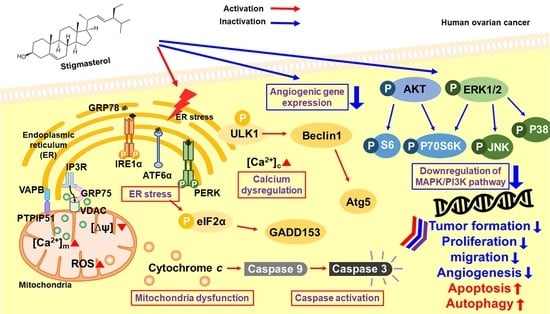
Stigmasterol Causes Ovarian Cancer Cell Apoptosis by Inducing Endoplasmic Reticulum and Mitochondrial Dysfunction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture
2.3. Western Blotting
2.4. Spheroid Assay
2.5. Annexin V and PI Staining
2.6. Cell Cycle Assay
2.7. JC-1 Staining
2.8. Measurement of Reactive Oxygen Species (ROS) Production
2.9. Cytosolic Ca2+ Level Analysis
2.10. Mitochondrial Ca2+ Level Analysis
2.11. Proliferation Assay
2.12. Migration Assay
2.13. Quantitative Real-Time PCR
2.14. Statistical Analysis
3. Results
3.1. Induction of Cell Apoptosis and Inhibition of Cell Aggregation by Stigmasterol in ES2 and OV90 Cells
3.2. Changes in Mitochondrial Function and ROS Levels by Stigmasterol in ES2 and OV90 Cells
3.3. Upregulation of Cytosolic and Mitochondrial Calcium Levels by Stigmasterol in ES2 and OV90 Cells
3.4. Regulation of Endoplasmic Reticulum Stress, the Endoplasmic Reticulum-Mitochondria Axis, and Autophagy by Stigmasterol in ES2 and OV90 Cells
3.5. Restriction of Growth and Inactivation of Intracellular Signals by Stigmasterol in ES2 and OV90 Cells
3.6. Calcium Regulation by Stigmasterol in the Death of ES2 and OV90 Cells
3.7. Decreased Migration Activity of ES2 and OV90 Cells Following Stigmasterol Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moreau, R.A.; Whitaker, B.D.; Hicks, K.B. Phytosterols, phytostanols, and their conjugates in foods: Structural diversity, quantitative analysis, and health-promoting uses. Prog. Lipid Res. 2002, 41, 457–500. [Google Scholar] [CrossRef]
- Garcia-Llatas, G.; Rodriguez-Estrada, M.T. Current and new insights on phytosterol oxides in plant sterol-enriched food. Chem. Phys. Lipids 2011, 164, 607–624. [Google Scholar] [CrossRef] [PubMed]
- Woyengo, T.A.; Ramprasath, V.R.; Jones, P.J. Anticancer effects of phytosterols. Eur. J. Clin. Nutr. 2009, 63, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Miras-Moreno, B.; Sabater-Jara, A.B.; Pedreno, M.A.; Almagro, L. Bioactivity of Phytosterols and Their Production in Plant in Vitro Cultures. J. Agr. Food Chem. 2016, 64, 7049–7058. [Google Scholar] [CrossRef]
- Ferrer, A.; Altabella, T.; Arro, M.; Boronat, A. Emerging roles for conjugated sterols in plants. Prog. Lipid Res. 2017, 67, 27–37. [Google Scholar] [CrossRef]
- Han, J.H.; Yang, Y.X.; Feng, M.Y. Contents of phytosterols in vegetables and fruits commonly consumed in China. Biomed. Environ. Sci. 2008, 21, 449–453. [Google Scholar] [CrossRef]
- Sundararaman, P.; Djerassi, C. A convenient synthesis of progesterone from stigmasterol. J. Org. Chem. 1977, 42, 3633–3634. [Google Scholar] [CrossRef]
- Kametani, T.; Furuyama, H. Synthesis of vitamin D3 and related compounds. Med. Res. Rev. 1987, 7, 147–171. [Google Scholar] [CrossRef]
- Cabral, C.E.; Klein, M. Phytosterols in the Treatment of Hypercholesterolemia and Prevention of Cardiovascular Diseases. Arq. Bras. Cardiol. 2017, 109, 475–482. [Google Scholar] [CrossRef]
