Enhanced Loading Efficiency and Mucoadhesion Properties of Gellan Gum Thin Films by Complexation with Hydroxypropyl-β-Cyclodextrin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Film Production
2.3. Phase Solubility Studies of Fluconazole with Hydroxypropyl--Cyclodextrin (HP--CD)
2.4. Preparation of Drug-Cyclodextrin Inclusion Complex
2.5. Mechanical and Mucoadhesion Properties of Films
2.6. Fluconazole Content Uniformity
2.7. Swelling Tests
2.8. In-Vitro Release Studies with Paddle Type Dissolution Apparatus (Usp II)
2.9. In-Vitro Release Studies with the Millifluidic Flow-Through Device (MFTD)
3. Transport Models
3.1. Swelling Modeling of Thin Films
3.2. Drug Release Modeling in the Usp II Apparatus
4. Results and Discussion
4.1. Rheological, Mechanical and Mucus-Adhesion Properties
4.2. Fluconazole Content Uniformity
4.3. Analysis of Phase Solubility of Fluconazole with HP--CD
4.4. Analysis of Swelling Tests
4.5. Analysis of Release Kinetics in Usp II (Paddle) Apparatus
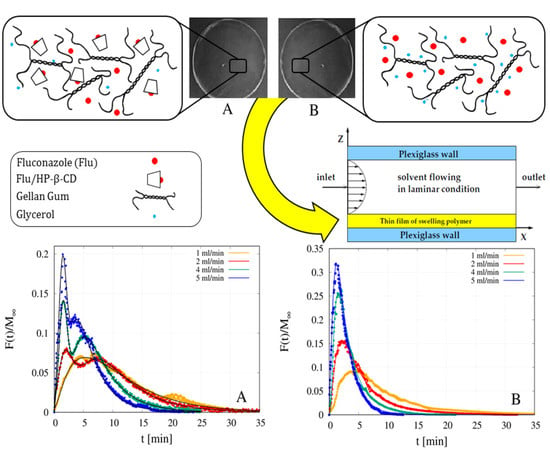
4.6. Analysis of Release Data from MFTD
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rouge, N.; Buri, P.; Doelker, E. Drug absorption sites in the gastro-intestinal tract and dosage forms for site-specific delivery. Int. J. Pharm. 1996, 136, 117–139. [Google Scholar] [CrossRef]
- Pinto, J.F. Site-specific drug delivery systems within the gastro-intestinal tract: From the mouth to the colon. Int. J. Pharm. 2010, 395, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.F.; Liu, F.; Brow, M.B. Advances in oral transmucosal drug delivery. J. Control. Release 2011, 153, 106–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madhav, N.V.S.; Shakya, A.K.; Singh, K. Oro-transmucosal drug delivery system: A review. J. Control. Release 2009, 140, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Dixit, R.P.; Puthli, S.P. Oral strip technology: Overview and future potential. J. Control. Release 2009, 139, 94–107. [Google Scholar] [CrossRef]
- Hearnden, V.; Sankar, V.; Hull, K.; Jaras, D.V.; Greenberg, M.; Kerr, A.R.; Lockhart, P.B.; Patton, L.L.; Porter, S.; Thornhill, M.H. New developments and opportunities in oral mucosal drug delivery for local and systemic disease. Adv. Drug Deliv. Rev. 2012, 64, 16–28. [Google Scholar] [CrossRef]
- Kathpalia, H.; Gupte, A. An introduction to fast dissolving oral thin film drug delivery systems: A review. Curr. Drug Deliv. 2013, 10, 667–684. [Google Scholar] [CrossRef]
- Gilhotra, R.M.; Ikram, M.; Srivastava, S.; Gilhotra, N. A clinical perspective on mucoadhesive buccal drug delivery systems. J. Biomed. Res. 2014, 28, 81–97. [Google Scholar] [CrossRef]
- Walsh, J.; Cram, A.; Woertz, K.; Breitkreutz, J.; Winzenburg, G.; Turner, R.; Tuleu, C. Playing hide and seek with poorly tasting pediatric medicines: Do not forget the excipients. Adv. Drug Deliv. Rev. 2014, 73, 14–33. [Google Scholar] [CrossRef] [Green Version]
- Karkia, S.; Kim, H.; Na, S.J.; Shina, D.; Jo, K.; Lee, J. Thin films as an emerging platform for drug delivery. Asian J. Pharm. Sci. 2016, 11, 559–574. [Google Scholar] [CrossRef] [Green Version]
- Borges, A.F.; Silva, C.; Coelho, J.F.J.; Simoes, S. Oral films: Current status and future perspectives: I-Galenical development and quality attributes. J. Control. Release 2015, 206, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.; Kim, K.; Kim, M.; Choi, D.H.; Jeong, S.H. Orally disintegrating films focusing on formulation, manufacturing process, and characterization. J. Pharm. Investig. 2017, 47, 183–201. [Google Scholar] [CrossRef]
