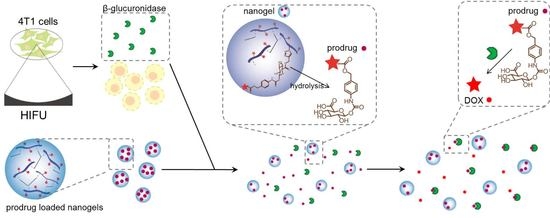
A Doxorubicin-Glucuronide Prodrug Released from Nanogels Activated by High-Intensity Focused Ultrasound Liberated β-Glucuronidase
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Synthesis of DOX-propGA3-Polymer
2.4. Characterization of the DOX-propGA3-Polymer Conjugate
2.5. Preparation of DOX-propGA3-Nanogels
2.6. Prodrug Conversion
2.7. Induction of β-Gus Liberated from 4T1 Cells by HIFU
2.8. Microscopy of Cells Exposed to HIFU
2.9. Determination of the β-Gus Activity
2.10. Conversion of DOX-propGA3-Nanogels into DOX by β-Gus Liberated from HIFU Treated Cells
2.11. In Vitro Cytotoxicity
2.12. Statistical Analysis
3. Results and Discussion
3.1. Synthesis of DOX-propGA3-Polymer Conjugate and DOX-propGA3-Nanogels
3.2. Conversion of Prodrug into DOX by Bovine β-Gus
3.3. Exposure of 4T1 Cells to HIFU and Conversion of Prodrug by HIFU Treated Cells
3.4. In Vitro Cytotoxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA A Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Speth, P.A.; van Hoesel, Q.G.; Haanen, C. Clinical pharmacokinetics of doxorubicin. Clin. Pharmacokinet. 1988, 15, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Beijers, A.J.; Jongen, J.L.; Vreugdenhil, G. Chemotherapy-induced neurotoxicity: The value of neuroprotective strategies. Neth. J. Med. 2012, 70, 18–25. [Google Scholar] [PubMed]
- Riddell, E.; Lenihan, D. The role of cardiac biomarkers in cardio-oncology. Curr. Probl. Cancer 2018, 42, 375–385. [Google Scholar] [CrossRef]
- Delahousse, J.; Skarbek, C.; Paci, A. Prodrugs as drug delivery system in oncology. Cancer Chemother. Pharmacol. 2019, 84, 937–958. [Google Scholar] [CrossRef] [PubMed]
- Denny, W.A. Prodrug strategies in cancer therapy. Eur. J. Med. Chem. 2001, 36, 577–595. [Google Scholar] [CrossRef]
- Kratz, F.; Muller, I.A.; Ryppa, C.; Warnecke, A. Prodrug strategies in anticancer chemotherapy. Chem. Med. Chem. 2008, 3, 20–53. [Google Scholar] [CrossRef] [PubMed]
- Tietze, L.F.; Krewer, B. Antibody-directed enzyme prodrug therapy: A promising approach for a selective treatment of cancer based on prodrugs and monoclonal antibodies. Chem. Biol. Drug Des. 2009, 74, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Houba, P.H.; Boven, E.; van der Meulen-Muileman, I.H.; Leenders, R.G.; Scheeren, J.W.; Pinedo, H.M.; Haisma, H.J. A novel doxorubicin-glucuronide prodrug DOX-GA3 for tumour-selective chemotherapy: Distribution and efficacy in experimental human ovarian cancer. Br. J. Cancer 2001, 84, 550–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houba, P.H.; Boven, E.; van der Meulen-Muileman, I.H.; Leenders, R.G.; Scheeren, J.W.; Pinedo, H.M.; Haisma, H.J. Pronounced antitumor efficacy of doxorubicin when given as the prodrug DOX-GA3 in combination with a monoclonal antibody beta-glucuronidase conjugate. Int. J. Cancer 2001, 91, 550–554. [Google Scholar] [CrossRef]
- Wang, S.M.; Chern, J.W.; Yeh, M.Y.; Ng, J.C.; Tung, E.; Roffler, S.R. Specific activation of glucuronide prodrugs by antibody-targeted enzyme conjugates for cancer therapy. Cancer Res. 1992, 52, 4484–4491. [Google Scholar] [PubMed]
