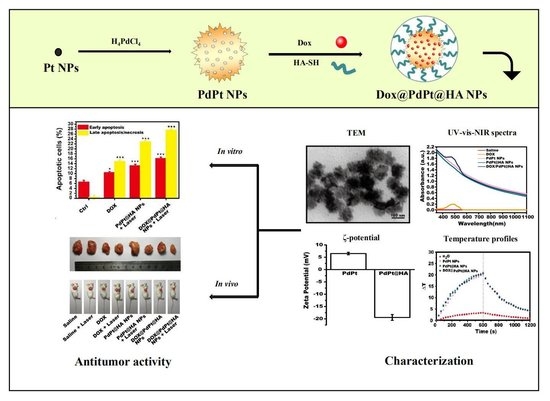
NIR Stimulus-Responsive PdPt Bimetallic Nanoparticles for Drug Delivery and Chemo-Photothermal Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PdPt NPs
2.3. Preparation of Thiol Functionalized HA (HA-SH)
2.4. Preparation of DOX@PdPt@HA
2.5. Characterization
2.6. Measurement of Photothermal Effect
2.7. Release of DOX from DOX@PdPt@HA NPs
2.8. Cell culture
2.9. MTT Assay
2.10. Cell Death Examined by AV-PI Double Staining
2.11. CD44-Mediated Delivery of DOX@PdPt@HA NPs
2.12. In Vivo Anti-Tumor Effect
2.13. H&E Staining and Immunohistochemistry Staining
2.14. Statistics
3. Results and Discussion
3.1. Preparation and Characterization of DOX@PdPt@HA NPs
3.2. DOX Release Behaviors
3.3. In Vitro Toxicity and Antitumor Efficacy
3.4. In Vivo Anti-Tumor Therapy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fan, W.; Yung, B.; Huang, P.; Chen, X. Nanotechnology for multimodal synergistic cancer therapy. Chem. Rev. 2017, 117, 13566–13638. [Google Scholar] [CrossRef] [PubMed]
- Kydd, J.; Jadia, R.; Velpurisiva, P.; Gad, A.; Paliwal, S.; Rai, P. Targeting strategies for the combination treatment of cancer using drug delivery systems. Pharmaceutics 2017, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, H.; Chen, Y.; Wang, Y.; Li, H.; Han, H.; Chen, T.; Jin, Q.; Ji, J. pH- and NIR light-responsive polymeric prodrug micelles for hyperthermia-assisted site-specific chemotherapy to reverse drug resistance in cancer treatment. Small 2016, 12, 2731–2740. [Google Scholar] [CrossRef]
- Lu, N.; Huang, P.; Fan, W.; Wang, Z.; Liu, Y.; Wang, S.; Zhang, G.; Hu, J.; Liu, W.; Niu, G.; et al. Tri-stimuli-responsive biodegradable theranostics for mild hyperthermia enhanced chemotherapy. Biomaterials 2017, 126, 39–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Ju, Y.; Zhao, L.; Chu, X.; Yang, W.; Tian, Y.; Sheng, F.; Lin, J.; Liu, F.; Dong, Y.; et al. Multistimuli-regulated photochemothermal cancer therapy remotely controlled via Fe5C2 nanoparticles. ACS Nano 2016, 10, 159–169. [Google Scholar] [CrossRef]
- Chen, Q.; Xu, L.; Liang, C.; Wang, C.; Peng, R.; Liu, Z. Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat. Commun. 2016, 7, 13193. [Google Scholar] [CrossRef]
- Chen, R.; Zhu, C.; Fan, Y.; Feng, W.; Wang, J.; Shang, E.; Zhou, Q.; Chen, Z. Polydopamine-based multifunctional platform for combined photothermal therapy, chemotherapy, and immunotherapy in malignant tumor treatment. ACS Appl. Bio Mater. 2019, 2, 874–883. [Google Scholar] [CrossRef]
- Hauck, T.S.; Jennings, T.L.; Yatsenko, T.; Kumaradas, J.C.; Chan, W.C.W. Enhancing the toxicity of cancer chemotherapeutics with gold nanorod hyperthermia. Adv. Mater. 2008, 20, 3832–3838. [Google Scholar] [CrossRef]