- Paolicelli, P.; Petralito, S.; Varani, G.; Nardoni, M.; Pacelli, S.; Di Muzio, L.; Tirilló, J.; Bartuli, C.; Cesa, S.; Casadei, M.A.; et al. Effect of glycerol on the physical and mechanical properties of thin gellan gum films for oral drug delivery. Int. J. Pharm. 2018, 547, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Price, D.J.; Ditzinger, F.; Koehl, N.J.; Jankovic, S.; Tsakiridou, G.; Nair, A.; Holm, R.; Kuentz, M.; Dressman, J.B.; Saal, C. Approaches to Increase Mechanistic Understanding and Aid in the Selection of Precipitation Inhibitors for Supersaturating Formulations—A PEARRL Review. J. Pharm. Pharmacol. 2019, 71, 483–509. [Google Scholar] [CrossRef] [Green Version]
- Kurkov, S.V.; Loftsson, T. Cyclodextrins. Int. J. Pharm. 2013, 453, 167–180. [Google Scholar] [CrossRef]
- Adrover, A.; Varani, G.; Paolicelli, P.; Petralito, S.; Di Muzio, L.; Casadei, M.A.; Tho, I. Experimental and Modeling Study of Drug Release from HPMC-Based Erodible Oral Thin Films. Pharmaceutics 2018, 10, 222. [Google Scholar] [CrossRef] [Green Version]
- Quaglia, F.; Varricchio, G.; Miro, A.; La Rotonda, M.I.; Larobina, D.; Mensitieri, G. Modulation of drug release from hydrogels by using cyclodextrins: The case of nicardipine-β-cyclodextrin system in crosslinked polyethylenglycol. J. Control. Release 2001, 71, 329–337. [Google Scholar] [CrossRef]
- Machín, R.; Ramón Isasi, J.; Vélaz, I. β-Cyclodextrin hydrogels as potential drug delivery systems. Carbohydr. Polym. 2012, 87, 2024–2030. [Google Scholar] [CrossRef]
- Canbolat, M.F.; Celebioglu, A.; Uyar, T. Drug delivery system based on cyclodextrin-naproxen inclusion complex incorporated in electrospun polycaprolactone nanofibers. Colloids Surfaces B Biointerfaces 2014, 115, 15–21. [Google Scholar] [CrossRef]
- Milcovich, G.; Antunes, F.E.; Grassi, M.; Asaro, F. Stabilization of unilamellar catanionic vesicles induced by β-cyclodextrins: A strategy for a tunable drug delivery depot. Int. J. Pharm. 2018, 548, 474–479. [Google Scholar] [CrossRef]
- Peh, K.K.; Wong, C.F. Polymeric films as vehicle for buccal delivery: Swelling, mechanical, and bioadhesive properties. J. Pharm. Pharm. Sci. 1999, 2, 53–61. [Google Scholar]
- Adrover, A.; Pedacchia, A.; Petralito, S.; Spera, R. In vitro dissolution testing of oral thin films: A comparison between USP 1, USP 2 apparatuses and a new millifluidic flow-through device. Chem. Eng. Res. Des. 2015, 95, 173–178. [Google Scholar] [CrossRef]
- Higuchi, T.; Connors, K.A. Phase solubility techniques. Adv. Anal. Chem. Instr. 1965, 4, 117–122. [Google Scholar]
- Petralito, S.; Zanardi, I.; Memoli, A.; Annesini, M.C.; Travagli, V. Solubility, spectroscopic properties and photostability of Rhein/cyclodextrin inclusion complex. Spectrochim. Acta. Part Mol. Biomol. Spectrosc. 2009, 74, 1254–1259. [Google Scholar] [CrossRef] [PubMed]
- Sunil, S.; Jambhekar; Breen, P. Cyclodextrins in pharmaceutical formulations I, structure and physiochemical properties, formation of complexes, and types of complex. Drug Deliv. Today 2016, 21, 356–362. [Google Scholar]
- Sunil, S.; Jambhekar; Breen, P. Cyclodextrins in pharmaceutical formulations II, solubilization, binding costant, and complexation efficiency. Drug Deliv. Today 2016, 21, 363–368. [Google Scholar]
- Petralito, S.; Zanardi, I.; Spera, R.; Memoli, A.; Travagli, V. Spectroscopic characterization of both aqueous and solid-state Diacerhein/hydroxypropyl-β-cyclodextrin inclusion complexes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 127, 355–360. [Google Scholar] [CrossRef]
- Pedacchia, A.; Adrover, A. Study of release kinetics and diffusion coefficients in swellable cellulosic thin films by means of a simple spectrophotometric technique. Chem. Eng. Res. Des. 2014, 92, 2550–2556. [Google Scholar] [CrossRef]
- Adrover, A.; Nobili, M. Release kinetics from oral thin films: Theory and experiments. Chem. Eng. Res. Des. 2015, 98, 188–211. [Google Scholar] [CrossRef]