- Sperker, B.; Werner, U.; Murdter, T.E.; Tekkaya, C.; Fritz, P.; Wacke, R.; Adam, U.; Gerken, M.; Drewelow, B.; Kroemer, H.K. Expression and function of beta-glucuronidase in pancreatic cancer: Potential role in drug targeting. Naunyn Schmiedeberg Arch. Pharmacol. 2000, 362, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Bosslet, K.; Straub, R.; Blumrich, M.; Czech, J.; Gerken, M.; Sperker, B.; Kroemer, H.K.; Gesson, J.P.; Koch, M.; Monneret, C. Elucidation of the mechanism enabling tumor selective prodrug monotherapy. Cancer Res. 1998, 58, 1195–1201. [Google Scholar] [PubMed]
- De Graaf, M.; Boven, E.; Scheeren, H.W.; Haisma, H.J.; Pinedo, H.M. Beta-glucuronidase-mediated drug release. Curr. Pharm. Des. 2002, 8, 1391–1403. [Google Scholar] [CrossRef] [PubMed]
- De Graaf, M.; Nevalainen, T.J.; Scheeren, H.W.; Pinedo, H.M.; Haisma, H.J.; Boven, E. A methylester of the glucuronide prodrug DOX-GA3 for improvement of tumor-selective chemotherapy. Biochem. Pharmacol. 2004, 68, 2273–2281. [Google Scholar] [CrossRef] [PubMed]
- Gentile, E.; Cilurzo, F.; Di Marzio, L.; Carafa, M.; Ventura, C.A.; Wolfram, J.; Paolino, D.; Celia, C. Liposomal chemotherapeutics. Future Oncol. 2013, 9, 1849–1859. [Google Scholar] [CrossRef] [PubMed]
- Shimanovich, U.; Bernardes, G.J.; Knowles, T.P.; Cavaco-Paulo, A. Protein micro-and nano-capsules for biomedical applications. Chem. Soc. Rev. 2014, 43, 1361–1371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siepmann, J.; Faham, A.; Clas, S.D.; Boyd, B.J.; Jannin, V.; Bernkop-Schnurch, A.; Zhao, H.; Lecommandoux, S.; Evans, J.C.; Allen, C.; et al. Lipids and polymers in pharmaceutical technology: Lifelong companions. Int. J. Pharm. 2019, 558, 128–142. [Google Scholar] [CrossRef] [PubMed]
- Van der Meel, R.; Sulheim, E.; Shi, Y.; Kiessling, F.; Mulder, W.J.M.; Lammers, T. Smart cancer nanomedicine. Nat. Nanotechnol. 2019, 14, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Yallapu, M.M.; Jaggi, M.; Chauhan, S.C. Design and engineering of nanogels for cancer treatment. Drug Discov. Today 2011, 16, 457–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.; Ichikawa, A.; Ikeuchi-Takahashi, Y.; Hattori, Y.; Onishi, H. Nanogels of succinylated glycol chitosan-succinyl prednisolone conjugate: Preparation, in vitro characteristics and therapeutic potential. Pharmaceutics 2019, 11, 333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, Y.; Hu, B.; Yuan, X.; Cai, L.; Gao, H.; Yang, Q. Nanogel: A versatile nano-delivery system for biomedical applications. Pharmaceutics 2020, 12, 290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chacko, R.T.; Ventura, J.; Zhuang, J.; Thayumanavan, S. Polymer nanogels: A versatile nanoscopic drug delivery platform. Adv. Drug Deliv. Rev. 2012, 64, 836–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel nanoparticles in drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef]
- Li, D.; van Nostrum, C.F.; Mastrobattista, E.; Vermonden, T.; Hennink, W.E. Nanogels for intracellular delivery of biotherapeutics. J. Control. Release 2017, 259, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Soni, K.S.; Desale, S.S.; Bronich, T.K. Nanogels: An overview of properties, biomedical applications and obstacles to clinical translation. J. Control. Release 2016, 240, 109–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, J.; Nakamura, H.; Maeda, H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Deliv. Rev. 2011, 63, 136–151. [Google Scholar] [CrossRef]