- Feng, S.; Dai, Z.; Liu, A.B.; Huang, J.; Narsipur, N.; Guo, G.; Kong, B.; Reuhl, K.; Lu, W.; Luo, Z.; et al. Intake of stigmasterol and beta-sitosterol alters lipid metabolism and alleviates NAFLD in mice fed a high-fat western-style diet. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 1274–1284. [Google Scholar] [CrossRef]
- Valitova, J.N.; Sulkarnayeva, A.G.; Minibayeva, F.V. Plant Sterols: Diversity, Biosynthesis, and Physiological Functions. Biochemistry 2016, 81, 819–834. [Google Scholar] [CrossRef] [PubMed]
- Tao, C.; Shkumatov, A.A.; Alexander, S.T.; Ason, B.L.; Zhou, M. Stigmasterol accumulation causes cardiac injury and promotes mortality. Commun. Biol. 2019, 2, 20. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Li, X.F.; Kang, K.H.; Ryu, B.; Kim, S.K. Stigmasterol isolated from marine microalgae Navicula incerta induces apoptosis in human hepatoma HepG2 cells. BMB Rep. 2014, 47, 433–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kangsamaksin, T.; Chaithongyot, S.; Wootthichairangsan, C.; Hanchaina, R.; Tangshewinsirikul, C.; Svasti, J. Lupeol and stigmasterol suppress tumor angiogenesis and inhibit cholangiocarcinoma growth in mice via downregulation of tumor necrosis factor-alpha. PLoS ONE 2017, 12, e0189628. [Google Scholar] [CrossRef] [Green Version]
- Pandey, P.; Bajpai, P.; Siddiqui, M.H.; Sayyed, U.; Tiwari, R.; Shekh, R.; Mishra, K.; Kapoor, V.K. Elucidation of the Chemopreventive Role of Stigmasterol Against Jab1 in Gall Bladder Carcinoma. Endocr. Metab. Immune. Disord Drug Targets 2019, 19, 826–837. [Google Scholar] [CrossRef]
- Ali, H.; Dixit, S.; Ali, D.; Alqahtani, S.M.; Alkahtani, S.; Alarifi, S. Isolation and evaluation of anticancer efficacy of stigmasterol in a mouse model of DMBA-induced skin carcinoma. Drug Des. Devel. Ther. 2015, 9, 2793–2800. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Yuan, D.; Yan, R.; Meng, L.; Zhang, Y.; Zhu, K. Stigmasterol exhibits potent antitumor effects in human gastric cancer cells mediated via inhibition of cell migration, cell cycle arrest, mitochondrial mediated apoptosis and inhibition of JAK/STAT signalling pathway. J. BUON 2018, 23, 1420–1425. [Google Scholar]
- Newill, H.; Loske, R.; Wagner, J.; Johannes, C.; Lorenz, R.L.; Lehmann, L. Oxidation products of stigmasterol interfere with the action of the female sex hormone 17beta-estradiol in cultured human breast and endometrium cell lines. Mol. Nutr. Food Res. 2007, 51, 888–898. [Google Scholar] [CrossRef]
- Ayaz, M.; Sadiq, A.; Wadood, A.; Junaid, M.; Ullah, F.; Zaman Khan, N. Cytotoxicity and molecular docking studies on phytosterols isolated from Polygonum hydropiper L. Steroids 2019, 141, 30–35. [Google Scholar] [CrossRef]
- Scholtysek, C.; Krukiewicz, A.A.; Alonso, J.L.; Sharma, K.P.; Sharma, P.C.; Goldmann, W.H. Characterizing components of the Saw Palmetto Berry Extract (SPBE) on prostate cancer cell growth and traction. Biochem. Biophys. Res. Commun. 2009, 379, 795–798. [Google Scholar] [CrossRef]
