech 2006, 7, E175–E183. [Google Scholar] [CrossRef] [Green Version]
- Buice, R.G.; Pinkston, P.; Lodder, R.A. Optimization of acoustic-resonance spectrometry for analysis of intact tablets and prediction of dissolution rate. Appl. Spectroscop. 1994, 48, 517–524. [Google Scholar] [CrossRef]
- Medendorp, J.; Lodder, R. Integrated Sensing and Processing and a Novel acoustic-Resonance Spectrometer; American Association of Pharmaceutical Sciences: Baltimore, MD, USA, 2004. [Google Scholar]
- Serris, E.; Périer-Camby, L.; Thomas, G.; Desfontaines, M.; Fantozzi, G. Acoustic emission of pharmaceutical powders during compaction. Powder Technol. 2002, 128, 296–299. [Google Scholar] [CrossRef]
- Martin, L.; Poret, J.; Danon, A.; Rosen, M. Effect of adsorbed water on the ultrasonic velocity in alumina powder compacts. J. Mater. Sci. Eng. A 1998, 252, 27–35. [Google Scholar] [CrossRef]
- Kaatze, U.; Wehrmann, B.; Pottel, R. Acoustical absorption spectroscopy of liquids between 0.15 and 3000 MHz. I. High resolution ultrasonic resonator method. J. Phys. E: Sci. Instrum. 1987, 20, 1025. [Google Scholar] [CrossRef]
- Bolotnikov, M.F.; Neruchev, Y.A. Speed of sound of hexane+ 1-chlorohexane, hexane+ 1-iodohexane, and 1-chlorohexane+ 1-iodohexane at saturation condition. J. Chem. Eng. Data 2003, 48, 411–415. [Google Scholar] [CrossRef]
- Austin, J.; Gupta, A.; Mcdonnell, R.; Reklaitis, G.V.; Harris, M.T. The use of near-infrared and microwave resonance sensing to monitor a continuous roller compaction process. J. Pharm. Sci. 2013, 102, 1895–1904. [Google Scholar] [CrossRef]
- Corredor, C.C.; Bu, D.; Both, D. Comparison of near infrared and microwave resonance sensors for at-line moisture determination in powders and tablets. Anal. Chim. Acta 2011, 696, 84–93. [Google Scholar] [CrossRef]
- Buschmüller, C.; Wiedey, W.; Döscher, C.; Dressler, J.; Breitkreutz, J. In-line monitoring of granule moisture in fluidized-bed dryers using microwave resonance technology. Eur. J. Pharm. Biopharm. 2008, 69, 380–387. [Google Scholar] [CrossRef]
- Hapgood, K.P.; Litster, J.D.; Smith, R. Nucleation regime map for liquid bound granules. Aiche J. 2003, 49, 350–361. [Google Scholar] [CrossRef]
- Kristensen, H.G.; Schaefer, T. Granulation: A review on pharmaceutical wet-granulation. Drug Dev. Ind. Pharm. 1987, 13, 803–872. [Google Scholar] [CrossRef]
- Shi, B.-H.; Chai, S.; Wang, L.-Y.; Lv, X.; Liu, H.-S.; Wu, H.-H.; Wang, W.; Yu, D.; Gong, J. Viscosity investigation of natural gas hydrate slurries with anti-agglomerants additives. Fuel 2016, 185, 323–338. [Google Scholar] [CrossRef]
- Medendorp, J.P.; Fackler, J.A.; Douglas, C.C.; Lodder, R.A. Integrated sensing and processing acoustic resonance spectrometry (ISP-ARS) for sample Classification. J. Pharm. Innov. 2007, 2, 125–134. [Google Scholar] [CrossRef]
- Nakamura, H.; Miyazaki, Y.; Sato, Y.; Iwasaki, T.; Watano, S. Numerical analysis of similarities of particle behavior in high shear mixer granulators with different vessel sizes. Adv. Powder Technol. 2009, 20, 493–501. [Google Scholar] [CrossRef]
- Pandey, P.; Bharadwaj, R. Predictive Modeling of Pharmaceutical Unit Operations; Woodhead Publishing: Cambridge, UK, 2016. [Google Scholar]
- Yan, B.; Regueiro, R. Large-scale dynamic and static simulations of complex-shaped granular materials using parallel three-dimensional discrete element method (DEM) on DoD supercomputers. Eng. Comput. 2018, 35, 1049–1084. [Google Scholar] [CrossRef]
- Pantaleev, S.; Yordanova, S.; Janda, A.; Marigo, M.; Ooi, J.Y. An experimentally validated DEM study of powder mixing in a paddle blade mixer. Powder Technol. 2017, 311, 287–302. [Google Scholar] [CrossRef] [Green Version]
- Yao, H.; Mori, Y.; Takabatake, K.; Sun, X.; Sakai, M. Numerical investigation on the influence of air flow in a die filling process. J. Taiwan Inst. Chem. Eng. 2018, 90, 9–17. [Google Scholar] [CrossRef]
- Wan, J.; Wang, F.; Yang, G.; Zhang, S.; Wang, M.; Lin, P.; Yang, L. The influence of orifice shape on the flow rate: A DEM and experimental research in 3D hopper granular flows. Powder Technol. 2018, 335, 147–155. [Google Scholar] [CrossRef]
- Thornton, A.R.; Krijgsman, D.; Fransen, R.; Briones, S.G.; Tunuguntla, D.R.; te Voortwis, A.; Luding, S.; Bokhove, O.; Weinhart, T. Mercury-DPM: Fast particle simulations in complex geometries. Enginsoft Newsl. Simul. Based Eng. Sci. 2013, 10, 48–53. [Google Scholar]
- Kozicki, J.; Donze, F.V. YADE-OPEN DEM: An open-source software using a discrete element method to simulate granular material. Eng. Comput. 2009. [Google Scholar] [CrossRef] [Green Version]
- Kloss, C.; Goniva, C. LIGGGHTS–open source discrete element simulations of granular materials based on Lammps. Suppl. Proc. Mater. Fabr. Prop. Charact. Modeling 2011, 2, 781–788. [Google Scholar]
- Garg, R.; Galvin, J.; Li, T.; Pannala, S. Open-source MFIX-DEM software for gas–solids flows: Part I—Verification studies. Powder Technol. 2012, 220, 122–137. [Google Scholar] [CrossRef]
- Escotet-Espinoza, M.S.; Foster, C.J.; Ierapetritou, M. Discrete element modeling (DEM) for mixing of cohesive solids in rotating cylinders. Powder Technol. 2018, 335, 124–136. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, F.; Zhang, J.; Zhang, J.; Zhang, J. Flow field analysis and parameter optimization of main and measured nozzles of differential pressure type gas momentum instrument based on CFD. In Proceedings of Journal of Physics: Conference Series; IOPscience: Bristol, UK, 2018; p. 012029. [Google Scholar]
- Lichtenegger, T.; Peters, E.A.J.F.; Kuipers, J.A.M.; Pirker, S. A recurrence CFD study of heat transfer in a fluidized bed. Chem. Eng. Sci. 2017, 172, 310–322. [Google Scholar] [CrossRef]
- Jacobsen, N.G.; Fuhrman, D.R.; Fredsøe, J. A wave generation toolbox for the open-source CFD library: OpenFoam®. Int. J. Numer. Methods Fluids 2012, 70, 1073–1088. [Google Scholar] [CrossRef]
- Parker, J.; LaMarche, K.; Chen, W.; Williams, K.; Stamato, H.; Thibault, S. CFD simulations for prediction of scaling effects in pharmaceutical fluidized bed processors at three scales. Powder Technol. 2013, 235, 115–120. [Google Scholar] [CrossRef]
- Mazor, A.; Perez-Gandarillas, L.; de Ryck, A.; Michrafy, A. Effect of roll compactor sealing system designs: A finite element analysis. Powder Technol. 2016, 289, 21–30. [Google Scholar] [CrossRef] [Green Version]