- Zhang, L.; Su, H.; Cai, J.; Cheng, D.; Ma, Y.; Zhang, J.; Zhou, C.; Liu, S.; Shi, H.; Zhang, Y.; et al. A multifunctional platform for tumor angiogenesis-targeted chemo-thermal therapy using polydopamine-coated gold nanorods. ACS Nano 2016, 10, 10404–10417. [Google Scholar] [CrossRef]
- Kang, S.; Shin, W.; Kang, K.; Choi, M.-H.; Kim, Y.-J.; Kim, Y.-K.; Min, D.-H.; Jang, H. Revisiting of Pd nanoparticles in cancer treatment: All-round excellence of porous Pd nanoplates in gene-thermo combinational therapy. ACS Appl. Mater. Interfaces 2018, 10, 13819–13828. [Google Scholar] [CrossRef]
- Fang, W.; Tang, S.; Liu, P.; Fang, X.; Gong, J.; Zheng, N. Pd nanosheet-covered hollow mesoporous silica nanoparticles as a platform for the chemo-photothermal treatment of cancer cells. Small 2012, 8, 3816–3822. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, C.; Liu, H.; Fu, C.; Tan, L.; Wang, S.; Fu, S.; Liu, X.; Meng, X.; Liu, H. Multifunctional carbon-silica nanocapsules with gold core for synergistic photothermal and chemo-cancer therapy under the guidance of bimodal imaging. Adv. Funct. Mater. 2016, 26, 4252–4261. [Google Scholar] [CrossRef]
- Zheng, T.; Li, G.G.; Zhou, F.; Wu, R.; Zhu, J.-J.; Wang, H. Gold-nanosponge-based multistimuli-responsive drug vehicles for targeted chemo-photothermal therapy. Adv. Mater. 2016, 28, 8218–8226. [Google Scholar] [CrossRef] [PubMed]
- Pacardo, D.B.; Neupane, B.; Rikard, S.M.; Lu, Y.; Mo, R.; Mishra, S.R.; Tracy, J.B.; Wang, G.; Ligler, F.S.; Gu, Z. A dual wavelength-activatable gold nanorod complex for synergistic cancer treatment. Nanoscale 2015, 7, 12096–12103. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Hu, K.; Ma, R.; Yan, Y.; Ni, B.; Zhang, Y.; Wen, L.; Zhang, Q.; Cheng, Y. Cancer therapy: Dendritic platinum-copper alloy nanoparticles as theranostic agents for multimodal imaging and combined chemophotothermal therapy. Adv. Funct. Mater. 2016, 26, 5950. [Google Scholar] [CrossRef] [Green Version]
- Long, N.V.; Asaka, T.; Matsubara, T.; Nogami, M. Shape-controlled synthesis of Pt-Pd core-shell nanoparticles exhibiting polyhedral morphologies by modified polyol method. Acta Mater. 2011, 59, 2901–2907. [Google Scholar] [CrossRef]
- Lee, H.; Habas, S.E.; Somorjai, G.A.; Yang, P. Localized Pd overgrowth on cubic Pt nanocrystals for enhanced electrocatalytic oxidation of formic acid. J. Am. Chem. Soc. 2008, 130, 5406–5407. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Vara, M.; Luo, M.; Huang, H.; Ruditskiy, A.; Park, J.; Bao, S.; Liu, J.; Howe, J.; Chi, M. Pd@Pt core-shell concave decahedra: A class of catalysts for the oxygen reduction reaction with enhanced activity and durability. J. Am. Chem. Soc. 2015, 137, 15036–15042. [Google Scholar] [CrossRef]
- Wei, J.; Li, J.; Sun, D.; Li, Q.; Ma, J.; Chen, X.; Zhu, X.; Zheng, N. A novel theranostic nanoplatform based on Pd@Pt-PEG-Ce6 for enhanced photodynamic therapy by modulating tumor hypoxia microenvironment. Adv. Funct. Mater. 2018, 28, 1706310. [Google Scholar] [CrossRef]
