Cellulose/Collagen Dressings for Diabetic Foot Ulcer: A Review
Abstract
:1. Introduction
1.1. Diabetic Foot Ulcer
1.2. Diabetic Foot Ulcer Scenario in Malaysia
1.3. Socioeconomic Burden
1.4. Current Treatment for Diabetic Foot Ulcer
1.5. Contraindication and Complications of Current Available Treatment
2. Collagen
3. Collagen-Based Treatment for DFU
4. Cellulose
5. Cellulose as a Protective Barrier
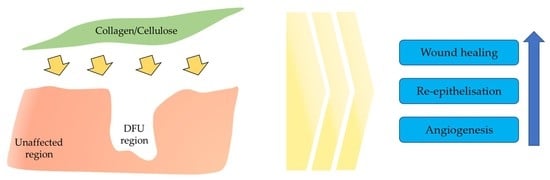
6. Cellulose/Collagen Dressing for DFU
7. Synergistic Effect of Cellulose/Collagen Dressing
8. Advantages of Cellulose/Collagen Dressings
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethical Approval and Consent to Participate
Abbreviations
| DFU | Diabetic foot ulcer |
| TCC | Total Contact Cast |
| NPWT | Negative Pressure wound therapy |
| AM | Amniotic membrane |
| DM | Diabetes mellitus |
| ORC | oxidized regenerated cellulose |
| GF | growth factor |
| FCG | formulated collagen gel |
| BC | bacterial cellulose |
| MC | Microbial cellulose |
| siRNA | small interfering RNA |
| CC | carboxymethyl cellulose |
References
- Mishra, S.C.; Chhatbar, K.C.; Kashikar, A.; Mehndiratta, A. Diabetic foot. BMJ 2017, 359, j5064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fauzi, A.A.; Chung, T.Y.; Latif, L.A. Risk factors of diabetic foot Charcot arthropathy: A case-control study at a Malaysian tertiary care centre. Singap. Med. J. 2016, 57, 198–203. [Google Scholar] [CrossRef] [Green Version]
- Tsourdi, E.; Barthel, A.; Rietzsch, H.; Reichel, A.; Bornstein, S.R. Current aspects in the pathophysiology and treatment of chronic wounds in diabetes mellitus. Biomed. Res. Int. 2013, 2013, 385641. [Google Scholar] [CrossRef] [Green Version]
- Jeyaraman, K.; Berhane, T.; Hamilton, M.; Chandra, A.P.; Falhammar, H. Mortality in patients with diabetic foot ulcer: A retrospective study of 513 cases from a single Centre in the Northern Territory of Australia. BMC Endocr. Disord. 2019, 19, 1. [Google Scholar] [CrossRef]
- Chun, D.; Kim, S.; Kim, J.; Yang, H.-J.; Kim, J.H.; Cho, J.; Yi, Y.; Kim, W.J.; Won, S.H. Epidemiology and Burden of Diabetic Foot Ulcer and Peripheral Arterial Disease in Korea. J. Clin. Med. 2019, 8, 748. [Google Scholar] [CrossRef] [Green Version]
- Rakin, E. Business Insider Malaysia. Malaysia July 25, 2018, Malaysia Has the Highest Rate of Diabetes in Asia—Doctors Have Classified the Disease as Another’ Silent Killer. Available online: https://www.businessinsider.my/malaysia-highest-rate-diabetes-silent-killer-asia (accessed on 24 June 2020).
- Malaysia Has 3.6 Million Diabetics, Says Dzulkefly|The Star Online. 2019. Available online: https://www.thestar.com.my/news/nation/2019/03/27/malaysia-has-36-million-diabeticssays-dzulkefly (accessed on 24 May 2020).
- Murugappan, R. Kuala Lumpur 2019. Caring for Chronic Wounds Can be Complicated. The Star. Available online: https://www.thestar.com.my/lifestyle/health/2019/06/12/caring-for-chronic-wounds (accessed on 14 June 2020).