- Diarra, H.; Mazel, V.; Busignies, V.; Tchoreloff, P. FEM simulation of the die compaction of pharmaceutical products: Influence of visco-elastic phenomena and comparison with experiments. Int. J. Pharm. 2013, 453, 389–394. [Google Scholar] [CrossRef]
- Dhondt, G.; Wittig, K. Calculix: A Free Software three-Dimensional Structural Finite Element Program; Mtu Aero Engines Gmbh: Munich, Germany, 1998. [Google Scholar]
- Tamrakar, A.; Ramachandran, R. Cfd–dem–pbm coupled model development and validation of a 3d top-spray fluidized bed wet granulation process. Comput. Chem. Eng. 2019, 125, 249–270. [Google Scholar] [CrossRef]
- Barrasso, D.; Ramachandran, R. Qualitative assessment of a multi-scale, compartmental PBM-DEM model of a continuous twin-screw wet granulation process. J. Pharm. Innov. 2016, 11, 231–249. [Google Scholar] [CrossRef]
- Barrasso, D.; Tamrakar, A.; Ramachandran, R. Model order reduction of a multi-scale PBM-DEM description of a wet granulation process via ANN. Procedia Eng. 2015, 102, 1295–1304. [Google Scholar] [CrossRef] [Green Version]
- Sen, M.; Barrasso, D.; Singh, R.; Ramachandran, R. A multi-scale hybrid CFD-DEM-PBM description of a fluid-bed granulation process. Processes 2014, 2, 89–111. [Google Scholar] [CrossRef] [Green Version]
- Sampat, C. Parallel Solution to Multi-Scale, Multi-Dimensional Coupled DEM-PBM Model for High Shear Granulation Using High Performance Computing; Rutgers University-School of Graduate Studies: New Brunswick, NJ, USA, 2019. [Google Scholar]
- Lourenço, V.; Lochmann, D.; Reich, G.; Menezes, J.C.; Herdling, T.; Schewitz, J. A quality by design study applied to an industrial pharmaceutical fluid bed granulation. Eur. J. Pharm. Biopharm. 2012, 81, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Börner, M.; Bück, A.; Tsotsas, E. DEM-CFD investigation of particle residence time distribution in top-spray fluidised bed granulation. Chem. Eng. Sci. 2017, 161, 187–197. [Google Scholar] [CrossRef]
- Hayashi, K.; Nakamura, H.; Watano, S. Numerical study on granule aggregation and breakage in fluidized bed granulation by a novel PBM with DEM-CFD coupling approach. Powder Technol. 2020, 360, 1321–1336. [Google Scholar] [CrossRef]
- Thiry, J.; Krier, F.; Evrard, B. A review of pharmaceutical extrusion: Critical process parameters and scaling-up. Int. J. Pharm. 2015, 479, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Litster, J.; Ennis, B. The Science and Engineering of Granulation processes; Springer Science & Business Media: New York, NY, USA, 2004; Volume 15. [Google Scholar]
- Ding, Y.L.; Forster, R.N.; Seville, J.P.K.; Parker, D.J. Scaling relationships for rotating drums. Chem. Eng. Sci. 2001, 56, 3737–3750. [Google Scholar] [CrossRef]
- Dewicki, G.; Mustoe, G. Bulk material belt conveyor transfer point simulation of material flow using DEM. In Proceedings of the Third International Conference on DEMs, Santa Fe, NM, USA, 23–25 September 2002; pp. 1–11. [Google Scholar]
- Campbell, G.A.; Clancy, D.J.; Zhang, J.X.; Gupta, M.K.; Oh, C.K. Closing the gap in series scale up of high shear wet granulation process using impeller power and blade design. Powder Technol. 2011, 205, 184–192. [Google Scholar] [CrossRef]
- Gijón-Arreortúa, I.; Tecante, A. Mixing time and power consumption during blending of cohesive food powders with a horizontal helical double-ribbon impeller. J. Food Eng. 2015, 149, 144–152. [Google Scholar] [CrossRef]
- Hapgood, K.P.; Litster, J.D.; White, E.T.; Mort, P.R.; Jones, D.G. Dimensionless spray flux in wet granulation: Monte-Carlo simulations and experimental validation. Powder Technol. 2004, 141, 20–30. [Google Scholar] [CrossRef]
- Litster, J.D.; Hapgood, K.; Michaels, J.N.; Sims, A.; Roberts, M.; Kameneni, S.; Hsu, T. Liquid distribution in wet granulation: Dimensionless spray flux. Powder Technol. 2001, 114, 32–39. [Google Scholar] [CrossRef]
- Rekhi, G.S.; Caricofe, R.; Parikh, D.; Augsburger, L. A new approach to scale-up of a high-shear granulation process. Pharm. Technol. 1996, 20, 1–10. [Google Scholar] [CrossRef]
- Bock, T.K.; Kraas, U. Experience with the Diosna mini-granulator and assessment of process scalability. Eur. J. Pharm. Biopharm. 2001, 52, 297–303. [Google Scholar] [CrossRef]
- Horsthuis, G.; Van Laarhoven, J.; Van Rooij, R.; Vromans, H. Studies on upscaling parameters of the Gral high shear granulation process. Int. J. Pharm. 1993, 92, 143–150. [Google Scholar] [CrossRef]
- Holm, P. Effect of impeller and chopper design on granulation in a high speed mixer. Drug Dev. Ind. Pharm. 1987, 13, 1675–1701. [Google Scholar] [CrossRef]
- Sirois, P.J.; Craig, G.D. Scaleup of a high-shear granulation process using a normalized impeller work parameter. Pharm. Dev. Technol. 2000, 5, 365–374. [Google Scholar] [CrossRef]
- Bardin, M.; Knight, P.C.; Seville, J.P.K. On control of particle size distribution in granulation using high-shear mixers. Powder Technol. 2004, 140, 169–175. [Google Scholar] [CrossRef]
- Sato, Y.; Okamoto, T.; Watano, S. Scale-up of high shear granulation based on agitation power. Chem. Pharm. Bull. 2005, 53, 1547–1550. [Google Scholar] [CrossRef] [Green Version]
- Landin, M.; York, P.; Cliff, M.; Rowe, R.; Wigmore, A. Scale-up of a pharmaceutical granulation in fixed bowl mixer-granulators. Int. J. Pharm. 1996, 133, 127–131. [Google Scholar] [CrossRef]
- Ax, K.; Feise, H.; Sochon, R.; Hounslow, M.; Salman, A. Influence of liquid binder dispersion on agglomeration in an intensive mixer. Powder Technol. 2008, 179, 190–194. [Google Scholar] [CrossRef]
- Hassanpour, A.; Kwan, C.; Ng, B.; Rahmanian, N.; Ding, Y.; Antony, S.; Jia, X.; Ghadiri, M. Effect of granulation scale-up on the strength of granules. Powder Technol. 2009, 189, 304–312. [Google Scholar] [CrossRef]
- Cavinato, M.; Artoni, R.; Bresciani, M.; Canu, P.; Santomaso, A.C. Scale-up effects on flow patterns in the high shear mixing of cohesive powders. Chem. Eng. Sci. 2013, 102, 1–9. [Google Scholar] [CrossRef]
- André, C.; Demeyre, J.F.; Gatumel, C.; Berthiaux, H.; Delaplace, G. Derivation of dimensionless relationships for the agitation of powders of different flow behaviours in a planetary mixer. Powder Technol. 2014, 256, 33–38. [Google Scholar] [CrossRef]
- Faure, A.; Grimsey, I.M.; Rowe, R.C.; York, P.; Cliff, M.J. Applicability of a scale-up methodology for wet granulation processes in Collette Gral high shear mixer-granulators. Eur. J. Pharm. Sci. 1999, 8, 85–93. [Google Scholar] [CrossRef]