- Denny, W.A. Tumor-activated prodrugs-a new approach to cancer therapy. Cancer Investig. 2004, 22, 604–619. [Google Scholar] [CrossRef] [PubMed]
- Han, H.K.; Amidon, G.L. Targeted prodrug design to optimize drug delivery. Aaps Pharmsci. 2000, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; McLeod, H.L. Strategies for enzyme/prodrug cancer therapy. Clin. Cancer Res. 2001, 7, 3314–3324. [Google Scholar]
- Antunes, I.F.; Haisma, H.J.; Elsinga, P.H.; Di Gialleonardo, V.; van Waarde, A.; Willemsen, A.T.; Dierckx, R.A.; de Vries, E.F. Induction of beta-glucuronidase release by cytostatic agents in small tumors. Mol. Pharm. 2012, 9, 3277–3285. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Hernandez, E.; Hess, M.; Melen, G.J.; Theek, B.; Talelli, M.; Shi, Y.; Ozbakir, B.; Teunissen, E.A.; Ramirez, M.; Moeckel, D.; et al. PEG-pHPMAm-based polymeric micelles loaded with doxorubicin-prodrugs in combination antitumor therapy with oncolytic vaccinia viruses. Polym. Chem. 2014, 5, 1674–1681. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kale, V.; Chen, M. Gene-directed enzyme prodrug therapy. AAPS J. 2015, 17, 102–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagshawe, K.D.; Sharma, S.K.; Springer, C.J.; Rogers, G.T. Antibody directed enzyme prodrug therapy (ADEPT). A review of some theoretical, experimental and clinical aspects. Ann. Oncol. 1994, 5, 879–891. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Bagshawe, K.D. Antibody directed enzyme prodrug therapy (ADEPT): Trials and tribulations. Adv. Drug Deliv. Rev. 2017, 118, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Besse, H.C.; Römhild, K.; Wang, B.; Sun, Q.; Omata, D.; Ozbakir, B.; Shi, Y.; Bos, C.; Scheeren, H.W.; Storm, G.; et al. Ultrasound directed enzyme prodrug therapy. Manuscript in preparation.
- Hill, C.R.; Ter-Haar, G.R. Review article: High intensity focused ultrasound-potential for cancer treatment. Br. J. Radiol. 1995, 68, 1296–1303. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tezcan, O.; Li, D.; Beztsinna, N.; Lou, B.; Etrych, T.; Ulbrich, K.; Metselaar, J.M.; Lammers, T.; Hennink, W.E. Overcoming multidrug resistance using folate receptor-targeted and pH-responsive polymeric nanogels containing covalently entrapped doxorubicin. Nanoscale 2017, 9, 10404–10419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talelli, M.; Morita, K.; Rijcken, C.J.; Aben, R.W.; Lammers, T.; Scheeren, H.W.; van Nostrum, C.F.; Storm, G.; Hennink, W.E. Synthesis and characterization of biodegradable and thermosensitive polymeric micelles with covalently bound doxorubicin-glucuronide prodrug via click chemistry. Bioconjugate Chem. 2011, 22, 2519–2530. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; van Steenbergen, M.J.; Li, D.; van de Dikkenberg, J.B.; Lammers, T.; van Nostrum, C.F.; Metselaar, J.M.; Hennink, W.E. Polymeric nanogels with tailorable degradation behavior. Macromol. Biosci. 2016, 16, 1122–1137. [Google Scholar] [CrossRef] [Green Version]