- Mafauzy, M.; Hussein, Z.; Chan, S.P. The Status of Diabetes Control in Malaysia: Results of Diabcare 2008. Med. J. Malays. 2011, 66, 175–181. [Google Scholar]
- Raghav, A.; Khan, Z.A.; Labala, R.K.; Ahmad, J.; Noor, S.; Mishra, B.K. Financial burden of diabetic foot ulcers to world: A progressive topic to discuss always. Ther. Adv. Endocrinol. Metab. 2018, 9, 29–31. [Google Scholar] [CrossRef]
- Lam, A.W.; Zaim, M.R.; Helmy, H.H.; Ramdhan, I.M. Economic Impact of Managing Acute Diabetic Foot Infection in a Tertiary Hospital in Malaysia. Malays. Orthop. J. 2014, 8, 46–49. [Google Scholar] [CrossRef]
- Raspovic, A.; Landorf, K.B. A survey of offloading practices for diabetes-related plantar neuropathic foot ulcers. J. Foot Ankle Res. 2014, 7, 35. [Google Scholar] [CrossRef]
- Whitelaw, S. The total contact cast : Controversy in offloading the diabetic foot. Br. J. Community Nurs. 2012, 17, 16–20. [Google Scholar] [CrossRef]
- Shaw, M. Foot Ulcers: A Different Technique to Total Contact Casting for Healing Chronic Foot Ulcers. J. Nurs. Healthc. 2017, 1, 1–4. [Google Scholar]
- Yadav, S.L. To evaluate the efficacy of Total Contact Cast (TCC) compared to Patellar Tendon Bearing (PTB) cast with walking iron in the treatment of neuropathic plantar foot ulcer. In Prosthetics and Orthotics International; SAGE Publications: Lyon, France, 2015; p. 483. [Google Scholar]
- Ranaweera, A. Negative Pressure Wound Therapy. DermNet N. Z. 2013. Available online: https://dermnetnz.org/topics/negative-pressure-wound-therapy/ (accessed on 15 May 2020).
- Ma, Z.; Li, Z.; Shou, K.; Jian, C.; Li, P.; Niu, Y.; Qi, B.; Yu, A. Negative pressure wound therapy: Regulating blood flow perfusion and microvessel maturation through microvascular pericytes. Int. J. Mol. Med. 2017, 40, 1415–1425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meloni, M.; Izzo, V.; Vainieri, E.; Giurato, L.; Ruotolo, V.; Uccioli, L. Management of negative pressure wound therapy in the treatment of diabetic foot ulcers. World J. Orthop. 2015, 6, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Voelker, R. Diabetic Foot Ulcers Heal With Shock Wave Therapy. JAMA 2018, 319, 649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inserro, A. FDA Approves First Shock Wave Device to Treat Diabetic Foot Ulcers. AJMC. 2017. Available online: https://www.ajmc.com/view/fda-approves-first-shock-wave-device-to-treat-diabetic-foot-ulcers (accessed on 23 July 2020).
- Snyder, R.; Galiano, R.; Mayer, P.; Rogers, L.C.; Alvarez, O. Diabetic foot ulcer treatment with focused shockwave therapy: Two multicentre, prospective, controlled, double-blinded, randomised phase III clinical trials. J. Wound Care 2018, 27, 822–836. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Liang, X.M.; Song, G.M.; Zhao, Y.; Yang, X.L. Maggot debridement therapy for the treatment of diabetic foot ulcers: A meta-analysis. J. Wound Care 2013, 22, 462–469. [Google Scholar] [CrossRef]
- Azad, A.K.; Yee, B.-L.; Azizi, W.M.; Adham, S. Maggot debridement therapy for diabetic foot ulcer: Experience from Maggot treatment Centers. Asian J. Pharm. Pharmacol. 2016, 2, 23–25. [Google Scholar]
- Netten, J.J.V.; Clark, D.; Lazzarini, P.A.; Janda, M.; Reed, L.F. The validity and reliability of remote diabetic foot ulcer assessment using mobile phone images. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Naik, G.; Harding, K. Maggot debridement therapy: The current perspectives. Chronic Wound Care Manag. Res. 2017, 4, 121–128. [Google Scholar] [CrossRef] [Green Version]
- Ali, R.; Qureshi, A.; Yaqoob, M.Y.; Shakil, M. Total contact cast for neuropathic diabetic foot ulcers. J. Coll. Physicians Surg. Pak. 2008, 18, 695–698. [Google Scholar]
- Applewhite, A.J. Following the Evidence for Total Contact Casting as First-Line Treatment of DFUs in the Wound Clinic. Today’s Wound Clin. 2016. Available online: https://www.todayswoundclinic.com/articles/following-evidence-total-contact-casting-first-line-treatment-dfus-wound-clinic (accessed on 16 June 2020).
