Anticancer Activity of Half-Sandwich Ru, Rh and Ir Complexes with Chrysin Derived Ligands: Strong Effect of the Side Chain in the Ligand and Influence of the Metal
Abstract
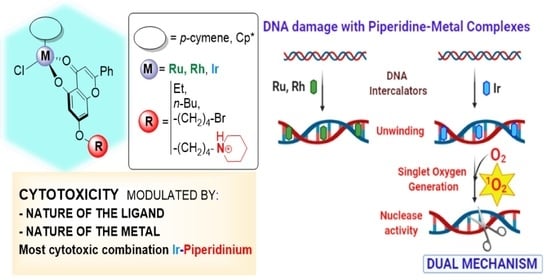
:1. Introduction
2. Materials and Methods
2.1. Crystal Data for HL2
2.2. General Procedure for Stability Studies by NMR Spectroscopy
2.3. Cell Lines Culture
2.4. Antiproliferative Activity
2.5. Cellular Uptake Studies
2.6. Intracellular ROS Generation
2.7. Apoptosis Detection
2.8. Cell Cycle Arrest
2.9. Statistical Analysis
2.10. UV-Vis Spectroscopy
2.11. Circular Dichroism Spectroscopy
2.12. Viscosity Measurements
2.13. Native Polyacrylamide Gel Electrophoresis (PAGE)
2.14. Plasmidic Electrophoresis Assays
3. Results and Discussion
3.1. Synthesis and Characterization of the Ligands and Complexes
3.2. Cytotoxic Activity
3.3. NMR Stability Studies of the Complexes with L4 in DMSO-d6 and DMSO-d6/D2O (3:2)
3.3.1. NMR Stability Studies of the Complexes Containing L4 in DMSO-d6
3.3.2. NMR Stability Studies of the Complexes with L4 in DMSO-d6/D2O (3:2)
3.4. UV-Vis Stability Studies of the Complexes with L4 in DMSO and Aqueous Solution
3.5. Determination of the pKa Values of HL4, L4-Ru, L4-Rh and L4-Ir
3.6. Interaction with DNA
3.6.1. Thermal Denaturation by UV-Vis
3.6.2. Circular Dichroism (CD)
3.6.3. Viscosity
3.6.4. Agarose Gel Electrophoresis
3.7. Intracellular ROS Generation
3.8. Apoptosis
3.9. Cell Cycle Studies
3.10. Cellular Uptake
3.11. Interaction with BSA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heffeter, P.; Jungwirth, U.; Jakupec, M.; Hartinger, C.; Galanski, M.; Elbling, L.; Micksche, M.; Keppler, B.; Berger, W. Resistance against novel anticancer metal compounds: Differences and similarities. Drug Resist. Updates 2008, 11, 1–16. [Google Scholar] [CrossRef]
- Van Rijt, S.H.; Sadler, P.J. Current applications and future potential for bioinorganic chemistry in the development of anticancer drugs. Drug Discov. Today 2009, 14, 1089–1097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medici, S.; Peana, M.; Nurchi, V.M.; Lachowicz, J.I.; Crisponi, G.; Zoroddu, M.A. Noble metals in medicine: Latest advances. Coord. Chem. Rev. 2015, 284, 329–350. [Google Scholar] [CrossRef]
- Hartinger, C.G.; Dyson, P. Bioorganometallic chemistry—From teaching paradigms to medicinal applications. Chem. Soc. Rev. 2009, 38, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Sadler, P.J. Advances in the design of organometallic anticancer complexes. J. Organomet. Chem. 2017, 839, 5–14. [Google Scholar] [CrossRef]
- Gasser, G.; Ott, I.; Metzler-Nolte, N. Organometallic Anticancer Compounds. J. Med. Chem. 2010, 54, 3–25. [Google Scholar] [CrossRef]
- Hartinger, C.G.; Metzler-Nolte, N.; Dyson, P.J. Challenges and Opportunities in the Development of Organometallic Anticancer Drugs. Organometallics 2012, 31, 5677–5685. [Google Scholar] [CrossRef]
- Thota, S.; Rodrigues, D.A.; Crans, D.; Barreiro, E.J. Ru(II) Compounds: Next-Generation Anticancer Metallotherapeutics? J. Med. Chem. 2018, 61, 5805–5821. [Google Scholar] [CrossRef]
- Jakupec, M.A.; Galanski, M.; Arion, V.B.; Hartinger, C.; Keppler, B. Antitumour metal compounds: More than theme and variations. Dalton Trans. 2008, 183–194. [Google Scholar] [CrossRef]
- Tremlett, W.D.; Goodman, D.M.; Steel, T.R.; Kumar, S.; Wieczorek-Błauż, A.; Walsh, F.P.; Sullivan, M.P.; Hanif, M.; Hartinger, C.G. Design concepts of half-sandwich organoruthenium anticancer agents based on bidentate bioactive ligands. Coord. Chem. Rev. 2021, 445, 213950. [Google Scholar] [CrossRef]
- Sun, Q.; Li, Y.; Shi, H.; Wang, Y.; Zhang, J.; Zhang, Q. Ruthenium Complexes as Promising Candidates against Lung Cancer. Molecules 2021, 26, 4389. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, S.A.; Harrypersad, S.; Sahyon, H.A.; Abu El-Magd, M.; Walsby, C.J. Ruthenium(II)/(III) DMSO-Based Complexes of 2-Aminophenyl Benzimidazole with In Vitro and In Vivo Anticancer Activity. Molecules 2020, 25, 4284. [Google Scholar] [CrossRef] [PubMed]
- Simović, A.R.; Masnikosa, R.; Bratsos, I.; Alessio, E. Chemistry and reactivity of ruthenium(II) complexes: DNA/protein binding mode and anticancer activity are related to the complex structure. Coord. Chem. Rev. 2019, 398, 113011. [Google Scholar] [CrossRef]
- Omondi, R.O.; Ojwach, S.O.; Jaganyi, D. Review of comparative studies of cytotoxic activities of Pt(II), Pd(II), Ru(II)/(III) and Au(III) complexes, their kinetics of ligand substitution reactions and DNA/BSA interactions. Inorg. Chim. Acta 2020, 512, 119883. [Google Scholar] [CrossRef]
- Allardyce, C.S.; Dyson, P.J. Ruthenium in medicine: Current clinical uses and future prospects. Platin. Met. Rev. 2001, 45, 62–69. [Google Scholar]
- Clarke, M.J. Ruthenium metallopharmaceuticals. Coord. Chem. Rev. 2002, 232, 69–93. [Google Scholar] [CrossRef]
- Kenny, R.G.; Marmion, C.J. Toward Multi-Targeted Platinum and Ruthenium Drugs—A New Paradigm in Cancer Drug Treatment Regimens? Chem. Rev. 2019, 119, 1058–1137. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Gupta, P.; Chen, Y.; Wang, E.; Ji, L.; Chao, H.; Chen, Z.-S. The development of anticancer ruthenium(II) complexes: From single molecule compounds to nanomaterials. Chem. Soc. Rev. 2017, 46, 5771–5804. [Google Scholar] [CrossRef]
- Kostova, I. Ruthenium Complexes as Anticancer Agents. Curr. Med. Chem. 2006, 13, 1085–1107. [Google Scholar] [CrossRef]
- Alessio, E.; Mestroni, G.; Bergamo, A.; Sava, G. Ruthenium Antimetastatic Agents. Curr. Top. Med. Chem. 2004, 4, 1525–1535. [Google Scholar] [CrossRef]
- Bergamo, A.; Gaiddon, C.; Schellens, J.; Beijnen, J.; Sava, G. Approaching tumour therapy beyond platinum drugs: Status of the art and perspectives of ruthenium drug candidates. J. Inorg. Biochem. 2012, 106, 90–99. [Google Scholar] [CrossRef]
- Steel, T.R.; Walsh, F.; Wieczorek-Błauż, A.; Hanif, M.; Hartinger, C.G. Monodentately-coordinated bioactive moieties in multimodal half-sandwich organoruthenium anticancer agents. Coord. Chem. Rev. 2021, 439, 213890. [Google Scholar] [CrossRef]
- Alessio, E. Thirty Years of the Drug Candidate NAMI-A and the Myths in the Field of Ruthenium Anticancer Compounds: A Personal Perspective. Eur. J. Inorg. Chem. 2016, 2017, 1549–1560. [Google Scholar] [CrossRef]
- Hartinger, C.G.; Jakupec, M.; Zorbas-Seifried, S.; Groessl, M.; Egger, A.; Berger, W.; Zorbas, H.; Dyson, P.; Keppler, B.K. KP1019, A New Redox-Active Anticancer Agent—Preclinical Development and Results of a Clinical Phase I Study in Tumor Patients. Chem. Biodivers. 2008, 5, 2140–2155. [Google Scholar] [CrossRef] [PubMed]
- Pages, B.J.; Ang, D.L.; Wright, E.P.; Aldrich-Wright, J. Metal complex interactions with DNA. Dalton Trans. 2014, 44, 3505–3526. [Google Scholar] [CrossRef] [PubMed]
- Ang, W.H.; Casini, A.; Sava, G.; Dyson, P.J. Organometallic ruthenium-based antitumor compounds with novel modes of action. J. Organomet. Chem. 2011, 696, 989–998. [Google Scholar] [CrossRef]
- Renfrew, A.K.; Juillerat-Jeanneret, L.; Dyson, P.J. Adding diversity to ruthenium(II)–arene anticancer (RAPTA) compounds via click chemistry: The influence of hydrophobic chains. J. Organomet. Chem. 2011, 696, 772–779. [Google Scholar] [CrossRef]
- Berndsen, R.H.; Weiss, A.; Abdul, U.K.; Wong, T.J.; Meraldi, P.; Griffioen, A.W.; Dyson, P.; Nowak-Sliwinska, P. Combination of ruthenium(II)-arene complex [Ru(η6-p-cymene)Cl2(pta)] (RAPTA-C) and the epidermal growth factor receptor inhibitor erlotinib results in efficient angiostatic and antitumor activity. Sci. Rep. 2017, 7, srep43005. [Google Scholar] [CrossRef]
- Nhukeaw, T.; Hongthong, K.; Dyson, P.J.; Ratanaphan, A. Cellular responses of BRCA1-defective HCC1937 breast cancer cells induced by the antimetastasis ruthenium(II) arene compound RAPTA-T. Apoptosis 2019, 24, 612–622. [Google Scholar] [CrossRef]
- Murray, B.; Babak, M.; Hartinger, C.; Dyson, P. The development of RAPTA compounds for the treatment of tumors. Coord. Chem. Rev. 2016, 306, 86–114. [Google Scholar] [CrossRef]
- Peacock, A.; Habtemariam, A.; Fernández, R.; Walland, V.; Fabbiani, F.P.A.; Parsons, S.; Aird, R.E.; Jodrell, D.I.; Sadler, P.J. Tuning the Reactivity of Osmium(II) and Ruthenium(II) Arene Complexes under Physiological Conditions. J. Am. Chem. Soc. 2006, 128, 1739–1748. [Google Scholar] [CrossRef]
- Peacock, A.; Sadler, P.J. Medicinal Organometallic Chemistry: Designing Metal Arene Complexes as Anticancer Agents. Chem.-Asian J. 2008, 3, 1890–1899. [Google Scholar] [CrossRef]
- Leijen, S.; Burgers, S.A.; Baas, P.; Pluim, D.; Tibben, M.; van Werkhoven, E.; Alessio, E.; Sava, G.; Beijnen, J.H.; Schellens, J.H.M. Phase I/II study with ruthenium compound NAMI-A and gemcitabine in patients with non-small cell lung cancer after first line therapy. Investig. New Drugs 2014, 33, 201–214. [Google Scholar] [CrossRef] [Green Version]
- Monro, S.; Colón, K.L.; Yin, H.; Roque, J., III; Konda, P.; Gujar, S.; Thummel, R.P.; Lilge, L.; Cameron, C.G.; McFarland, S.A. Transition Metal Complexes and Photodynamic Therapy from a Tumor-Centered Approach: Challenges, Opportunities, and Highlights from the Development of TLD1433. Chem. Rev. 2019, 119, 797–828. [Google Scholar] [CrossRef]
- Geldmacher, Y.; Splith, K.; Kitanovic, I.; Alborzinia, H.; Can, S.; Rubbiani, R.; Nazif, M.A.; Wefelmeier, P.; Prokop, A.; Ott, I.; et al. Cellular impact and selectivity of half-sandwich organorhodium(III) anticancer complexes and their organoiridium(III) and trichloridorhodium(III) counterparts. J. Biol. Inorg. Chem. 2012, 17, 631–646. [Google Scholar] [CrossRef]
- Liu, Z.; Romero-Canelón, I.; Qamar, B.; Hearn, J.M.; Habtemariam, A.; Barry, N.P.E.; Pizarro, A.M.; Clarkson, G.J.; Sadler, P.J. The Potent Oxidant Anticancer Activity of Organoiridium Catalysts. Angew. Chem. Int. Ed. 2014, 53, 3941–3946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, P.-Y.; Ho, C.-L.; Wong, W.-Y. Recent advances of iridium(III) metallophosphors for health-related applications. Coord. Chem. Rev. 2020, 413, 213267. [Google Scholar] [CrossRef]
- Tensi, L.; Macchioni, A. Extremely Fast NADH-Regeneration Using Phosphonic Acid as Hydride Source and Iridium-pyridine-2-sulfonamidate Catalysts. ACS Catal. 2020, 10, 7945–7949. [Google Scholar] [CrossRef]
- Li, J.; Guo, L.; Tian, Z.; Tian, M.; Zhang, S.; Xu, K.; Qian, Y.; Liu, Z. Novel half-sandwich iridium(III) imino-pyridyl complexes showing remarkable in vitro anticancer activity. Dalton Trans. 2017, 46, 15520–15534. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Kong, D.; He, X.; Guo, L.; Ge, X.; Liu, X.; Zhang, H.; Li, J.; Yang, Y.; Liu, Z. Mitochondria-targeted half-sandwich rutheniumII diimine complexes: Anticancer and antimetastasis via ROS-mediated signalling. Inorg. Chem. Front. 2018, 5, 2100–2105. [Google Scholar] [CrossRef]
- Aird, R.E.; Cummings, J.; Ritchie, A.; Muir, M.; Morris, R.E.; Chen, H.; Sadler, P.J.; Jodrell, D.I. In vitro and in vivo activity and cross resistance profiles of novel ruthenium (II) organometallic arene complexes in human ovarian cancer. Br. J. Cancer 2002, 86, 1652–1657. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Habtemariam, A.; Pizarro, A.M.; Fletcher, S.A.; Kisova, A.; Vrana, O.; Salassa, L.; Bruijnincx, P.; Clarkson, G.J.; Brabec, V.; et al. Organometallic Half-Sandwich Iridium Anticancer Complexes. J. Med. Chem. 2011, 54, 3011–3026. [Google Scholar] [CrossRef] [PubMed]
- Yellol, J.G.; Pérez, S.A.; Buceta, A.; Yellol, G.S.; Donaire, A.; Szumlas, P.; Bednarski, P.J.; Makhloufi, G.; Janiak, C.; Espinosa, A.; et al. Novel C,N-Cyclometalated Benzimidazole Ruthenium(II) and Iridium(III) Complexes as Antitumor and Antiangiogenic Agents: A Structure–Activity Relationship Study. J. Med. Chem. 2015, 58, 7310–7327. [Google Scholar] [CrossRef] [PubMed]
- Petruzzella, E.; Braude, J.P.; Aldrich-Wright, J.R.; Gandin, V.; Gibson, D. A Quadruple-Action Platinum(IV) Prodrug with Anticancer Activity Against KRAS Mutated Cancer Cell Lines. Angew. Chem. Int. Ed. 2017, 56, 11539–11544. [Google Scholar] [CrossRef] [PubMed]
- Ott, I.; Kircher, B.; Bagowski, C.P.; Vlecken, D.H.W.; Ott, E.B.; Will, J.; Bensdorf, K.; Sheldrick, W.S.; Gust, R. Modulation of the Biological Properties of Aspirin by Formation of a Bioorganometallic Derivative. Angew. Chem. Int. Ed. 2009, 48, 1160–1163. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, G.R.; Lehár, J.; Keith, C.T. Multi-target therapeutics: When the whole is greater than the sum of the parts. Drug Discov. Today 2007, 12, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.V.; Mistry, B.; Syed, R.; Rathi, A.K.; Lee, Y.-J.; Sung, J.-S.; Shinf, H.-S.; Keum, Y.-S. Chrysin-piperazine conjugates as antioxidant and anticancer agents. Eur. J. Pharm. Sci. 2016, 88, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Lemanska, K.; Szymusiak, H.; Tyrakowska, B.; Zielinski, R.; Soffers, A.E.; Rietjens, I.M.C.M. The influence of pH on antioxidant properties and the mechanism of antioxidant action of hydroxyflavones. Free Radic. Biol. Med. 2001, 31, 869–881. [Google Scholar] [CrossRef]
- Pietta, P.-G. Flavonoids as Antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Cushnie, T.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Harborne, J.B.; Williams, C.A. Advances in flavonoid research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef]
- Middleton, E.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 4, 52. [Google Scholar]
- Galati, G.; O’Brien, P.J. Potential toxicity of flavonoids and other dietary phenolics: Significance for their chemopreventive and anticancer properties. Free Radic. Biol. Med. 2004, 37, 287–303. [Google Scholar] [CrossRef]
- Williams, R.J.; Spencer, J.P.; Rice-Evans, C. Flavonoids: Antioxidants or signalling molecules? Free Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Rusak, G.; Piantanida, I.; Mašić, L.; Kapuralin, K.; Durgo, K.; Kopjar, N. Spectrophotometric analysis of flavonoid-DNA interactions and DNA damaging/protecting and cytotoxic potential of flavonoids in human peripheral blood lymphocytes. Chem. Interact. 2010, 188, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Grazul, M.; Budzisz, E. Biological activity of metal ions complexes of chromones, coumarins and flavones. Coord. Chem. Rev. 2009, 253, 2588–2598. [Google Scholar] [CrossRef]
- Małecka, M.; Skoczyńska, A.; Goodman, D.M.; Hartinger, C.G.; Budzisz, E. Biological properties of ruthenium(II)/(III) complexes with flavonoids as ligands. Coord. Chem. Rev. 2021, 436, 213849. [Google Scholar] [CrossRef]
- Dolatabadi, J.E.N. Molecular aspects on the interaction of quercetin and its metal complexes with DNA. Int. J. Biol. Macromol. 2011, 48, 227–233. [Google Scholar] [CrossRef]
- Gaur, R.; Mishra, L. Bi-nuclear Ru(II) complexes of bis-chalcone and bis-flavonol: Synthesis, characterization, photo cleavage of DNA and Topoisomerase I inhibition. RSC Adv. 2013, 3, 12210–12219. [Google Scholar] [CrossRef]
- Marques, J.; Silva, A.M.; Marques, M.P.M.; Braga, S.S. Ruthenium(II) trithiacyclononane complexes of 7,3′,4′-trihydroxyflavone, chrysin and tectochrysin: Synthesis, characterisation, and cytotoxic evaluation. Inorg. Chim. Acta 2019, 488, 71–79. [Google Scholar] [CrossRef]
- Wang, Y.; Bian, L.; Chakraborty, T.; Ghosh, T.; Chanda, P.; Roy, S. Construing the Biochemical and Molecular Mechanism Underlying the In Vivo and In Vitro Chemotherapeutic Efficacy of Ruthenium-Baicalein Complex in Colon Cancer. Int. J. Biol. Sci. 2019, 15, 1052–1071. [Google Scholar] [CrossRef] [Green Version]
- Thangavel, P.; Viswanath, B.; Kim, S. Synthesis and characterization of kaempferol-based ruthenium (II) complex: A facile approach for superior anticancer application. Mater. Sci. Eng. C 2018, 89, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Das, R.; Ghosh, B.; Chakraborty, T. Deciphering the biochemical and molecular mechanism underlying the in vitro and in vivo chemotherapeutic efficacy of ruthenium quercetin complex in colon cancer. Mol. Carcinog. 2018, 57, 700–721. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak, M.; Fabijańska, M.; Chęcińska, L.; Studzian, K.; Markowicz-Piasecka, M.; Sikora, J.; Mikiciuk-Olasik, E.; Ochocki, J. Small differences in structure, a large difference in activity—Comparing a new Ru(II)-3-hydroxyiminoflavanone complex with analogous Ru(II) compounds. Inorg. Chim. Acta 2017, 457, 69–80. [Google Scholar] [CrossRef]
- Garcia, J.P.; Lakshmi, B.A.; Kim, S. Potential anticancer applications of the novel naringin-based ruthenium (II) complex. 3 Biotech 2019, 9, 181. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, B.A.; Bae, J.-Y.; An, J.H.; Kim, S. Facile design and spectroscopic characterization of novel bio-inspired Quercetin-conjugated tetrakis (dimethylsulfoxide)dichlororuthenium(II) complex for enhanced anticancer properties. Inorg. Chim. Acta 2019, 495, 118989. [Google Scholar] [CrossRef]
- Medina, J.J.M.; Naso, L.G.; Pérez, A.L.; Rizzi, A.; Okulik, N.B.; Ferrer, E.G.; Williams, P.A. Apigenin oxidovanadium(IV) cation interactions. Synthesis, spectral, bovine serum albumin binding, antioxidant and anticancer studies. J. Photochem. Photobiol. A Chem. 2017, 344, 84–100. [Google Scholar] [CrossRef]
- Naso, L.G.; Lezama, L.; Valcarcel, M.; Salado, C.; Villacé, P.; Kortazar, D.; Ferrer, E.G.; Williams, P.A. