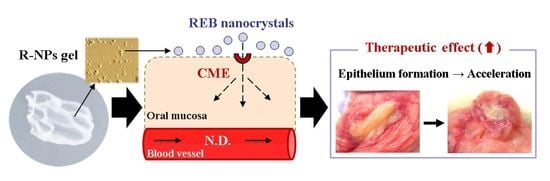
Hydrogel Formulations Incorporating Drug Nanocrystals Enhance the Therapeutic Effect of Rebamipide in a Hamster Model for Oral Mucositis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Preparation of REB Hydrogel
2.3. Characteristics of REB Hydrogels
2.4. Permeation Study from REB Hydrogels
2.5. REB Contents in Hamsters Treated with REB Hydrogels
2.6. Measurement of Wound Area in the Hamster Model for Oral Mucositis
2.7. Measurement of Wound Area in the Hamster Model for Oral Mucositis
2.8. Statistical Analysis
3. Results
3.1. Design and Characteristics of the R-NPs Gel
3.2. Endocytic Uptake of REB Nanocrystals into Cheek Pouch Tissue
3.3. Effect of REB Hydrogel on Oral Wound Healing in the Hamster Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sciubba, J.J.; Goldenberg, D. Oral complications of radiotherapy. Lancet Oncol. 2006, 7, 175–183. [Google Scholar] [CrossRef]
- Rodríguez-Caballero, A.; Torres-Lagares, D.; Robles-García, M.; Pachón-Ibáñez, J.; González-Padilla, D.; Gutiérrez-Pérez, J.L. Cancer treatment-induced oral mucositis: A critical review. Int. J. Oral Maxillofac. Surg. 2012, 41, 225–238. [Google Scholar] [CrossRef]
- Donnelly, J.P.; Blijlevens, N.M.; Verhagen, C.A. Can anything be done about oral mucositis? Ann. Oncol. 2003, 14, 505–507. [Google Scholar] [CrossRef]
- Sonis, S.T. Oral mucositis in cancer therapy. J. Support. Oncol. 2004, 2, 3–8. [Google Scholar]
- Trotti, A.; Bellm, L.A.; Epstein, J.B.; Frame, D.; Fuchs, H.J.; Gwede, C.K.; Komaroff, E.; Nalysnyk, L.; Zilberberg, M.D. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: A systematic literature review. Radiother. Oncol. 2003, 66, 253–262. [Google Scholar] [CrossRef]
- Sonis, S.T.; Elting, L.S.; Keefe, D.; Peterson, D.E.; Schubert, M.; Hauer-Jensen, M.; Bekele, B.N.; Raber-Durlacher, J.; Donnelly, J.P.; Rubenstein, E.B. Perspectives on cancer therapy-induced mucosal injury: Pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 2004, 100, 1995–2025. [Google Scholar] [CrossRef] [PubMed]
- Prescribing Information: Mucosta® Tablets 100 mg, Ohtsuka Japan Inc. 2017. Available online: https://www.pmda.go.jp/PmdaSearch/iyakuDetail/GeneralList/2329021 (accessed on 20 May 2020). (In Japanese).