- Messenger, G.; Masoetsa, R.; Hussain, I. A Narrative Review of the Benefits and Risks of Total Contact Casts in the Management of Diabetic Foot Ulcers. J. Am. Coll. Clin. Wound Spec. 2017, 9, 19–23. [Google Scholar] [CrossRef] [PubMed]
- García Oreja, S.; Navarro González-Moncayo, J.; Sanz Corbalán, I.; García Morales, E.; Álvaro Afonso, F.J.; Lázaro Martínez, J.L. Complications associated with the negative pressure therapy in the treatment of the diabetic foot ulcers: Retrospective case series. Rev. Española Podol. 2017, 28, e27–e31. [Google Scholar] [CrossRef]
- Liu, S.; He, C.Z.; Cai, Y.T.; Xing, Q.P.; Guo, Y.Z.; Chen, Z.L.; Su, J.L.; Yang, L.P. Evaluation of negative-pressure wound therapy for patients with diabetic foot ulcers: Systematic review and meta-analysis. Ther. Clin. Risk Manag. 2017, 13, 533–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jordan, A.; Khiyani, N.; Bowers, S.; Lukaszczyk, J.; Stawicki, S. Maggot debridement therapy: A practical review. Int. J. Acad. Med. 2018, 4, 34. [Google Scholar] [CrossRef]
- Total Contact Casting. Wikipedia. Creative Commons Attribution-ShareAlike License. 2018. Available online: https://en.wikipedia.org/wiki/Total_contact_casting (accessed on 27 June 2020).
- McIntosh, J. What Is Collagen, and Why Do People Use It? Med. News Today. 2017. Available online: https://www.medicalnewstoday.com/articles/262881 (accessed on 23 May 2020).
- Pawelec, K.M.; Best, S.M.; Cameron, R.E. Collagen: A network for regenerative medicine. J. Mater. Chem. B 2016, 4, 6484–6496. [Google Scholar] [CrossRef] [Green Version]
- Ricard-Blum, S. The Collagen Family. Cold Spring Harb. Perspect. Biol. 2011, 3, 1–19. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Mu, C.; Shi, J.; Zhang, Q.; Shi, B.; Lin, W. Modification of collagen with a natural cross-linker, procyanidin. Int. J. Biol. Macromol. 2011, 48, 354–359. [Google Scholar] [CrossRef]
- Lorenz, J.S.; Schnauß, J.; Glaser, M.; Sajfutdinow, M.; Schuldt, C.; Käs, J.A.; Smith, D.M. Synthetic Transient Crosslinks Program the Mechanics of Soft, Biopolymer-Based Materials. Adv. Mater. 2018, 30, e1706092. [Google Scholar] [CrossRef] [Green Version]
- Shah, R.; Stodulka, P.; Skopalova, K.; Saha, P. Dual crosslinked collagen/chitosan film for potential biomedical applications. Polymers 2019, 11, 2094. [Google Scholar] [CrossRef] [Green Version]
- Shoulders, M.D.; Raines, R.T. Collagen Structure and Stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, M.M.; Dornelles, R.C.P.; Mello, R.O.; Kubota, E.H.; Mazutti, M.A.; Kempka, A.P.; Demiate, I.M. Collagen extraction process. Int. Food Res. J. 2016, 23, 913–922. [Google Scholar]
- Yang, H.; Shu, Z. The extraction of collagen protein from pig skin. J. Chem. Pharm. Res. 2014, 6, 683–687. [Google Scholar]
- Moore, J. Examining The Potential Of Collagen Powders In The Diabetic Foot|Podiatry Today. Pod. Today 2015, 28, 18–22. [Google Scholar]
- Ulrich, D.; Smeets, R.; Unglaub, F.; Wöltje, M.; Pallua, N. Effect of oxidized regenerated cellulose/collagen matrix on proteases in wound exudate of patients with diabetic foot ulcers. J. Wound Ostomy Cont. Nurs. 2011, 38, 522–528. [Google Scholar] [CrossRef]
- Fleischli, J.G.; Laughlin, T.J.; Fleischli, J.W. Equine pericardium collagen wound dressing in the treatment of the neuropathic diabetic foot wound a pilot study. J. Am. Podiatr. Med. Assoc. 2009, 99, 301–305. [Google Scholar] [CrossRef]
- Blume, P.; Driver, V.R.; Tallis, A.J.; Kirsner, R.S.; Kroeker, R.; Payne, W.G.; Wali, S.; Marston, W.; Dove, C.; Engler, R.L.; et al. Formulated collagen gel accelerates healing rate immediately after application in patients with diabetic neuropathic foot ulcers. Wound Repair Regen. 2011, 19, 302–308. [Google Scholar] [CrossRef] [Green Version]