- Liu, X.; Li, Q.; Zhou, J.; Zhang, S. ATP-binding cassette transporter A7 accelerates epithelial-to-mesenchymal transition in ovarian cancer cells by upregulating the transforming growth factor-beta signaling pathway. Oncol. Lett. 2018, 16, 5868–5874. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.; Lee, J.Y.; Yang, C.; Song, G.; Lim, W. Fucoidan Derived from Fucus vesiculosus Inhibits the Development of Human Ovarian Cancer via the Disturbance of Calcium Homeostasis, Endoplasmic Reticulum Stress, and Angiogenesis. Mar. Drugs 2020, 18, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lian, I.A.; Loset, M.; Mundal, S.B.; Fenstad, M.H.; Johnson, M.P.; Eide, I.P.; Bjorge, L.; Freed, K.A.; Moses, E.K.; Austgulen, R. Increased endoplasmic reticulum stress in decidual tissue from pregnancies complicated by fetal growth restriction with and without pre-eclampsia. Placenta 2011, 32, 823–829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez, J.; Tait, S.W. Mitochondrial apoptosis: Killing cancer using the enemy within. Br. J. Cancer 2015, 112, 957–962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinton, P.; Giorgi, C.; Siviero, R.; Zecchini, E.; Rizzuto, R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene 2008, 27, 6407–6418. [Google Scholar] [CrossRef] [Green Version]
- Azimi, I.; Roberts-Thomson, S.J.; Monteith, G.R. Calcium influx pathways in breast cancer: Opportunities for pharmacological intervention. Br. J. Pharmacol. 2014, 171, 945–960. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.G.; Pathan, N.; Ethell, I.M.; Krajewski, S.; Yamaguchi, Y.; Shibasaki, F.; McKeon, F.; Bobo, T.; Franke, T.F.; Reed, J.C. Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science 1999, 284, 339–343. [Google Scholar] [CrossRef]
- Moloney, J.N.; Cotter, T.G. ROS signalling in the biology of cancer. Semin. Cell Dev. Biol. 2018, 80, 50–64. [Google Scholar] [CrossRef]
- Yoon, M.J.; Lee, A.R.; Jeong, S.A.; Kim, Y.S.; Kim, J.Y.; Kwon, Y.J.; Choi, K.S. Release of Ca2+ from the endoplasmic reticulum and its subsequent influx into mitochondria trigger celastrol-induced paraptosis in cancer cells. Oncotarget 2014, 5, 6816–6831. [Google Scholar] [CrossRef] [Green Version]
- Larman, M.; Lucy, J. The endoplasmic reticulum: Structure and function. Mol. Membr. Biol. 1999, 16, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Osaki, M.; Oshimura, M.; Ito, H. PI3K-Akt pathway: Its functions and alterations in human cancer. Apoptosis 2004, 9, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Cheaib, B.; Auguste, A.; Leary, A. The PI3K/Akt/mTOR pathway in ovarian cancer: Therapeutic opportunities and challenges. Chin. J. Cancer 2015, 34, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, T.; Ohata, H.; Sato, A.; Yamawaki, K.; Enomoto, T.; Okamoto, K. Tumor-derived spheroids: Relevance to cancer stem cells and clinical applications. Cancer Sci. 2017, 108, 283–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, K.; Kim, M.; Gilbert, C.A.; Simpkins, F.; Ince, T.A.; Slingerland, J.M. VEGFA activates an epigenetic pathway upregulating ovarian cancer-initiating cells. EMBO Mol. Med. 2017, 9, 304–318. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Han, X. The urokinase plasminogen activator system in breast cancer invasion and metastasis. Biomed. Pharm. 2013, 67, 179–182. [Google Scholar] [CrossRef]