- Lalla, R.V.; Bowen, J.; Barasch, A.; Elting, L.; Epstein, J.; Keefe, D.M.; McGuire, D.B.; Migliorati, C.; Nicolatou-Galitis, O.; Peterson, D.E.; et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 2014, 120, 1453–1461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bensinger, W.; Schubert, M.; Ang, K.K.; Brizel, D.; Brown, E.; Eilers, J.G.; Elting, L.; Mittal, B.B.; Schattner, M.A.; Spielberger, R.; et al. NCCN Task Force Report. prevention and management of mucositis in cancer care. J. Natl. Compr. Cancer Netw. 2008, 6, S1–S21; quiz S22–S24. [Google Scholar]
- Peterson, D.E.; Boers-Doets, C.B.; Bensadoun, R.J.; Herrstedt, J. ESMO Guidelines Committee Management of oral and gastrointestinal mucosal injury: ESMO Clinical Practice Guidelines for diagnosis, treatment, and follow-up. Ann. Oncol. 2015, 26, v139–v151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murakami, K.; Okajima, K.; Uchiba, M.; Harada, N.; Johno, M.; Okabe, H.; Takatsuki, K. Rebamipide attenuates indomethacin-induced gastric mucosal lesion formation by inhibiting activation of leukocytes in rats. Dig. Dis. Sci. 1997, 42, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; Naito, Y.; Tanigawa, T.; Kondo, M. Free radical scavenging activity of the novel anti-ulcer agent rebsamipide studied by electron spin resonance. Arzneimittelforschung 1993, 43, 363–366. [Google Scholar] [PubMed]
- Nanke, Y.; Kobashigawa, T.; Yago, T.; Kawamoto, M.; Yamanaka, H.; Kotake, S. Rebamipide, an Amino Acid Analog of 2(1H)-Quinolinone, Inhibits the Formation of Human Osteoclasts. BioMed Res. Int. 2016, 2016, 6824719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, H.; Fukuda, K.; Ishida, W.; Harada, Y.; Sumi, T.; Fukushima, A. Rebamipide increases barrier function and attenuates TNFalpha-induced barrier disruption and cytokine expression in human corneal epithelial cells. Br. J. Ophthalmol. 2013, 97, 912–916. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, T.; Chiba, H.; Satomi, T.; Matsuo, A.; Kaneko, T.; Chikazu, D.; Miyamatsu, H. Preventive effect of rebamipide gargle on chemoradiotherpy-induced oral mucositis in patients with oral cancer: A pilot study. J. Oral Maxillofac. Res. 2011, 2, e3. [Google Scholar] [CrossRef] [PubMed]
- Nabeta, I.; Nakamura, K.; Kimura, M.; Kaya, M.; Tsuneizumi, M.; Nakagami, K.; Kawarasaki, T.; Honma, M. The effect of rebamipide for prevention of mucositis associated with anthracycline chemotherapy for breast cancer. J. Jpn. Soc. Hosp. Pharm. 2010, 46, 1629–1634. (In Japanese) [Google Scholar]
- Chaitanya, B.; Pai, K.M.; Yathiraj, P.H.; Fernandes, D.; Chhaparwal, Y. Rebamipide gargle in preventive management of chemo-radiotherapy induced oral mucositis. Oral Oncol. 2017, 72, 179–182. [Google Scholar] [CrossRef]
- Yokota, T.; Ogawa, T.; Takahashi, S.; Okami, K.; Fujii, T.; Tanaka, K.; Iwae, S.; Ota, I.; Ueda, T.; Monden, N.; et al. Efficacy and safety of rebamipide liquid for chemoradiotherapy-induced oral mucositis in patients with head and neck cancer: A multicenter, randomized, double-blind, placebo-controlled, parallel-group phase II study. BMC Cancer 2017, 17, 314. [Google Scholar] [CrossRef] [Green Version]
- Ishihara, K.; Komuro, Y.; Nishiyama, N.; Yamasaki, K.; Hotta, K. Effect of rebamipide on mucus secretion by endogenous prostaglandin-independent mechanism in rat gastric mucosa. Arzneimittelforschung 1992, 42, 1462–1466. [Google Scholar]
- Yamasaki, K.; Kanbe, T.; Chijiwa, T.; Ishiyama, H.; Morita, S. Gastric mucosal protection by OPC-12759, a novel antiulcer compound, in the rat. Eur. J. Pharmacol. 1987, 142, 23–29. [Google Scholar] [CrossRef]