- Fauzi, M.B.; Lokanathan, Y.; Nadzir, M.M.; Aminuddin, S.; Ruszymah, B.H.I.; Chowdhury, S.R. Attachment, proliferation, and morphological properties of human dermal fibroblasts on ovine tendon collagen scaffolds: A comparative study. Malays. J. Med. Sci. 2017, 24, 33–43. [Google Scholar] [CrossRef]
- Munish, T.; Ramneesh, G.; Sanjeev, S.; Jasdeep, S.; Jaspal, S.; Nikhil, G. Collagen Based Dressing and Standard Dressing in Diabetic Foot Ulcer. J. Evol. Med. Dent. Sci. 2015, 4, 3614–3621. [Google Scholar] [CrossRef]
- Chalimidi, K.R.; Kumar, Y.; Kini, U.A. Efficacy of Collagen Particles in Chronic Non Healing Ulcers. J. Clin. Diagn. Res. 2015, 9, PC03. [Google Scholar] [CrossRef]
- Shanmugam, S.; Professor, A.; Assistant Professor, S. A comprehensive study on effect of collagen dressing in diabetic foot ulcer. Int. Arch. Integr. Med. 2017, 4, 163–167. [Google Scholar]
- Lee, D.-S.; Lee, Y.-N.; Han, S.-K.; Namgoong, S. Effect of Collagen Dressing on Diabetic Wound Healing—A Pilot Study. J. Korean Wound Manag. Soc 2015, 11, 1–10. [Google Scholar]
- Wen, Y.; Wang, H.; Chen, B.; Chen, Y.; Zhang, T.; Xu, T.; Sun, W. Association of Information Sources and Knowledge on HIV/AIDS in Rural China. Int. J. Collab. Res. Intern. Med. Public Health 2015, 7, 13–23. [Google Scholar]
- Pei, Y.; Yang, J.; Liu, P.; Xu, M.; Zhang, X.; Zhang, L. Fabrication, properties and bioapplications of cellulose/collagen hydrolysate composite films. Carbohydr. Polym. 2013, 92, 1752–1760. [Google Scholar] [CrossRef] [PubMed]
- Sahana, T.G.; Rekha, P.D. Biopolymers: Applications in wound healing and skin tissue engineering. Mol. Biol. Rep. 2018, 45, 2857–2867. [Google Scholar] [CrossRef]
- Lavanya, D.; Kulkarni, P.; Dixit, M.; Raavi, P.K. Sources of cellulose and their applications—A review. Int. J. Drug Formul. Res. 2011, 2, 19–38. [Google Scholar]
- Manizate, F.; Fuller, A.; Gendics, C.; Lantis, J.C. A prospective, single-center, nonblinded, comparative, postmarket clinical evaluation of a bovine-derived collagen with ionic silver dressing versus a carboxymethylcellulose and ionic silver dressing for the reduction of bioburden in variable-etiology, bi. Adv. Ski. Wound Care 2012, 25, 220–225. [Google Scholar] [CrossRef]
- Mohamad, N.; Loh, E.Y.X.; Fauzi, M.B.; Ng, M.H.; Mohd Amin, M.C.I. In vivo evaluation of bacterial cellulose/acrylic acid wound dressing hydrogel containing keratinocytes and fibroblasts for burn wounds. Drug Deliv. Transl. Res. 2018, 9, 444–452. [Google Scholar] [CrossRef]
- Portal, O.; Clark, W.A.; Levinson, D.J. Microbial Cellulose Wound Dressing in the Treatment of Nonhealing Lower Extremity Ulcers|Wounds Research. Wounds 2009, 21, 1–3. [Google Scholar]
- Galateanu, B.; Bunea, M.-C.; Stanescu, P.; Vasile, E.; Casarica, A.; Iovu, H.; Hermenean, A.; Zaharia, C.; Costache, M. In Vitro Studies of Bacterial Cellulose and Magnetic Nanoparticles Smart Nanocomposites for Efficient Chronic Wounds Healing. Stem Cells Int. 2015, 2015, 195096. [Google Scholar] [CrossRef] [Green Version]
- Portela, R.; Leal, C.R.; Almeida, P.L.; Sobral, R.G. Bacterial cellulose: A versatile biopolymer for wound dressing applications. Microb. Biotechnol. 2019, 12, 586–610. [Google Scholar] [CrossRef]
- Xi Loh, E.Y.; Fauzi, M.B.; Ng, M.H.; Ng, P.Y.; Ng, S.F.; Ariffin, H.; Mohd Amin, M.C.I. Cellular and Molecular Interaction of Human Dermal Fibroblasts with Bacterial Nanocellulose Composite Hydrogel for Tissue Regeneration. ACS Appl. Mater. Interfaces 2018, 10, 1–57. [Google Scholar] [CrossRef] [PubMed]