- Landin, M.; York, P.; Cliff, M.; Rowe, R. Scaleup of a pharmaceutical granulation in planetary mixers. Pharm. Dev. Technol. 1999, 4, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Watano, S.; Okamoto, T.; Sato, Y.; Osako, Y. Scale-up of high shear granulation based on the internal stress measurement. Chem. Pharm. Bull. 2005, 53, 351–354. [Google Scholar] [CrossRef] [Green Version]
- Côté, P.; Abatzoglou, N. Powder and other divided solids mixing. Scale-up and parametric study of a ribbon blender used in pharmaceutical powders mixing. Pharm. Dev. Technol. 2006, 11, 29–45. [Google Scholar] [CrossRef]
- Watano, S.; Sato, Y.; Miyanami, K. Scale-Up of Agitation Fluidized Bed Granulation. IV. Scale-Up Theory Based on the Kinetic Energy Similarity. Chem. Pharm. Bull. 1995, 43, 1227–1230. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, A.O.; Penlington, R.; Busawon, K.; Morgan, A. A novel modeling approach to the mixing process in twin-screw extruders. In Proceedings of the PPS-29, The 29th International Conference of the Polymer Processing Society, Nuremberg, Germany, 15–19 July 2013; pp. 29–38. [Google Scholar]
- Bier, H. Determination of the uncritical quantity of granulating liquid by power consumption measurement on planetary mixers. Pharm. Ind. 1979, 41, 375–380. [Google Scholar]
- Leuenberger, H.; Bier, H.; Sucker, H. Determination of the liquid requirement for a conventional granulation process. Ger. Chem. Eng. 1981, 4, 13–18. [Google Scholar]
- Watano, S.; Tanaka, T.; Miyanami, K. A method for process monitoring and determination of operational end-point of consumption in agitation granulation. Adv. Powder Technol. 1995, 6, 91–102. [Google Scholar] [CrossRef]
- Luypaert, J.; Massart, D.L.; Vander Heyden, Y. Near-infrared spectroscopy applications in pharmaceutical analysis. Talanta 2007, 72, 865–883. [Google Scholar] [CrossRef]
- Frake, P.; Greenhalgh, D.; Grierson, S.; Hempenstall, J.; Rudd, D. Process control and end-point determination of a fluid bed granulation by application of near infra-red spectroscopy. Int. J. Pharm. 1997, 151, 75–80. [Google Scholar] [CrossRef]
- Wikström, H.; Marsac, P.J.; Taylor, L.S. In-line monitoring of hydrate formation during wet granulation using Raman spectroscopy. J. Pharm. Sci. 2005, 94, 209–219. [Google Scholar] [CrossRef]
- Wilms, A.; Knop, K.; Kleinebudde, P. Combination of a rotating tube sample divider and dynamic image analysis for continuous on-line determination of granule size distribution. Int. J. Pharm. 2019, 1, 100029. [Google Scholar] [CrossRef]
- Narang, A.S.; Sheverev, V.; Freeman, T.; Both, D.; Stepaniuk, V.; Delancy, M.; Millington-Smith, D.; Macias, K.; Subramanian, G. Process analytical technology for high shear wet granulation: Wet mass consistency reported by in-line drag flow force sensor is consistent with powder rheology measured by at-line FT4 powder rheometer®. J. Pharm. Sci. 2016, 105, 182–187. [Google Scholar] [CrossRef]
- Rowe, R.; Sadeghnejad, G. The rheology of microcrystalline cellulose powder/water mixes—measurement using a mixer torque rheometer. Int. J. Pharm. 1987, 38, 227–229. [Google Scholar] [CrossRef]
- Jørgensen, A.C.; Rantanen, J.; Luukkonen, P.; Laine, S.; Yliruusi, J. Visualization of a Pharmaceutical Unit Operation: Wet Granulation. Anal. Chem. 2004, 76, 5331–5338. [Google Scholar] [CrossRef]
- De Beer, T.R.M.; Bodson, C.; Dejaegher, B.; Walczak, B.; Vercruysse, P.; Burggraeve, A.; Lemos, A.; Delattre, L.; Heyden, Y.V.; Remon, J.P.; et al. Raman spectroscopy as a process analytical technology (PAT) tool for the in-line monitoring and understanding of a powder blending process. J. Pharm. Biomed. Anal. 2008, 48, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Rantanen, J.; Lehtola, S.; Rämet, P.; Mannermaa, J.-P.; Yliruusi, J. On-line monitoring of moisture content in an instrumented fluidized bed granulator with a multi-channel NIR moisture sensor. Powder Technol. 1998, 99, 163–170. [Google Scholar] [CrossRef]
- Roßteuscher-Carl, K.; Fricke, S.; Hacker, M.C.; Schulz-Siegmund, M. In-line monitoring of particle size in a fluid bed granulator: Investigations concerning positioning and configuration of the sensor. Int. J. Pharm. 2014, 466, 31–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fonteyne, M.; Vercruysse, J.; Díaz, D.C.; Gildemyn, D.; Vervaet, C.; Remon, J.P.; Beer, T.D. Real-time assessment of critical quality attributes of a continuous granulation process. Pharm. Dev. Technol. 2013, 18, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; Román-Ospino, A.D.; Panikar, S.S.; O’Callaghan, C.; Gilliam, S.J.; Ramachandran, R.; Muzzio, F.J. Advanced process design and understanding of continuous twin-screw granulation via implementation of in-line process analytical technologies. Adv. Powder Technol. 2019, 30, 879–894. [Google Scholar] [CrossRef]
- Chaudhury, A.; Wu, H.; Khan, M.; Ramachandran, R. A mechanistic population balance model for granulation processes: Effect of process and formulation parameters. Chem. Eng. Sci. 2014, 107, 76–92. [Google Scholar] [CrossRef]
- Liu, L.; Litster, J. Population balance modelling of granulation with a physically based coalescence kernel. Chem. Eng. Sci. 2002, 57, 2183–2191. [Google Scholar] [CrossRef]
- Chan, E.L.; Washino, K.; Reynolds, G.K.; Gururajan, B.; Hounslow, M.J.; Salman, A.D. Blade-granule bed stress in a cylindrical high shear granulator: Further characterisation using DEM. Powder Technol. 2016, 300, 92–106. [Google Scholar] [CrossRef]
- Fries, L.; Antonyuk, S.; Heinrich, S.; Dopfer, D.; Palzer, S. Collision dynamics in fluidised bed granulators: A DEM-CFD study. Chem. Eng. Sci. 2013, 86, 108–123. [Google Scholar] [CrossRef]
- Dhenge, R.M.; Washino, K.; Cartwright, J.J.; Hounslow, M.J.; Salman, A.D. Twin screw granulation using conveying screws: Effects of viscosity of granulation liquids and flow of powders. Powder Technol. 2013, 238, 77–90. [Google Scholar] [CrossRef]
- Rahmanian, N.; Ng, B.; Hassanpour, A.; Ding, Y.; Antony, J.; Jia, X.; Ghadiri, M.; van der Wel, P.; Krug-Polman, A.; York, D. Scale-up of high-shear mixer granulators. Kona Powder Part. J. 2008, 26, 190–204. [Google Scholar] [CrossRef] [Green Version]
- Remy, B.; Glasser, B.J.; Khinast, J.G. The effect of mixer properties and fill level on granular flow in a bladed mixer. Aiche J. 2010, 56, 336–353. [Google Scholar] [CrossRef]