- Jacob, A.; Prekeris, R. The regulation of MMP targeting to invadopodia during cancer metastasis. Front. Cell Dev. Biol. 2015, 3, 4. [Google Scholar] [CrossRef] [Green Version]









| Primary Antibodies | Dilution | Supplier | Catalog Number |
|---|---|---|---|
| Cleaved caspase-3 | 1:1000 | Cell Signaling | 9664 |
| Cleaved caspase-9 | 1:1000 | Cell Signaling | 9501 |
| Cytochrome c | 1:1000 | Cell Signaling | 11940 |
| BAK | 1:1000 | Cell Signaling | 12105 |
| BAX | 1:1000 | Cell Signaling | 2774 |
| Phospho-PERK (Thr981) | 1:1000 | Santa Cruz | sc-32577 |
| Phospho-eIF2α (Ser51) | 1:1000 | Cell Signaling | 3398 |
| IRE1α | 1:1000 | Cell Signaling | 3294 |
| GADD153 | 1:1000 | Santa Cruz | sc-7351 |
| ATF6α | 1:1000 | Santa Cruz | sc-166659 |
| GRP78 | 1:1000 | Santa Cruz | sc-13968 |
| TUBA | 1:1000 | Santa Cruz | sc-5286 |
| VDAC | 1:1000 | Cell Signaling | 4661 |
| IP3R1 | 1:1000 | Invitrogen | PA1-901 |
| IP3R2 | 1:1000 | Santacruz | sc-398434 |
| VAPB | 1:1000 | Invitrogen | PA5-53023 |
| FAM82A2 | 1:1000 | Abcam | ab182105 |
| Phospho-ULK1 (SER555) | 1:1000 | Cell Signaling | 5869 |
| ULK1 | 1:1000 | Cell Signaling | 8054 |
| BECN1 | 1:1000 | Cell Signaling | 3495 |
| ATG5 | 1:1000 | Cell Signaling | 12994 |
| Phospho-AKT (SER473) | 1:1000 | Cell Signaling | 4060 |
| AKT | 1:1000 | Cell Signaling | 9272 |
| Phospho-P70S6K (Thr421/Ser424) | 1:1000 | Cell Signaling | 9204 |
| P70S6K | 1:1000 | Cell Signaling | 9202 |
| Phospho-S6 (Ser235/236) | 1:1000 | Cell Signaling | 2211 |
| S6 | 1:1000 | Cell Signaling | 2217 |
| Phospho-ERK1/2(Thr202/Tyr204) | 1:1000 | Cell Signaling | 9101 |
| ERK1/2 | 1:1000 | Cell Signaling | 4695 |
| Phospho-JNK (Thr183/Tyr185) | 1:1000 | Cell Signaling | 4668 |
| JNK | 1:1000 | Cell Signaling | 9252 |
| Phospho-P38 (Thr180/Tyr182) | 1:1000 | Cell Signaling | 4511 |
| P38 | 1:1000 | Cell Signaling | 9212 |
| Primer | Sequence | Size | |
|---|---|---|---|
| VEGFA | Forward | 5′-TTGTACAAGATCCGCAGACG-3′ | 100 bp |
| Reverse | 5′-TCACATCTGCAAGTACGTTCG-3′ | ||
| PLAU | Forward | 5′-CAACTGCCCAAAGAAATTCG-3′ | 148 bp |
| Reverse | 5′-AAGGACAGTGGCAGAGTTCC-3′ | ||
| MMP2 | Forward | 5′-GGCATTCAGGAGCTCTATGG-3′ | 137 bp |
| Reverse | 5′-ATCTCACCACGGATCTGAGC-3′ | ||
| MMP9 | Forward | 5′-TTGACAGCGACAAGAAGTGG-3′ | 145 bp |
| Reverse | 5′-TCAGTGAAGCGGTACATAGGG-3′ | ||
| MMP14 | Forward | 5′-GCAGAAGTTTTACGGCTTGC-3′ | 117 bp |
| Reverse | 5′-ACATTGGCCTTGATCTCAGC-3′ | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, H.; Song, G.; Lim, W. Stigmasterol Causes Ovarian Cancer Cell Apoptosis by Inducing Endoplasmic Reticulum and Mitochondrial Dysfunction. Pharmaceutics 2020, 12, 488. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12060488
Bae H, Song G, Lim W. Stigmasterol Causes Ovarian Cancer Cell Apoptosis by Inducing Endoplasmic Reticulum and Mitochondrial Dysfunction. Pharmaceutics. 2020; 12(6):488. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12060488
Chicago/Turabian StyleBae, Hyocheol, Gwonhwa Song, and Whasun Lim. 2020. "Stigmasterol Causes Ovarian Cancer Cell Apoptosis by Inducing Endoplasmic Reticulum and Mitochondrial Dysfunction" Pharmaceutics 12, no. 6: 488. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12060488