- Dumont, I.J.; Lepeut, M.; Segalen, C.; Guillemin, Y.; Gouze, J.N. Use of gbt013, a collagen-based dressing, for the healing of diabetic foot ulcers a case series. J. Am. Podiatr. Med. Assoc. 2018, 108, 419–429. [Google Scholar] [CrossRef]
- Kloeters, O.; Unglaub, F.; de Laat, E.; van Abeelen, M.; Ulrich, D. Prospective and randomised evaluation of the protease-modulating effect of oxidised regenerated cellulose/collagen matrix treatment in pressure sore ulcers. Int. Wound J. 2015, 13, 1231–1236. [Google Scholar] [CrossRef] [PubMed]
- Song, S.H.; Kim, J.E.; Koh, E.K.; Sung, J.E.; Lee, H.A.; Yun, W.B.; Hong, J.T.; Hwang, D.Y. Selenium-loaded cellulose film derived from Styela clava tunic accelerates the healing process of cutaneous wounds in streptozotocin-induced diabetic Sprague–Dawley rats. J. Dermatol. Treat. 2018, 29, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-X.; Dong, J.-Y.; Li, Y.-H.; Zhong, J.; Yu, H.; Yu, Q.-Q.; Lei, M. Fabrication of Ag–ZnO@ carboxymethyl cellulose/K-carrageenan/graphene oxide/konjac glucomannan hydrogel for effective wound dressing in nursing care for diabetic foot ulcers. Appl. Nanosci. 2020, 10, 729–738. [Google Scholar] [CrossRef]
- Solway, D.R.; Clark, W.A.; Levinson, D.J. A parallel open-label trial to evaluate microbial cellulose wound dressing in the treatment of diabetic foot ulcers. Int. Wound J. 2011, 8, 69–73. [Google Scholar] [CrossRef]
- Loh, E.Y.X.; Mohamad, N.; Fauzi, M.B.; Ng, M.H.; Ng, S.F.; Mohd Amin, M.C.I. Development of a bacterial cellulose-based hydrogel cell carrier containing keratinocytes and fibroblasts for full-thickness wound healing. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Singla, R.; Soni, S.; Patial, V.; Kulurkar, P.M.; Kumari, A.; Mahesh, S.; Padwad, Y.S.; Yadav, S.K. In vivo diabetic wound healing potential of nanobiocomposites containing bamboo cellulose nanocrystals impregnated with silver nanoparticles. Int. J. Biol. Macromol. 2017, 105, 45–55. [Google Scholar] [CrossRef]
- Gottrup, F.; Cullen, B.M.; Karlsmark, T.; Bischoff-Mikkelsen, M.; Nisbet, L.; Gibson, M.C. Randomized controlled trial on collagen/oxidized regenerated cellulose/silver treatment. Wound Repair Regen. 2013, 21, 216–225. [Google Scholar] [CrossRef]
- Griffin, L.; Carter, M.J.; D’Agostino, R.; McGowan, L.D. Comparative Effectiveness of Two Collagen-containing Dressings: Oxidized Regenerated Cellulose (ORC)/Collagen/Silver-ORC Dressing Versus Ovine Collagen Extracellular Matrix|Wounds Research. Wounds 2019, 31, E73–E76. [Google Scholar]
- Hickey, R.J.; Pelling, A.E. Cellulose biomaterials for tissue engineering. Front. Bioeng. Biotechnol. 2019, 7, 45. [Google Scholar] [CrossRef] [Green Version]
- Pinho, E.; Soares, G. Functionalization of cotton cellulose for improved wound healing. J. Mater. Chem. B 2018, 6, 1887–1898. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Yang, L.; Pan, C.; Saw, P.E.; Ren, M.; Lan, B.; Wu, J.; Wang, X.; Zeng, T.; Zhou, L.; et al. Naturally-occurring bacterial cellulose-hyperbranched cationic polysaccharide derivative/MMP-9 siRNA composite dressing for wound healing enhancement in diabetic rats. Acta Biomater. 2020, 18, 298–314. [Google Scholar] [CrossRef]
- Serafica, G.; Mormino, G.; Oster, G.A.; Lentz, K.E.; Koehler, K.P. Microbial Cellulose Wound Dressing for Treating Chronic Wounds. U.S. Patent 7,704,523, 27 April 2010. [Google Scholar]
- Rangaraj, A.; Harding, K.; Leaper, D. Role of collagen in wound management. Wounds UK 2011, 7, 54–63. [Google Scholar]