- Hassanpour, A.; Tan, H.; Bayly, A.; Gopalkrishnan, P.; Ng, B.; Ghadiri, M. Analysis of particle motion in a paddle mixer using Discrete Element Method (DEM). Powder Technol. 2011, 206, 189–194. [Google Scholar] [CrossRef]
- Chandratilleke, G.R.; Yu, A.; Bridgwater, J. A DEM study of the mixing of particles induced by a flat blade. Chem. Eng. Sci. 2012, 79, 54–74. [Google Scholar] [CrossRef]
- Fries, L.; Antonyuk, S.; Heinrich, S.; Palzer, S. DEM–CFD modeling of a fluidized bed spray granulator. Chem. Eng. Sci. 2011, 66, 2340–2355. [Google Scholar] [CrossRef]
- Barrasso, D.; Eppinger, T.; Pereira, F.E.; Aglave, R.; Debus, K.; Bermingham, S.K.; Ramachandran, R. A multi-scale, mechanistic model of a wet granulation process using a novel bi-directional PBM–DEM coupling algorithm. Chem. Eng. Sci. 2015, 123, 500–513. [Google Scholar] [CrossRef]
- Teng, Y.; Qiu, Z.; Wen, H. Systematical approach of formulation and process development using roller compaction. Eur. J. Pharm. Biopharm. 2009, 73, 219–229. [Google Scholar] [CrossRef]
- Rowe, J.M.; Crison, J.R.; Carragher, T.J.; Vatsaraj, N.; Mccann, R.J.; Nikfar, F. Mechanistic Insights into the Scale-Up of the Roller Compaction Process: A Practical and Dimensionless Approach. J. Pharm. Sci. 2013, 102, 3586–3595. [Google Scholar] [CrossRef]
- Pietsch, W. Size enlargement by agglomeration. In Handbook of Powder Science & Technology; Springer: New York, NY, USA, 1997; pp. 202–377. [Google Scholar]
- Sheskey, P.; Pacholke, K.; Sackett, G.; Maher, L.; Polli, J. Roll compaction granulation of a controlled-release matrix tablet formulation containing HPMC: Effect of process scale-up on robustness of tablets, tablet stability and predicted in vivo performance. Pharm. Technol. 2000, 24, 30–52. [Google Scholar]
- Johanson, J. A rolling theory for granular solids. J. Appl. Mech. 1965, 32, 842–848. [Google Scholar] [CrossRef]
- Bi, M.; Alvarez-Nunez, F.; Alvarez, F. Evaluating and Modifying Johanson’s Rolling Model to Improve its Predictability. J. Pharm. Sci. 2014, 103, 2062–2071. [Google Scholar] [CrossRef]
- Reynolds, G.; Ingale, R.; Roberts, R.; Kothari, S.; Gururajan, B. Practical application of roller compaction process modeling. Comput. Chem. Eng. 2010, 34, 1049–1057. [Google Scholar] [CrossRef]
- Toson, P.; Lopes, D.G.; Paus, R.; Kumar, A.; Geens, J.; Stibale, S.; Quodbach, J.; Kleinebudde, P.; Hsiao, W.-K.; Khinast, J. Model-based approach to the design of pharmaceutical roller-compaction processes. Int. J. Pharm. 2019, 1, 100005. [Google Scholar] [CrossRef]
- Souihi, N.; Reynolds, G.; Tajarobi, P.; Wikström, H.; Haeffler, G.; Josefson, M.; Trygg, J. Roll compaction process modeling: Transfer between equipment and impact of process parameters. Int. J. Pharm. 2015, 484, 192–206. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Bruwer, M.-J.; MacGregor, J.F.; Rathore, S.S.; Reed, D.E.; Champagne, M.J. Scale-up of a pharmaceutical roller compaction process using a joint-Y partial least squares model. Ind. Eng. Chem. Res. 2011, 50, 10696–10706. [Google Scholar] [CrossRef]
- U.S: Food and Drug Administration. Quality by Design for ANDAs: An Example for Immediate-Release Dosage Forms; U.S. Department of Health and Human Service FDA: Rockville, MD, USA, 2012.
- Boersen, N.; Belair, D.; Peck, G.E.; Pinal, R. A dimensionless variable for the scale up and transfer of a roller compaction formulation. Drug Dev. Ind. Pharm. 2016, 42, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Sprockel, O.L. A practical approach for the scale-up of roller compaction process. Eur. J. Pharm. Biopharm. 2016, 106, 15–19. [Google Scholar] [CrossRef]
- Gupta, A.; Peck, G.E.; Miller, R.W.; Morris, K.R. Nondestructive measurements of the compact strength and the particle-size distribution after milling of roller compacted powders by near-infrared spectroscopy. J. Pharm. Sci. 2004, 93, 1047–1053. [Google Scholar] [CrossRef]
- Acevedo, D.; Muliadi, A.; Giridhar, A.; Litster, J.D.; Romañach, R.J. Evaluation of three approaches for real-time monitoring of roller compaction with near-infrared spectroscopy. Aaps Pharmscitech 2012, 13, 1005–1012. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Peck, G.E.; Miller, R.W.; Morris, K.R. Real-time near-infrared monitoring of content uniformity, moisture content, compact density, tensile strength, and young’s modulus of roller compacted powder blends. J. Pharm. Sci. 2005, 94, 1589–1597. [Google Scholar] [CrossRef]
- Khorasani, M.; Amigo, J.M.; Sun, C.C.; Bertelsen, P.; Rantanen, J. Near-infrared chemical imaging (NIR-CI) as a process monitoring solution for a production line of roll compaction and tableting. Eur. J. Pharm. Biopharm. 2015, 93, 293–302. [Google Scholar] [CrossRef]
- Akseli, I.; Iyer, S.; Lee, H.P.; Cuitiño, A.M. A quantitative correlation of the effect of density distributions in roller-compacted ribbons on the mechanical properties of tablets using ultrasonics and X-ray tomography. Aaps Pharmscitech 2011, 12, 834–853. [Google Scholar] [CrossRef]
- Miguélez-Morán, A.M.; Wu, C.-Y.; Dong, H.; Seville, J.P. Characterisation of density distributions in roller-compacted ribbons using micro-indentation and X-ray micro-computed tomography. Eur. J. Pharm. Biopharm. 2009, 72, 173–182. [Google Scholar] [CrossRef]
- Wiedey, R.; Kleinebudde, P. Infrared thermography—A new approach for in-line density measurement of ribbons produced from roll compaction. Powder Technol. 2018, 337, 17–24. [Google Scholar] [CrossRef]
- Gupta, A.; Peck, G.E.; Miller, R.W.; Morris, K.R. Influence of ambient moisture on the compaction behavior of microcrystalline cellulose powder undergoing uni-axial compression and roller-compaction: A comparative study using near-infrared spectroscopy. J. Pharm. Sci. 2005, 94, 2301–2313. [Google Scholar] [CrossRef] [PubMed]
- Samanta, A.K.; Karande, A.D.; Ng, K.Y.; Heng, P.W.S. Application of near-infrared spectroscopy in real-time monitoring of product attributes of ribbed roller compacted flakes. Aaps Pharmscitech 2013, 14, 86–100. [Google Scholar] [CrossRef] [Green Version]
- Wiedey, R.; Kleinebudde, P. The density distribution in ribbons from roll compaction. Chem. Ing. Tech. 2017, 89, 1017–1024. [Google Scholar] [CrossRef] [Green Version]
- Muliadi, A.R.; Litster, J.D.; Wassgren, C.R. Modeling the powder roll compaction process: Comparison of 2-D finite element method and the rolling theory for granular solids (Johanson’s model). Powder Technol. 2012, 221, 90–100. [Google Scholar] [CrossRef]