- Ramaekers, P.; de Greef, M.; van Breugel, J.M.; Moonen, C.T.; Ries, M. Increasing the HIFU ablation rate through an MRI-guided sonication strategy using shock waves: Feasibility in the in vivo porcine liver. Phys. Med. Biol. 2016, 61, 1057–1077. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, R.A.; Kavanagh, T.A.; Bevan, M.W. GUS fusions: Beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987, 6, 3901–3907. [Google Scholar] [CrossRef]
- Hein, C.D.; Liu, X.M.; Wang, D. Click chemistry, a powerful tool for pharmaceutical sciences. Pharm. Res. 2008, 25, 2216–2230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Presolski, S.I.; Hong, V.P.; Finn, M.G. Copper-catalyzed azide-alkyne click chemistry for bioconjugation. Curr. Protoc. Chem. Biol. 2011, 3, 153–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hein, J.E.; Fokin, V.V. Copper-catalyzed azide-alkyne cycloaddition (CuAAC) and beyond: New reactivity of copper(I) acetylides. Chem. Soc. Rev. 2010, 39, 1302–1315. [Google Scholar] [CrossRef]
- Jain, S.; Drendel, W.B.; Chen, Z.W.; Mathews, F.S.; Sly, W.S.; Grubb, J.H. Structure of human beta-glucuronidase reveals candidate lysosomal targeting and active-site motifs. Nat. Struct. Biol. 1996, 3, 375–381. [Google Scholar] [CrossRef]
- Naz, H.; Islam, A.; Waheed, A.; Sly, W.S.; Ahmad, F.; Hassan, I. Human beta-glucuronidase: Structure, function, and application in enzyme replacement therapy. Rejuvenation Res. 2013, 16, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Mindell, J.A. Lysosomal acidification mechanisms. Annu. Rev. Physiol. 2012, 74, 69–86. [Google Scholar] [CrossRef] [Green Version]
- Deshmukh, M.; Chao, P.; Kutscher, H.L.; Gao, D.; Sinko, P.J. A series of alpha-amino acid ester prodrugs of camptothecin: In vitro hydrolysis and A549 human lung carcinoma cell cytotoxicity. J. Med. Chem. 2010, 53, 1038–1047. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| IC50 with PBS (nM) | IC50 with Bovine β-Gus (nM) | IC50 with Supernatant of Cells Exposed to HIFU (nM) | |
|---|---|---|---|
| DOX | 2000 ± 300 | 1700 ± 200 | 1600 ± 300 |
| DOX-propGA3 | >100,000 | 5500 ± 1100 * | 5600 ± 1400 * |
| DOX-propGA3-polymer | >100,000 | 24,100 ± 4700 * | 2100 ± 1800 * |
| DOX-propGA3 nanogels | >100,000 | 10,300 ± 1800 * | 9900 ± 1100 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Besse, H.C.; Chen, Y.; Scheeren, H.W.; Metselaar, J.M.; Lammers, T.; Moonen, C.T.W.; Hennink, W.E.; Deckers, R. A Doxorubicin-Glucuronide Prodrug Released from Nanogels Activated by High-Intensity Focused Ultrasound Liberated β-Glucuronidase. Pharmaceutics 2020, 12, 536. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12060536
Besse HC, Chen Y, Scheeren HW, Metselaar JM, Lammers T, Moonen CTW, Hennink WE, Deckers R. A Doxorubicin-Glucuronide Prodrug Released from Nanogels Activated by High-Intensity Focused Ultrasound Liberated β-Glucuronidase. Pharmaceutics. 2020; 12(6):536. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12060536
Chicago/Turabian StyleBesse, Helena C., Yinan Chen, Hans W. Scheeren, Josbert M. Metselaar, Twan Lammers, Chrit T. W. Moonen, Wim E. Hennink, and Roel Deckers. 2020. "A Doxorubicin-Glucuronide Prodrug Released from Nanogels Activated by High-Intensity Focused Ultrasound Liberated β-Glucuronidase" Pharmaceutics 12, no. 6: 536. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12060536