- Li, B.; Wang, J.H.C. Fibroblasts and myofibroblasts in wound healing: Force generation and measurement. J. Tissue Viability 2011, 20, 108–120. [Google Scholar] [CrossRef] [Green Version]
- Goulding, V. Wound healing. Diabet. Foot J. 2015, 18, 75–80. [Google Scholar]
- How Diabetes Impacts Wound Healing. Wound Source. 2017. Available online: https://www.woundsource.com/blog/how-diabetes-impacts-wound-healing#:~:text=High%20blood%20glucose%20causes%20stiffening,complications%20in%20diabetes%20wound%20healing (accessed on 12 July 2020).
- Nguyen, T.T.; Mobashery, S.; Chang, M. Roles of matrix metalloproteinases in cutaneous wound healing. In Wound Healing—New Insights into Ancient Challenges; Alexandrescu, D.V., Ed.; InTech: London, UK, 2016; pp. 37–70. [Google Scholar] [CrossRef] [Green Version]
- Auf Dem Keller, U.; Sabino, F. Matrix metalloproteinases in impaired wound healing. Met. Med. 2015, 2, 8. [Google Scholar] [CrossRef] [Green Version]
- Häkkinen, L.; Larjava, H.; Koivisto, L. Granulation tissue formation and remodeling. Endod. Top. 2012, 24, 94–129. [Google Scholar] [CrossRef]
- Tahergorabi, Z.; Khazaei, M. Imbalance of angiogenesis in diabetic complications: The mechanisms. Int. J. Prev. Med. 2012, 3, 827–838. [Google Scholar] [CrossRef]
- Park, S.U.; Lee, B.K.; Kim, M.S.; Park, K.K.; Sung, W.J.; Kim, H.Y.; Han, D.G.; Shim, J.S.; Lee, Y.J.; Kim, S.H.; et al. The possibility of microbial cellulose for dressing and scaffold materials. Int. Wound J. 2012, 11, 35–43. [Google Scholar] [CrossRef]
- Basu, P.; Narendrakumar, U.; Arunachalam, R.; Devi, S.; Manjubala, I. Characterization and Evaluation of Carboxymethyl Cellulose-Based Films for Healing of Full-Thickness Wounds in Normal and Diabetic Rats. ACS Omega 2018, 3, 12622–12632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nurwito, B.S.; Maulida, H.N. Electrospun fibers as a wound dressing material using combination of cellulose acetate/collagen seeding stem cell. Asian J. Microbiol. Biotechnol. Environ. Sci. Pap. 2018, 20, 43–47. [Google Scholar]
- Vatankhah, E.; Prabhakaran, M.P.; Jin, G.; Mobarakeh, L.G.; Ramakrishna, S. Development of nanofibrous cellulose acetate/gelatin skin substitutes for variety wound treatment applications. J. Biomater. Appl. 2014, 28, 909–921. [Google Scholar] [CrossRef] [PubMed]
- Kilic Bektas, C.; Kimiz, I.; Sendemir, A.; Hasirci, V.; Hasirci, N. A bilayer scaffold prepared from collagen and carboxymethyl cellulose for skin tissue engineering applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 1764–1784. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Raines, R.T. Collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef] [Green Version]
- Naomi, R.; Ratanavaraporn, J.; Fauzi, M.B. Comprehensive Review of Hybrid Collagen and Silk Fibroin for Cutaneous Wound Healing. Materials 2020, 13, 3097. [Google Scholar] [CrossRef]
- Wu, S.; Applewhite, A.J.; Niezgoda, J.; Snyder, R.; Shah, J.; Cullen, B.; Schultz, G.; Harrison, J.; Hill, R.; Howell, M.; et al. Oxidized regenerated cellulose/collagen dressings: Review of evidence and recommendations. Adv. Ski. Wound Care 2017, 30, S1–S18. [Google Scholar] [CrossRef] [Green Version]
- Singh, O.; Gupta, S.; Soni, M.; Moses, S.; Shukla, S.; Mathur, R. Collagen dressing versus conventional dressings in burn and chronic wounds: A retrospective study. J. Cutan. Aesthet. Surg. 2011, 4, 16. [Google Scholar] [CrossRef]
- Matthews, K.H. Drug delivery dressings. In Advanced Wound Repair Therapies; Farrar, D., Ed.; Elsevier Inc.: York, UK, 2011; pp. 361–394. ISBN 9781845697006. [Google Scholar]