- Muliadi, A.R.; Litster, J.D.; Wassgren, C.R. Validation of 3-D finite element analysis for predicting the density distribution of roll compacted pharmaceutical powder. Powder Technol. 2013, 237, 386–399. [Google Scholar] [CrossRef]
- Michrafy, A.; Diarra, H.; Dodds, J.A.; Michrafy, M. Experimental and numerical analyses of homogeneity over strip width in roll compaction. Powder Technol. 2011, 206, 154–160. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, J.C.; Winstead, D.; Zavaliangos, A. Understanding variation in roller compaction through finite element-based process modeling. Comput. Chem. Eng. 2010, 34, 1058–1071. [Google Scholar] [CrossRef]
- Michrafy, A.; Diarra, H.; Dodds, J.A.; Michrafy, M.; Penazzi, L. Analysis of strain stress state in roller compaction process. Powder Technol. 2011, 208, 417–422. [Google Scholar] [CrossRef] [Green Version]





















| Type | Name | Equation | Nomenclature |
|---|---|---|---|
| Mechanical unit operation | Reynolds, Re | = velocity = characteristic length v = liquid volume = gravitational acceleration F = force = density | |
| Froude, Fr | |||
| Newton, Ne | |||
| Heat transfer | Nusselt, Nu | = convective heat transfer coefficient = characteristic length = thermal conductivity = time = density = heat capacity at constant pressure | |
| Fourier, Fo | |||
| Prandtl, Pr | |||
| Mass transfer | Sherwood, Sh | = mass transfer coefficient = characteristic length = vessel diameter v = liquid volume = velocity = axial dispersion coefficient | |
| Schmidt, Sc | |||
| Bodenstein, Bo |
| Type | Process Parameters | QAs of Granule |
|---|---|---|
| High-shear, Low-shear | Order of addition Pre-blending time Granulation time Impeller and chopper (speed, configuration and location) Spray nozzle type and location Liquid/solid ratio Binder addition method Binder temperature Vessel temperature Fill level | Content, Content uniformity, Granule size, Granule size distribution, Granule hardness, Tapped density, Bulk density, Moisture content, Flow properties, True density |
| Twin-screw | Screw speed Screw configuration/type/size Liquid/solid ratio Feed rate Fill level Number of die holes | |
| Fluidized bed |
Blending time Spray nozzle (type, quantity, pattern, configuration) Binder addition method Binder fluid temperature Binder addition rate Inlet air (Flow rate, volume, temperature) Filter (properties, size) Shaking intervals Product temperature |
| Equipment Type | Model (Manufacturer) | Scale and Parameter Conditions | Dimensionless Number | Measured QAs | Ref. |
|---|---|---|---|---|---|
| High shear/ Low shear | Eirich R02 (EIRICH Machines, Inc., Hardheim, Germany) | Spray nozzle: 0.3, 0.5 and 1.2 mm Amount of binder: 225 g Rotation speed: 1500 rpm | Spray flux | Particle size distribution | [5] |
| Cyclomix high-shear granulators (Hosokawa Micron BV, Doetinchem, Nederland) | Vessel size: 1, 5, 10, 250 and 500 L Fill level: 40% | Constant Froude number Constant shear stress Constant impeller tip speed | Strength of granules | [107] | |
| SPG (Fuji Paudal Co., Ltd., Osaka, Japan) | Vessel size: 2–112 L | Impeller tip speed | Particle flow and collision energy determined from DEM | [28] | |
| MiPro (ProCepT, Zelzate, Belgium), Fielder PMA 65 (GEA Niro Aeromatic Fielder, Paris, France) | Vessel size: 1.9 and 66 L Fill level: 20 and 40% | Modified dimensionless torque number relevant of Froude number, fill ratio and, impeller clearance at the vessel base | Observation of the surface velocity of the powder bed. | [108] | |
| TRIAXE system (TriaProcess, Albi, France) | Vessel size: 48 L Gyrational speed of agitator: 25–800 rpm Rotational speed of agitator: 25–800 rpm Fill level: 10 and 20% | Modified Froude and Power numbers | Power consumption | [109] | |
| Diosna P1–6 (Diosna Dierks und Söhne GmbH, Osnabrück, Germany) | Vessel size: 1.23–100 L Impeller tip speed: 3.8 and 7.5 m/s Fill ratios: 32.2% | Constant impeller tip speed Constant Froude number | Size distribution of the granules | [99] | |
| Collette Gral (GEA-Colette, Wommelgem, Belgium) | Vessel size: 8, 25, 75 and 600 L | Dimensionless power relationships | Consistency in a commercial mixer torque rheometer | [110] | |
| MP 20, 90, MPH 200 (GEA Pharma Systems-Collette, Wommelgem, Belgium) | Vessel size: 5–200 L | Dimensionless numbers of Power, Reynolds and Froude number | Power consumption | [111] | |
| Fielder PMA (GEA Niro Aeromatic Fielder, Paris, France) | Vessel size: 25, 100 and 600 L | Dimensionless numbers of Power, Reynolds and Froude number | Power consumption | [105] | |
| Fielder PMA (GEA Niro Aeromatic Fielder, Paris, France) | Vessel size: 10, 65, 150 and 300 L Impeller tip speed: about 5.1 and 10.3 m/s Chopper speed: 3000 rpm | Constant impeller tip speed Binder solution proportional to batch size Maintains granulation time proportions about impeller speeds that vary with batch size | Percent loss on drying, granule size distribution, bulk and tap densities | [98] | |
| Ploughshare Mixer (Gebrüder Lödige Maschinenbau GmbH, Paderborn, Germany) | Vessel size: 40 and 140 L Fill level: 40% | Constant Froude number Constant relative swept volume Constant impeller tip speed | Granule bulk density, sphericity, packing coefficient | [9] | |
| Collette Gral (GEA-Colette, Wommelgem, Belgium) | Vessel size: 8, 75 and 300 L | Constant Froude number Constant relative swept volume Constant impeller tip speed | Power consumption, temperature of the mass | [100] | |
| SPG-10, 25, 200, 400 (Fuji Paudal Co., Ltd., Osaka, Japan) | Vessel size: 9.8, 25.7, 205.9 and 401.8 L Binder content: 22% Operating time: 10 min Chopper speed: 3000 rpm | Constant tip speed | Strength, size distribution and compressibility of granules | [112] | |
| Ribbon granulator | S-50 mixer (S. Howes LLC, New York, USA) | Vessel size: 0.5 and 100 ft3 Fill level: 40–80% Impeller speeds: 20, 40 and 60 rpm | Adjusting the rotation speed to have the same number of Froude in each vessel size | Content uniformity | [113] |
| Helical double-ribbon impeller blender (Custom-made) | Fill level: 8–50% Impeller speeds: 25, 50 and 75 rpm | Correlation between the power number and the cohesion number | Power consumption Content uniformity | [95] | |
| Fluidized bed | NQ-125. 230, 500 (Fuji Paidal Co., Ltd., Osaka, Japan) | Vessel diameter: 125, 230 and 500 mm | The constant ratio of kinetic energy by agitator rotation | Moisture content | [114] |