- Wang, L.; Yamauchi, Y. p53-mediated autophagy adjustment is involved in the protection of silibinin against murine dermal inflammation and epidermal apoptosis induced by UVB irradiation. J. Am. Chem. Soc. 2013, 135, 16762–16765. [Google Scholar] [CrossRef]
- Fang, C.; Zhao, J.; Jiang, R.; Wang, J.; Zhao, G.; Geng, B. Engineering of hollow PdPt nanocrystals via reduction kinetic control for their superior electrocatalytic performances. ACS Appl. Mater. Interfaces 2018, 10, 29543–29551. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Fu, J.; Li, R.; Zhang, F.; Ling, G.; Zhang, P. A potential carrier for anti-tumor targeted delivery-hyaluronic acid nanoparticles. Carbohyd. Polym. 2019, 208, 356–364. [Google Scholar] [CrossRef]
- Cai, Z.; Zhang, H.; Wei, Y.; Cong, F. Hyaluronan-inorganic nanohybrid materials for biomedical applications. Biomacromolecules 2017, 18, 1677–1696. [Google Scholar] [CrossRef]
- Huang, G.; Huang, H. Hyaluronic acid-based biopharmaceutical delivery and tumor-targeted drug delivery system. J. Control. Release 2018, 278, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Dai, Y.; Gao, H. Development and application of hyaluronic acid in tumor targeting drug delivery. Acta Pharm. Sin. B. 2019, 9, 1099–1112. [Google Scholar] [CrossRef]
- Dosio, F.; Arpicco, S.; Stella, B.; Fattal, E. Hyaluronic acid for anticancer drug and nucleic acid delivery. Adv. Drug Deliv. Rev. 2016, 97, 204–236. [Google Scholar] [CrossRef]
- Yin, T.; Wang, Y.; Chu, X.; Fu, Y.; Wang, L.; Zhou, J.; Tang, X.; Liu, J.; Huo, M. Free adriamycin-loaded pH/reduction dual-responsive hyaluronic acid-adriamycin prodrug micelles for efficient cancer therapy. ACS Appl. Mater. Interfaces. 2018, 10, 35693–35704. [Google Scholar] [CrossRef]
- Li, H.; Li, H.; Yu, W.; Huang, S.; Liu, Y.; Zhang, N.; Yuan, J.; Xu, X.; Duan, S.; Hu, Y. PEGylated hyaluronidase/NIR induced drug controlled release system for synergetic chemo-photothermal therapy of hepatocellular carcinoma. Eur. J. Pharm. Sci. 2019, 133, 127–136. [Google Scholar] [CrossRef]
- Oommen, O.P.; Duehrkop, C.; Nilsson, B.; Hilborn, J.; Varghese, O.P. Multifunctional hyaluronic acid and chondroitin sulfate nanoparticles: Impact of glycosaminoglycan presentation on receptor mediated cellular uptake and immune activation. ACS Appl. Mater. Interfaces 2016, 8, 20614–20624. [Google Scholar] [CrossRef]
- El-Dakdouki, M.H.; Zhu, D.C.; El-Boubbou, K.; Kamat, M.; Chen, J.; Li, W.; Huang, X. Development of multifunctional hyaluronan-coated nanoparticles for imaging and drug delivery to cancer cells. Biomacromolecules 2012, 13, 1144–1151. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Mok, H.; Lee, S.; Oh, Y.-K.; Park, T.G. Target-specific intracellular delivery of siRNA using degradable hyaluronic acid nanogels. J. Control. Release 2007, 119, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Maney, V.; Singh, M. An in vitro assessment of novel chitosan/bimetallic PtAu nanocomposites as delivery vehicles for doxorubicin. Nanomedicine 2017, 12, 2625–2640. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.J.; Buriak, J.M. Best practices for the reporting of colloidal inorganic nanomaterials. Chem. Mater. 2015, 27, 4911–4913. [Google Scholar] [CrossRef] [Green Version]