- Pilehvar-Soltanahmadi, Y.; Dadashpour, M.; Mohajeri, A.; Fattahi, A.; Sheervalilou, R.; Zarghami, N. An overview on application of natural substances incorporated with electrospun nanofibrous scaffolds to development of innovative wound dressings. Mini-Rev. Med. Chem. 2017, 18, 414–427. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.R.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef]
- Li, H.; Cheng, W.; Liu, K.; Chen, L.; Huang, Y.; Wang, X.; Lv, Z.; He, J.; Li, C. Reinforced collagen with oxidized microcrystalline cellulose shows improved hemostatic effects. Carbohydr. Polym. 2017, 165, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Hermans, M.H.E.; Bolton, L.L. Air exposure versus occlusion: Merits and disadvantages of different dressings. J. Wound Care 1993, 2, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.J. Use of Oxidized Regenerated Cellulose (ORC)/Collagen/Silver-ORC Dressings alone or subsequent to advanced wound therapies in complex wounds. Wounds 2020, 32, 37–43. [Google Scholar]
- Growney Kalaf, E.A.; Hixon, K.R.; Kadakia, P.U.; Dunn, A.J.; Sell, S.A. Electrospun biomaterials for dermal regeneration. In Electrospun Materials for Tissue Engineering and Biomedical Applications: Research, Design and Commercialization; Kny, E., Uyar, T., Eds.; Woodhead Publishing: Sawston, Cambridge, UK, 2017; pp. 179–231. ISBN 9780081022221. [Google Scholar]
- Moraes, P.R.; Saska, S.; Barud, H.; Lima, L.R.; Martins, V.D.; Plepis, A.M.; Ribeiro, S.J.; Gaspar, A.M. Bacterial cellulose/collagen hydrogel for wound healing. Mater. Res. 2016, 19, 106–116. [Google Scholar] [CrossRef] [Green Version]





| Author | Aim | Study Design | Sample Size | Follow Up | Findings | Conclusion |
|---|---|---|---|---|---|---|
| Manizate and co-workers (2012) [55] | Compare the efficiency of bovine native collagen with silver ion (Ag) and sodium carboxymethylcellulose with Ag | Comparative, post-market clinical evaluation | 10 patients | 1st and 4th week | -50% of the wound exhibit S. Aureus -By the 4th week, the bacteria load increases up to 1.53 × 105 ppm | -Bovine native collagen dressing shows rapid wound closure. |
| Gottrup and co-workers (2013) [68] | Compare collagen/oxidised regenerated cellulose (ORC)/silver therapy to standard treatment | Randomized control trial | 39 patients | Every 2 week for 14 weeks | -Decreased concentration of elastase, MMP-9 -low MMP-9:TIMP-1 concentration -Absence of infection and adverse effects | -Collagen/ORC/silver therapy shows improved wound healing. |
| Ulrich and co-workers (2011) [43] | Evaluate the effect of collagen matrix/oxidized regenerated cellulose in wound exudate of DFU patients | Comparative clinical study | 32 patients | 14th, 28th, 42th, and 56th day | -Reduced level of MMP-2 -Reduction in plasmin, elastase and gelatinase | -Wound size reduction on 14th and 28th days in ORC treated groups. |
| Griffin and co-workers (2019) [69] | Comparative study between the effectiveness of oxidized regenerated cellulose and ovine collagen extracellular matrix | Comparative study | 3230 patients | 4th, 8th, 12th, and 16th week | -82% of the healed wound with ORC dressing -15.2% of a worsened wound with ovine collagen extracellular matrix dressing | -ORC decreases healing duration by improving granulation tissue formation in a short period. |
| Dumont and co-workers (2018) [61] | Evaluate the effectiveness of collagen-based dressing for DFU patients | Clinical follow-up | 6 male & 1 female | 38th to 64th day | -Increased formation of granulation tissue -complete surface healing at the wound site | -Fast skin restoration -Decreased healing tine. -Decreased rate of infection. |