| Twin-screw | - | Feed rate: 10, 15 and 20 kg/h Screw speed: 200 and 400 rpm | Dimensionless number about mean residence time and mean time delay | Residence time, mean residence time, fill level | [115] |
| Equipment Type | Model (Manufacturer) | PAT Tool (Model) | Detail | Measured QAs | Ref. |
|---|---|---|---|---|---|
| High-shear/ Low shear | MiPro (ProCepT, Zelzate, Belgium) | Power consumption | Impeller torque was measuring at bench-scale Current value of the motor at pilot scale | Impeller torque | [108] |
| Image analysis (FastCam PCI 1000) | Measuring the powder surface velocity using high speed camera | Particle surface velocity | |||
| PharmaConnect™ (GEA, Düsseldorf, Germany) | Power consumption (in-line DFF 2 sensor) | Measurement range of ± 3 N Measured at 500 point per second with DFF sensor | Impeller torque | [123] | |
| Fielder PMA (GEA Niro Aeromatic Fielder, Paris, France) | Power consumption (mixer-torque rheometer, Caleva MTR) | Measures impeller torque when rotating at 50 rpm Mix for 30 s, then log data for 30 s | Impeller torque | [8] | |
| Lodige granulator (Gebrüder Lödige Maschinenbau GmbH, Paderborn, Germany) | FBRM 1 and parsumTM | Measured in the 790 nm Scanning beam velocity was 4 m/s | Particle size distribution | [44] | |
| Loedige M5 high-shear mixer (Gebrüder Lödige Maschinenbau GmbH, Paderborn, Germany) | Power consumption (Sineax Type PQ 502), Temperature | Power consumption is calculated by the actual current consumption of the motor |
Power consumption Granule temperature | [93] | |
| Jacketed beken duples mixer (Beken Engineering, London, UK) | Power consumption (Plasti-Coder rotational torque rheometer) | Torque measurement after 3–5 min of mixing | Impeller torque | [124] | |
| MiPro (ProCepT, Zelzate, Belgium) | NIR 3 (Foss Nirsystems Model 6500 Rapid Content Analyzer) | Measured in the range of 1100–2500 nm Spectrum was obtained of 32 scans | Moisture content | [125] | |
| SPG25 (Fuji Paudal Co., Ltd., Osaka, Japan) | Power consumption (Digital power meter) | Torque of impeller and chopper measured |
Particle size distribution Granule moisture content Granule temperature | [40] | |
| Image analysis (image processing system comprising a CCD 4 camera) | Use of high energy Xenon lamps flashing in 1us intervals | ||||
| SPG25, 200 and 400 (Fuji Paudal Co., Ltd., Osaka, Japan) | Power consumption (digital torque meter) | Calculation of agitation power per unit volume using shaft torque. | Shaft torque | [104] | |
| MiPro (ProCepT, Zelzate, Belgium) | Raman (Renishaw InVia Raman mapping instrument) | Center of the measurement range was settled at 1100 cm−1 45 mW laser source at 785 nm | Distribution of binder within the granules | [38] | |
| Collette Gral-10 (GEA-Colette, Wommelgem, Belgium), | NIR (Multi-Purpose Analyzer FT-NIR spectrometer), | Laser wavelength was the 785 nm line from a 785 nm | Content uniformity | [126] | |
| Raman (RamanRxn1 spectrometer) | Measured in the range of 12,500–4000 cm−1 with 8 cm−1 resolution Spectrum was obtained the average of 16 scans | ||||
| Fluidized bed | ConsiGma™ system (GEA-Colette, Wommelgem, Belgium) | FBRM (FBRM M680) |
Probe including a scraper unit Scan speed can be adjusted from 2 to 8 m/s | Particle size distribution | [43] |
| Glatt GPCG 30/50 (Glatt GmbH, Binzen, Germany) | NIR (NIRSystems 6500 spectrophotometer) | Measured in the range of 1100–2500 nm every 2.5 min | Moisture content | [120] | |
| FD-3S (Powrex, Hyogo, Japan) | NIR (WET EYE, WETRON) | The 1990 nm wavelength for water detection The 1740 nm wavelength for correction of the background level The 2145 nm wavelength as a non-water-sensitive reference | Moisture content | [127] | |
| Custom apparatus | |||||
| NQ-160 (Fuji Paidal Co., Ltd., Osaka, Japan) | Image analysis | Use of air purge to prevent visual disturbances due to powder attachment |
Particle size distribution, Granule shape | [39] | |
| GPCG 3 granulator (Glatt GmbH, Binzen, Germany) | ParsumTM (IPP-70 Se SFT-sensor) |
The sensor was installed at three different insertion depths The rotation angle of the probe changed in steps of 45 ° | Particle size distribution | [128] | |
| AGM-2A-PJ (Hosokawa Micron Ltd., Osaka, Japan) | Acoustic emissions (AE-901S) | The receptor used had a resonance point of 140 kHz | Particle fluidization | [48] | |
| Twin-screw | ConsiGma-25 Unit (GEA Pharma systems, Collette, Wommelgem, Belgium) | Raman (RamanRxn1 spectrometer) |
400 mW laser source at 785 nm. Measured at a resolution of 4 cm−1 with an exposure time of 30 s | Solid-state behavior | [129] |
| NIR (Fourier-Transform NIR spectrometer) | Measured in the range of 4500–10,000 cm−1 with 16 cm−1 resolutions. Spectrum was obtained averaged over 16 scans | ||||
| In-line Spatial Filter Velocimetry probe | Semiconductor laser diode probe radiating visible light at a wavelength of 670 nm. | Particle size distribution | |||
| Thermo Scientific™ Pharma 11 twin-screw granulator (Thermo Fisher Scientific, Karlsruhe, Germany) | Eyecon™ | The integration time was 60 s The maximum detection diameter was 3000 µm the analysis block size was 501 pixels | Particle size distribution | [130] | |
| TS16 Quick Extruder (QUICK 2000 Ltd., Tiszavasvári, Hungary) | Raman (Kaiser RamanRxn2 Hybrid in situ analyzer) | Measured in the range of 200–1890 cm−1 with a 4 cm−1 resolution Spectrum pixel was 1690 | Content uniformity of powder and tablet | [37] |
| Equipment Type | Simulation Tool | Summary | Predicted Attributes | Ref. |
|---|---|---|---|---|
| High-shear/ Low-shear | DEM | Parameter study of particle shape and impeller geometries Comparison of different vessel sizes (0.173, 1.39 11.1 and 88.7 L) | Blade-bed stress and bed surface velocities | [133] |
| PBM–DEM | Wet granulation processes were simulated by the coupled model of PBM and DEM The coupled model demonstrated sensitivity to the impeller speed | Particle size distributions and collision rate functions | [84] | |
| DEM | Granulator (SPG, Fuji Paudal Co., Ltd.) was used Identification of kinematic and dynamic similarities using DEM Comparison of different vessel sizes (simulated 2, 10, 26, 112 and 206 L) | Internal particle flow Particle collision energy | [28] | |