- Leng, C.; Zhang, X.; Xu, F.; Yuan, Y.; Pei, H.; Sun, Z.; Li, L.; Bao, Z. Engineering gold nanorod-gopper sulfide heterostructures with enhanced photothermal conversion efficiency and photostability. Small 2018, 14, 1703077. [Google Scholar] [CrossRef]
- Park, H.K.; Lee, S.J.; Oh, J.S.; Lee, S.G.; Jeong, Y.I.; Lee, H.C. Smart nanoparticles based on hyaluronic acid for redox-responsive and CD44 receptor-mediated targeting of tumor. Nanoscale Res. Lett. 2015, 10, 288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bi, H.; Dai, Y.; Lv, R.; Zhong, C.; He, F.; Gai, S.; Gulzar, A.; Yang, G.; Yang, P. Doxorubicin-conjugated cus nanoparticles for efficient synergistic therapy triggered by near-infrared light. Dalton Trans. 2016, 45, 5101–5110. [Google Scholar] [CrossRef]
- Xu, W.; Wang, J.; Qian, J.; Hou, G.; Wang, Y.; Ji, L.; Suo, A. NIR/pH dual-responsive polysaccharide-encapsulated gold nanorods for enhanced chemo-photothermal therapy of breast cancer. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109854. [Google Scholar] [CrossRef]
- Thorn, C.F.; Oshiro, C.; Marsh, S.; Hernandez-Boussard, T.; McLeod, H.; Klein, T.R.; Altman, R.B. Doxorubicin pathways: Pharmacodynamics and adverse effects. Pharmacogenetics Genom. 2011, 21, 440–446. [Google Scholar] [CrossRef]
- Melamed, J.R.; Edelstein, R.S.; Day, E.S. Elucidating the fundamental mechanisms of cell death triggered by photothermal therapy. ACS Nano 2015, 9, 6–11. [Google Scholar] [CrossRef]
- Liu, C.; Chen, Z.; Wang, Z.; Li, W.; Ju, E.; Yan, Z.; Liu, Z.; Ren, J.; Qu, X. A graphitic hollow carbon nitride nanosphere as a novel photochemical internalization agent for targeted and stimuli-responsive cancer therapy. Nanoscale 2016, 8, 12570–12578. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Ru, Z.; Song, W.; Sun, L. A ROS-responsive polymeric prodrug nanosystem with self-amplified drug release for PSMA (−) prostate cancer specific therapy. J. Nanobiotechnol. 2019, 17, 91. [Google Scholar] [CrossRef] [Green Version]
- Feng, W.; Li, H.; Xu, K.; Chen, Y.; Pan, L.; Mei, Y.; Cai, H.; Jiang, Y.; Chen, T.; Feng, D. SHCBP1 is over-expressed in breast cancer and is important in the proliferation and apoptosis of the humanmalignant breast cancer cell line. Gene 2016, 587, 91–97. [Google Scholar] [CrossRef]
- Rajasekharreddy, P.; Rani, P.U. Biosynthesis and characterization of Pd and Pt nanoparticles using Piper betle L. plant in a photoreduction method. J. Clust. Sci. 2014, 25, 1377–1388. [Google Scholar] [CrossRef]