| Kloeters and co-workers (2015) [62] | Evaluate the effectiveness of oxidized regenerated collagen-cellulose matrix in pressure ulcer | Clinical assessment | 33 patients | Weekly for 12 weeks | -Decreased level of plasmin and elastase activity -reduction in the surface area of the wound -Absence of infection and intolerance towards oxidized regenerated cellulose/collagen matrix dressing. | -Notable fast healing rate. |
| Solway and co-workers (2011) [65] | Study the effectiveness of microbial cellulose in DFU | Parallel open-label trial | 34 patients | Weekly till complete wound closure | -Increased formation of granulation tissue and maintenance of moist environment at the wound area -High tensile strength and crystallinity of the microbial cellulose | -Rapid wound healing with a short period of re epithelisation |
| Li and co-workers (2020) [72] | Access the efficiency of naturally occurring bacterial cellulose-hyper branched cationic polysaccharide derivative on wound healing of diabetic rats | In vivo study | Not specified | 1st, 4th and 7th day | -Good viability of cell -Low concentration of LDH -No effect on apoptosis -Inhibition in MMP-9 | -Increased wound healing rate. |
| Song and co-workers (2018) [63] | Evaluate the effect of Selenium-loaded cellulose film in diabetic induced rats | In vivo study | 48 male rats | 3rd and 12th day | -Low elongation, high tensile strength, excellent microporous structure and high-water absorption capacity -Absence of toxicity | -Notable rapid wound healing. -Notable stimulation in the angiogenesis pathway. |
| Li and co-workers (2020) [64] | Evaluate the effectiveness of carboxymethyl cellulose/K-carrageena/graphene oxide/konjac glucomannan hydrogel in diabetic induced mice | In vitro and in vivo study | 18 mice | 4th, 7th, 14th and 21st day | -The presence of permeable surface, high mechanical strength and great swelling capacity, supports the viability of the cell and has bactericidal property. | -Notable rapid wound recuperating. -Advanced fibroblast production and rapid re-epithelialization were seen. |
| Cellulose/Collagen Dressing | Conventional Dressing |
|---|---|
| Reduced reactive oxygen species in the wound [89] | Slow granulation tissue deposition [90] |
| Ability to absorb wound exudates [59] | High possibility for pathogenic organism to harbor [90] |
| Accelerates wound healing/promote rapid healing [91] | Dry, so it’s impossible to retain moist microenvironment [92] |
| Reduced length of stay in hospital [52] | Loss efficiency when loaded with absorbed wound exudates [93] |
| Shortened course of treatment [94] | Often requires extra care and frequent changing [95] |
| Improvement in wound reduction area [96] | |
| Rapid granulation tissue formation [90] | |
| Improved re-epithelisation and GF concentration [88] | |
| Absence/reduced bacterial invasion at the wound site [97] | |
| Cost effective [89] | |
| Easy application and good adherence to the wound bed [98] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naomi, R.; Fauzi, M.B. Cellulose/Collagen Dressings for Diabetic Foot Ulcer: A Review. Pharmaceutics 2020, 12, 881. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12090881
Naomi R, Fauzi MB. Cellulose/Collagen Dressings for Diabetic Foot Ulcer: A Review. Pharmaceutics. 2020; 12(9):881. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12090881
Chicago/Turabian StyleNaomi, Ruth, and Mh Busra Fauzi. 2020. "Cellulose/Collagen Dressings for Diabetic Foot Ulcer: A Review" Pharmaceutics 12, no. 9: 881. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12090881