- Kleine, A.; Kluge, S.; Peskar, B.M. Stimulation of prostaglandin biosynthesis mediates gastroprotective effect of rebamipide in rats. Dig. Dis. Sci. 1993, 38, 1441–1449. [Google Scholar] [CrossRef]
- Nagano, C.; Azuma, A.; Ishiyama, H.; Sekiguchi, K.; Imagawa, K.; Kikuchi, M. Rebamipide suppresses formyl-methionyl-leucylphenylalanine (fMLP)-induced superoxide production by inhibiting fMLP-receptor binding in human neutrophils. J. Pharmacol. Exp. Ther. 2001, 297, 388–394. [Google Scholar]
- Kobayashi, T.; Zinchuk, V.S.; del Saz, E.G.; Jiang, F.; Yamasaki, Y.; Kataoka, S.; Okada, T.; Tsunawaki, S.; Seguchi, H. Suppressive effect of rebamipide, an antiulcer agent, against activation of human neutrophils exposed to formyl-methionyl-leucyl-phenylalanine. Histol. Histopathol. 2000, 15, 1067–1076. [Google Scholar]
- Masamune, A.; Yoshida, M.; Sakai, Y.; Shimosegawa, T. Rebamipide inhibits ceramide-induced interleukin-8 production in Kato III human gastric cancer cells. J. Pharmacol. Exp. Ther. 2001, 298, 485–492. [Google Scholar]
- Kim, C.D.; Kim, H.H.; Hong, K.W. Inhibitory effect of rebamipide on the neutrophil adherence stimulated by conditioned media from helicobacter pylori-infected gastric epithelial cells. J. Pharmacol. Exp. Ther. 1999, 288, 133–138. [Google Scholar]
- Arakawa, T.; Kobayashi, K.; Yoshikawa, T.; Tarnawski, A. Rebamipide: Overview of its mechanisms of action and efficacy in mucosal protection and ulcer healing. Dig. Dis. Sci. 1998, 43, 5S–13S. [Google Scholar]
- Tarnawski, A.; Arakawa, T.; Kobayashi, K. Rebamipide treatment activates epidermal growth factor and its receptor expression in normal and ulcerated gastric mucosa in rats: One mechanism for its ulcer healing action? Dig. Dis. Sci. 1998, 43, 90S–98S. [Google Scholar]
- Shioya, Y.; Kashiyama, E.; Okada, K.; Kusumoto, N.; Abe, Y. Metabolic fate of the anti-ulcer agent, (±)-2-(4-chlorobenzoylamino)-3-[2(1H)-quinolinon-4-yl]propionic acid (OPC-12759): Absorption, distribution, and excretion in rats and dogs. Iyakuhin Kenkyu 1989, 20, 522–533. [Google Scholar]
- Sanders, N.N.; De Smedt, S.C.; Van Rompaey, E.; Simoens, P.; De Baets, F.; Demeester, J. Cystic fibrosis sputum: A barrier to the transport of nanospheres. Am. J. Respir. Crit. Care Med. 2000, 162, 1905–1911. [Google Scholar] [CrossRef]
- Szentkuti, L. Light microscopical observations on luminally administered dyes, dextrans, nanospheres and microspheres in the pre-epithelial mucus gel layer of the rat distal colon. J. Control. Release 1997, 46, 233–242. [Google Scholar] [CrossRef]
- Norris, D.A.; Sinko, P.J. Effect of size, surface charge and hydrophobicity on the translocation of polyestyrene microspheres through gastrointestinal mucin. J. Appl. Polym. Sci. 1997, 63, 1481–1492. [Google Scholar] [CrossRef]
- Bravo-Osuna, I.; Vauthier, C.; Farabollini, A.; Palmieri, G.F.; Ponchel, G. Mucoadhesion mechanism of chitosan and thiolated chitosan-poly(isobutyl cyanoacrylate) core-shell nanoparticles. Biomaterials 2007, 28, 2233–2243. [Google Scholar] [CrossRef]
- Nagai, N.; Ishii, M.; Seiriki, R.; Ogata, F.; Otake, H.; Nakazawa, Y.; Okamoto, N.; Kanai, K.; Kawasaki, N. Novel Sustained-Release Drug Delivery System for Dry Eye Therapy by Rebamipide Nanoparticles. Pharmaceutics 2020, 12, 155. [Google Scholar] [CrossRef] [Green Version]
- Nagai, N.; Iwamae, A.; Tanimoto, S.; Yoshioka, C.; Ito, Y. Pharmacokinetics and Antiinflammatory Effect of a Novel Gel System Containing Ketoprofen Solid Nanoparticles. Biol. Pharm. Bull. 2015, 38, 1918–1924. [Google Scholar] [CrossRef] [Green Version]
- Nagai, N.; Ogata, F.; Otake, H.; Nakazawa, Y.; Kawasaki, N. Design of a transdermal formulation containing raloxifene nanoparticles for osteoporosis treatment. Int. J. Nanomed. 2018, 13, 5215–5229. [Google Scholar] [CrossRef] [Green Version]