- Piao, Y.; Liu, Y.; Xie, X. Change trends of organ weight background data in Sprague Dawley rats at different ages. J. Toxicol. Pathol. 2013, 26, 29–34. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Rong, Q.; Xu, M.; Zhang, Y.; Dong, Q.; Xiao, Y.; Liu, Q.; Chen, H.; Yang, X.; Yu, K.; et al. Safety pharmacology and subchronic toxicity of jinqing granules in rats. BMC Vet. Res. 2017, 13, 179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elmowafy, E.; El-Derany, M.O.; Biondo, F.; Tiboni, M.; Casettari, L.; Soliman, M.E. Quercetin loaded monolaurate sugar esters-based niosomes: Sustained release and mutual antioxidant-hepatoprotective interplay. Pharmaceutics 2020, 12, 143. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.L.; Wu, T.T.; Qin, Y.T.; Qi, Y.; Sun, Y.; Kong, M.; Jiang, X.; Qin, X.Y.; Shen, Y.Q.; Zhang, Z.P. A facile doxorubicin-dichloroacetate conjugate nanomedicine with high drug loading for safe drug delivery. Int. J. Nanomed. 2018, 13, 1281–1293. [Google Scholar] [CrossRef] [Green Version]







| Groups | Heart | Liver | Spleen | Lung | Kidney | Brain |
|---|---|---|---|---|---|---|
| Ctrl | 0.57 ± 0.02 | 6.15 ± 0.37 | 2.63 ± 0.08 | 0.99 ± 0.02 | 0.74 ± 0.01 | 2.09 ± 0.03 |
| Ctrl + Laser | 0.62 ± 0.04 | 5.98 ± 0.36 | 2.68 ± 0.05 | 0.99 ± 0.04 | 0.73 ± 0.03 | 2.15 ± 0.04 |
| DOX | 0.53 ± 0.03 | 5.43 ± 0.3 | 2.51 ± 0.06 | 0.98 ± 0.04 | 0.74 ± 0.06 | 2.13 ± 0.04 |
| DOX + Laser | 0.56 ± 0.04 | 5.44 ± 0.11 | 2.70 ± 0.12 | 1.00 ± 0.04 | 0.75 ± 0.03 | 2.20 ± 0.03 |
| PdPt@HA NPs | 0.57 ± 0.04 | 5.51± 0.25 | 2.63 ± 0.10 | 0.97 ± 0.05 | 0.76 ± 0.03 | 2.16 ± 0.06 |
| PdPt@HA NPs + Laser | 0.65 ± 0.07 | 5.42 ± 0.19 | 2.66 ± 0.06 | 1.00 ± 0.04 | 0.69 ± 0.03 | 2.18 ± 0.04 |
| DOX@PdPt@HA NPs | 0.60 ± 0.03 | 5.34 ± 0.19 | 2.62 ± 0.04 | 0.98 ± 0.06 | 0.67 ± 0.04 | 2.15 ± 0.04 |
| DOX@PdPt@HA NPs + Laser | 0.58 ± 0.04 | 5.40 ± 0.02 | 2.63 ± 0.03 | 0.96 ± 0.04 | 0.67 ± 0.02 | 2.14 ± 0.02 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, C.; Bao, Z.; Sun, M.; Wang, X.; Zhang, H.; Chen, W.; Sui, Y.; Li, J.; Zhuang, Y.; Wang, D. NIR Stimulus-Responsive PdPt Bimetallic Nanoparticles for Drug Delivery and Chemo-Photothermal Therapy. Pharmaceutics 2020, 12, 675. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12070675
Chu C, Bao Z, Sun M, Wang X, Zhang H, Chen W, Sui Y, Li J, Zhuang Y, Wang D. NIR Stimulus-Responsive PdPt Bimetallic Nanoparticles for Drug Delivery and Chemo-Photothermal Therapy. Pharmaceutics. 2020; 12(7):675. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12070675
Chicago/Turabian StyleChu, Chun, Zhihong Bao, Meng Sun, Xiaowei Wang, Hongyan Zhang, Weiguo Chen, Yang Sui, Ji Li, Yuanyuan Zhuang, and Dongkai Wang. 2020. "NIR Stimulus-Responsive PdPt Bimetallic Nanoparticles for Drug Delivery and Chemo-Photothermal Therapy" Pharmaceutics 12, no. 7: 675. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12070675