- Siepmann, J.; Peppas, N.A. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv. Drug Deliv. Rev. 2001, 48, 139–157. [Google Scholar] [CrossRef]
- Papanu, J.S.; Soane, D.S.; Bell, A.T.; Hess, D.M. Transport Models for swelling and dissolution of thin polymer films. J. Appl. Polym. Sci. 1989, 38, 859–885. [Google Scholar] [CrossRef]
- Tu, Y.-O.; Ouano, A.C. Model for the Kinematics of Polymer Dissolution. IBM J. Res. Dev. 1977, 21, 131–142. [Google Scholar] [CrossRef]
- Ranade, V.V.; Mashelkar, R.A. Convective Diffusion from a dissolving Polymeric Particle. AIChE J. 1995, 41, 666–676. [Google Scholar] [CrossRef]
- Narasimhan, B.; Peppas, N.A. Molecular Analysis of Drug Delivery Systems Controlled by Dissolution of the Polymer Carrier. J. Pharm. Sci. 1997, 86, 297–304. [Google Scholar] [CrossRef]
- D’Errico, G.; Ortona, O.; Capuano, F.; Vitagliano, V. Diffusion Coefficients for the Binary System Glycerol + Water at 25 °C. A Velocity Correlation Study. J. Chem. Eng. Data 2004, 49, 1665–1670. [Google Scholar] [CrossRef]
- Preis, M.; Knop, K.; Breitkreutz, J. Mechanical strength test for orodispersible and buccal films. Int. J. Pharm. 2014, 461, 22–29. [Google Scholar] [CrossRef]
- John, D.S. The basics and underlying mechanisms of mucoadhesion. Adv. Drug Deliv. Rev. 2005, 57, 1556–1568. [Google Scholar] [CrossRef]
- Li, J.; Zhang, S.; Zhou, Y.; Guan, S.; Zhang, L. Inclusion complexes of fluconazole with β-cyclodextrin and 2-hydroxypropyl-β-cyclodextrin in aqueous solution: Preparation, characterization and a structural insight. J. Incl. Phenom. Macrocycl. Chem. 2016, 84, 209–217. [Google Scholar] [CrossRef]
- Brewster, M.E.; Loftsson, T. Cyclodextrins as pharmaceutical solubilizers. Adv. Drug Deliv. Rev. 2007, 59, 645–666. [Google Scholar] [CrossRef]
- Paolicelli, P.; Varani, G.; Pacelli, S.; Ogliani, E.; Nardoni, M.; Petralito, S.; Adrover, A.; Casadei, M.A. Design and characterization of a biocompatible physical hydrogel based on scleroglucan for topical drug delivery. Carbohydr. Polym. 2017, 174, 960–969. [Google Scholar] [CrossRef]










| Film | GG-1%Gly | GG-2%Gly | GG-2% Gly-HP--CD | GG-6%Gly |
|---|---|---|---|---|
| Strength (N) | 0.5782 ± 0.0014 | 0.1274 ± 0.0016 | 0.6762 ± 0.0012 | 0.0052 ± 0.0014 |
| 0.5% Gly | 2%Gly | 2%Gly-HP--CD | 3%GLy | 3%Gly-HP--CD | 6%Gly | |
|---|---|---|---|---|---|---|
| 1.35 ± 0.05 | 3.3 ± 0.5 | 3.4 ± 0.2 | 5.2 ± 0.5 | 5.3 ± 0.4 | 11.5 ± 0.8 | |
| - | - | 0.45 ± 0.06 | - | 0.69 ± 0.05 | - | |
| - | 0.92 ± 0.08 | 3.02 ± 0.25 |
| Film | USP II | 5 mL/min | 4 mL/min | 3 mL/min | 2 mL/min | 1 mL/min |
|---|---|---|---|---|---|---|
| complex in GG-2%Gly | 4.58 | 7.21 | 9.62 | 10.96 | 13.67 | 16.33 |
| Flu in GG-6%Gly | 1.22 | 3.89 | 5.26 | 6.62 | 8.27 | 13.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adrover, A.; di Muzio, L.; Trilli, J.; Brandelli, C.; Paolicelli, P.; Petralito, S.; Casadei, M.A. Enhanced Loading Efficiency and Mucoadhesion Properties of Gellan Gum Thin Films by Complexation with Hydroxypropyl-β-Cyclodextrin. Pharmaceutics 2020, 12, 819. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12090819
Adrover A, di Muzio L, Trilli J, Brandelli C, Paolicelli P, Petralito S, Casadei MA. Enhanced Loading Efficiency and Mucoadhesion Properties of Gellan Gum Thin Films by Complexation with Hydroxypropyl-β-Cyclodextrin. Pharmaceutics. 2020; 12(9):819. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12090819
Chicago/Turabian StyleAdrover, Alessandra, Laura di Muzio, Jordan Trilli, Chiara Brandelli, Patrizia Paolicelli, Stefania Petralito, and Maria Antonietta Casadei. 2020. "Enhanced Loading Efficiency and Mucoadhesion Properties of Gellan Gum Thin Films by Complexation with Hydroxypropyl-β-Cyclodextrin" Pharmaceutics 12, no. 9: 819. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12090819