- Mäger, I.; Langel, K.; Lehto, T.; Eiríksdóttir, E.; Langel, U. The role of endocytosis on the uptake kinetics of luciferin-conjugated cell-penetrating peptides. Biochim. Biophys. Acta Biomembr. 2012, 1818, 502–511. [Google Scholar] [CrossRef] [Green Version]
- Hufnagel, H.; Hakim, P.; Lima, A.; Hollfelder, F. Fluid phase endocytosis contributes to transfection of DNA by PEI-25. Mol. Ther. 2009, 17, 1411–1417. [Google Scholar] [CrossRef]
- Malomouzh, A.I.; Mukhitov, A.R.; Proskurina, S.E.; Vyskocil, F.; Nikolsky, E.E. The effect of dynasore, a blocker of dynamin-dependent endocytosis, on spontaneous quantal and non-quantal release of acetylcholine in murine neuromuscular junctions. Dokl. Biol. Sci. 2014, 459, 330–333. [Google Scholar] [CrossRef]
- Nicolatou-Galitis, O.; Sarri, T.; Bowen, J.; Di Palma, M.; Kouloulias, V.E.; Niscola, P.; Riesenbeck, D.; Stokman, M.; Tissing, W.; Yeoh, E.; et al. Systematic review of anti-inflammatory agents for the management of oral mucositis in cancer patients. Support. Care Cancer 2013, 21, 3179–3189. [Google Scholar] [CrossRef] [Green Version]
- Nagai, N.; Ito, Y.; Okamoto, N.; Shimomura, Y. A nanoparticle formulation reduces the corneal toxicity of indomethacin eye drops and enhances its corneal permeability. Toxicology 2014, 319, 53–62. [Google Scholar] [CrossRef]
- Nagai, N.; Ito, Y. Therapeutic Effects of Gel Ointments containing Tranilast Nanoparticles on Paw Edema in Adjuvant-Induced Arthritis Rats. Biol. Pharm. Bull. 2014, 37, 96–104. [Google Scholar] [CrossRef] [Green Version]
- Ishii, M.; Fukuoka, Y.; Deguchi, S.; Otake, H.; Tanino, T.; Nagai, N. Energy-Dependent Endocytosis is Involved in the Absorption of Indomethacin Nanoparticles in the Small Intestine. Int. J. Mol. Sci. 2019, 20, 476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagai, N.; Ito, Y. Effect of Solid Nanoparticle of Indomethacin on Therapy for Rheumatoid Arthritis in Adjuvant-Induced Arthritis Rat. Biol. Pharm. Bull. 2014, 37, 1109–1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Byrne, J.D.; Napier, M.E.; DeSimone, J.M. More effective nanomedicines through particle design. Small 2011, 7, 1919–1931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rappoport, J.Z. Focusing on clathrin-mediated endocytosis. Biochem. J. 2008, 412, 415–423. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Li, J.; Lykotrafitis, G.; Bao, G.; Suresh, S. Size-dependent endocytosis of nanoparticles. Adv. Mater. 2009, 21, 419–424. [Google Scholar] [CrossRef] [Green Version]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagai, N.; Seiriki, R.; Deguchi, S.; Otake, H.; Hiramatsu, N.; Sasaki, H.; Yamamoto, N. Hydrogel Formulations Incorporating Drug Nanocrystals Enhance the Therapeutic Effect of Rebamipide in a Hamster Model for Oral Mucositis. Pharmaceutics 2020, 12, 532. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12060532
Nagai N, Seiriki R, Deguchi S, Otake H, Hiramatsu N, Sasaki H, Yamamoto N. Hydrogel Formulations Incorporating Drug Nanocrystals Enhance the Therapeutic Effect of Rebamipide in a Hamster Model for Oral Mucositis. Pharmaceutics. 2020; 12(6):532. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12060532
Chicago/Turabian StyleNagai, Noriaki, Ryotaro Seiriki, Saori Deguchi, Hiroko Otake, Noriko Hiramatsu, Hiroshi Sasaki, and Naoki Yamamoto. 2020. "Hydrogel Formulations Incorporating Drug Nanocrystals Enhance the Therapeutic Effect of Rebamipide in a Hamster Model for Oral Mucositis" Pharmaceutics 12, no. 6: 532. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12060532