| DEM | Comparison kinematic and dynamic similarities of different vessel sizes (1.0, 3.4, 8.1 and 16.0 L) | Particle collision energy (dynamic similarity) Particle velocity (kinematic similarity) | [64] | |
| DEM | Granulator (Mycromix, Bosch Packaging Technology) was used Confirmation of the effect of the size of the granulator (10 and 600 L) Confirmation of the influence of the number of blades (2 or 3 blades) | Shear forces Force distributions | [6] | |
| DEM | Granulator (Hosokawa Micron B.V.) was used Analysis of particle breakage and deformation behavior according to vessel size (1, 5 and 50 L) | Velocity fields of granules Stress fields of granules | [107] | |
| DEM | Identification of parameters affecting granule production using DEM Comparison of different vessel sizes (1, 5, 50 and 250 L) | Velocity fields of granules Stress fields of granules | [136] | |
| DEM | Effect of mixer size (1–300 L) and fill level (17%, 32% and 46%) | Granule of flow patterns Velocity field and stress of granule bed | [137] | |
| DEM | Comparative study of particle behavior using PEPT 1 and DEM | Internal flow fields and blending patterns | [138] | |
| DEM | The effects of blade rake angle and blade speed | Velocity fields of particles | [139] | |
| Fluidized bed | CFD–DEM–PBM | CFD–DEM–PBM coupled model for predicting fluidized bed granulation behavior | Average particle size Particle size distribution Moisture content | [85] |
| CFD | Predicting the behavior of fluidized bed granulators with different batch sizes (150 g, 2 kg and 45 kg) | Particle volume fraction Bed heights in granulator | [78] | |
| DEM–CFD | Modified DEM–CFD model using model for particle wetting. | The residence time distribution Average particle velocity and collisions | [140] | |
| CFD–DEM–PBM | Development and validation of a coupled CFD–DEM–PBM model The effect of process parameters, such as the inlet air flow rate, air temperature and spray rate | Particle velocities, temperature and collision frequencies form DEM Particle residence time from CFD Particle size distribution and moisture content change from PBM | [82] | |
| DEM–CFD | Particle motion and collision dynamics simulation using the coupled DEM–CFD model Comparison of Top-spray, Wurster-coater and Prismatic shaped spouted bed | Particle velocity Collision velocity between particles Collision velocity between particles and walls Collision frequency | [134] | |
| Twin-screw | DEM-PBM | Evaluate the effect of viscosity and amount of binder, as well as screw speed and type on granules using the coupled DEM-PBM model | Porosity and size distribution, liquid content for PBM Moisture content, porosity, average particle velocity and collision rate for DEM | [141] |
| DEM-PBM | Granulator (16 mm Prism EuroLab TSG 2, Thermo Fisher Scientific) was used Assess coagulation, breakage and consolidation during granulation using a coupled PBM–DEM model Evaluate the effect of screw configuration on granule quality attributes | Size distribution, liquid distribution and porosity of granules | [83] | |
| DEM | Granulator (16 mm Prism EuroLab TSG, Thermo Fisher Scientific) was used A study on the granulation performance according to the viscosity of binder solution and the rate of powder feeding | Surface velocities of dry and wet powders Particle size, porosity and strength | [135] |
| Equipment Model (Manufacturer) | Scale and Parameters Conditions | Dimensionless Number | Measured QAs and Predicted QAs | Ref. |
|---|---|---|---|---|
| Mini-Pactor (Gerteis Maschinen + Process Engineering AG, Jona, Switzerland) | Roll forces: 3.0–7.5 kN/cm Roll gaps: 1.5, 2.5 and 3.5 mm | Johanson’s model Johanson’s model was modified overestimation of maximum roll surface pressure | Ribbon density Maximum roll surface pressure | [147] |
| Gerteis (Gerteis, Rapperswil-Jona, Swiss), L.B. Bohle (L.B BOHLE, Ennigerloh, Germany) | Roll width: 25 and 100 mm Specific compaction force: 4, 6 and 8 kN/cm Roll gap: 1.5, 2.25 and 3 mm Roll speed: 2, 3 and 4 rpm | Reynolds model is applied to scale-up the process | Ribbon relative density. | [17] |
| WP 120 Pharma (Alexanderwerk, Remscheid, Germany) | Roll pressure: 40, 60 and 80 bar Roll speed: 5, 7.5 and 10 rpm Screw speed: 25, 30 and 35 rpm | Johanson’s model | Ribbon porosity | [150] |
| WP 120 Pharma (Alexanderwerk, Remscheid, Germany) | Roll width: 40 and 100 mm | Modified Bingham number | Ribbon density Ribbon solid fraction | [143] |
| WP 120 Pharma, WP 200 Pharma (Alexanderwerk, Remscheid, Germany) | Roll pressure: 40, 55 and 70 bar Roll speed: 4, 5, 8 and 12 rpm Screw speed: 19–53 rpm | Modified Johanson’s model | Ribbon density | [14] |
| Chilsonator IR-220, IR-520 (The Fitzpatrick Co., IL, USA) | Roll pressure: 3–12 kN/cm VFS/HFS 1: 4–100 Roll gap: 2–4 mm | Joint-Y partial least squares (JYPLS) | Ribbon density Ribbon solid fraction | [151] |
| WP 120 Pharma, WP 200 Pharma (Alexanderwerk, Germany) | Roll width: 25, 40 and 75 mm Roll diameter: 120 and 200 mm Roll pressure: 20–121 bar Roll gap: 1.2–4.0 mm | Maintain the ratio between the roller gap and the roller diameter Johanson’s model Constant ratio of screw speed to roller speed | Ribbon density | [152] |
| Mini-Pactor (Gerteis Maschinen + Process Engineering AG, Jona, Switzerland) | Roller speed: 1, 2 and 3 rpm Roll gap: 2, 3 and 4 mm Specific compaction force: 2, 3.5, 5, 7 and 9 | Johanson’s model Reynolds model | Ribbon density | [149] |
| WP 200 Pharma (Alexanderwerk, Remscheid, Germany) | Minimum gap width: 2 and 4 mm Inlet stress: 100 and 200 kPa Powder-roll friction coefficient: 0.35 and 0.5 | A study to modify the Johanson’s model compared to FEM simulation | Maximum roll pressure and ribbon relative density predicted | [18] |
| WP 120 Pharma (Alexanderwerk, Remscheid, Germany) | Roll speed: 4 and 12 rpm Roll pressure: 20 and 40 bar Screw speed: 20 rpm | Relationships between ribbon porosity, roll speed, roll pressure, screw speed, true density and roll diameter | Ribbon porosity | [153] |
| Chilsonator IR 220 (The Fitzpatrick Co., IL, USA) | Roll speed: 3 and 9 rpm Roll pressure: 700 and 1000 psi Screw speed: 19 and 21 rpm | |||
| WP 120 Pharma, WP 200 Pharma (Alexanderwerk, Remscheid, Germany) | Roll pressure: 50–70 bar Gap gap: 2.0–2.6 mm Milling speed: 40–80 rpm | Establish relationship between roller compaction parameters and ribbon thickness and density | Granules QAs: flow, bulk density and particle size distribution Ribbon attributes: ribbon density and thickness | [154] |
| Equipment (Manufacturer) | PAT Tool (Model) | Detail | Measured QA | Ref. |
|---|---|---|---|---|
| Laboratory-scale roller compactor (Alexanderwerk, Remscheid, Germany) | CDI non-contact diffuse reflectance spectrometer (SNIR 278, Control Development Inc. South Bend, IN) | Measured in the range of 1305–2205 nm Averaged over 8 scans | Ribbon density | [156] |
| WP 120 Pharma (Alexanderwerk, Remscheid, Germany) | In-line NIR 1 (Multieye) | Measured in the range of 4555–6600 cm−1 | Ribbon density Particle size Ribbon mechanical strength | [19] |
| Off-line NIR (PerkinElmer Spotlight 400 FT-IR) | Measured in the range of 4000–7800 cm−1 with a resolution of 2 cm−1 Averaged over 8 scans | |||
| Image analysis (Eyecon particle imager) | Measure particle size by capturing the color of the particle surface by shining red, green and blue LED on the particle | |||
| Raman (PerkinElmer RamanMicro 300) | 250 mW laser source at 785 nm. Measured in the range of 200-3200 cm−1 with an integration time of 5 s Averaging two scans | |||
| Die and punch set (Natoli Engineering Co., MO, USA) on a single station Laboratory Press (Carver, Inc., Wabash, Indiana). | NIR (Control Development) | Measured in the range of 1100–2200 cm−1 with a resolution of 4.4 nm Averaged over 16 scans | Content uniformity Moisture content Ribbon density Ribbon tensile strength Ribbon Young’s modulus | [157] |
| Chilsonator IR 220 (The Fitzpatrick Co., IL, USA) | NIR (Control Development) | Measured in the range of 1100–2200 nm with 1 nm intervals | Ribbon density | [155] |
| Chilsonator IR 220 (The Fitzpatrick Co., IL, USA) | NIR (Control Development) | Used 35 kW tungsten-halogen light source Measured in the range of 1100–2200 nm with a resolution of 4.4 nm. | Moisture content Ribbon density Ribbon tensile strength Ribbon Young’s modulus | [162] |
| Pharmapaktor L200/30P (Hosokawa Bepex, Leingarten, Germany) | NIR (NIR spectral sensor, MCS 611 NIR 2.2) | Measured in the range of 980–1900 nm with 1 nm resolution Spectra acquisition time of 100 ms. | Content uniformity Ribbon tensile strength Ribbon Young’s modulus, Ribbon density | [163] |
| WP 120 Pharma (Alexanderwerk, Remscheid, Germany) | NIR-CI 2 (Headwall Photonics model 1002A-00371) | Measured in the range of 1100–1700 nm with a resolution of 7 nm | Ribbon porosity distribution Content of API | [158] |
| WP 120 Pharma (Alexanderwerk, Remscheid, Germany) | NIR (Antaris II FT-NIR analyzer) | Spectrum was obtained of 64 scans Measured in the range of 10,000–4000 cm−1 with 8 cm−1 resolution | Moisture content | [58] |
| Microwave resonance (Sartorius LMA 320PA microwave moisture analyzer) | Operating at 2.5 GHz | |||
| Chilsonator IR-520 (The Fitzpatrick Co., IL, USA) | Microwave resonance (Panametrics, 5077PR) | Pulse repetition frequency was 1 kHz with 100 ns intervals | Young’s modulus Poisson’s ratio Porosity | [159] |
| X-ray micro-CT (SkyScan-1172 XRCT) | The spatial resolution was 14.8 μm/pixel Voltage of 50 kV and a currency of 100 μA | Ribbon density | ||
| Mini-Pactor (Gerteis Maschinen + Process Engineering AG, Jona, Switzerland) | X-ray micro-CT (CT alpha) | Scanned at a resolution of 80 mm per voxel All scans were performed in 1600 projections Voltage of 80 kV and a currency of 80 mA | Ribbon porosity | [164] |
| laboratory-scale instrumented roller compactor (developed at the University of Birmingham) | X-ray (X-ray micro-CT system) | Voltage and current were 50 kV and 98 μA The total sample rotation was set at 180° with an interval of 0.9 ° The spatial resolution is 11 μm/pixel | Ribbon density distribution | [160] |
| Mini-Pactor (Gerteis Maschinen + Process Engineering AG, Jona, Switzerland) | thermographic camera (optris PI 640, optris GmbH, D) | Monitoring frequency was 32 frames per second Distance between ribbon and the camera lens was 10–20 cm | Ribbon density distribution | [161] |
| Equipment Model (Manufacturer) | Simulation | Summary | Predicted Attributes | Ref. |
|---|---|---|---|---|
| WP 200 Pharma (Alexanderwerk, Remscheid, Germany) | FEM | FEM simulation was evaluated in comparison with the Johanson’s model | Normal stress Maximum ribbon relative density | [165] |
| Komarek B050PH laboratory press (K.R. Komarek Inc., IL, USA) | FEM | Comparative study of density distribution of ribbon by FEM and light transmission | Principal stress and density across the width of the strip. | [167] |
| Gerteis roll compactor: Mini-pactor 250/25 (Gerteis, Rapperswil-Jona, Swiss) | FEM | Investigate the effect of sealing system design on the density distribution of ribbon using FEM | Ribbon density distribution Roll pressure | [79] |
| Komarek B050H Laboratory Press (K.R. Komarek Inc., IL, USA) | DEM-FEM | Study on the effect of screw feed rate using DEM-FEM coupling approach | Roll pressure Ribbon relative density | [23] |
| WP 120 Pharma (Alexanderwerk, Remscheid, Germany) | FEM | Comparison of the results of ribbon density with experimental measurements of FEM simulations | Ribbon density | [166] |
| RC100 (Roland Research Devices, Inc., NJ, USA) | FEM | Investigate ribbon characteristics according to various process parameters with FEM | Roll pressure Ribbon density Feed stress Roll friction on roll force | [168] |
| WP 200 Pharma (Alexanderwerk, Remscheid, Germany) | FEM | Improvement of prediction accuracy of predicted ribbon density at Johanson’s roll compaction model using FEM | Roll pressure Ribbon relative density | [18] |
| Komarek B050PH laboratory press (K.R. Komarek Inc., IL, USA) | FEM | Study the mechanism of powder transport and ribbon density by predicting the pressure distribution between particles and roller | Roll pressure distribution, shear stress and nip angle | [169] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, E.H.; Park, Y.S.; Kim, M.-S.; Choi, D.H. Model-Based Scale-up Methodologies for Pharmaceutical Granulation. Pharmaceutics 2020, 12, 453. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12050453
Jang EH, Park YS, Kim M-S, Choi DH. Model-Based Scale-up Methodologies for Pharmaceutical Granulation. Pharmaceutics. 2020; 12(5):453. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12050453
Chicago/Turabian StyleJang, Eun Ha, Yun Sang Park, Min-Soo Kim, and Du Hyung Choi. 2020. "Model-Based Scale-up Methodologies for Pharmaceutical Granulation" Pharmaceutics 12, no. 5: 453. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12050453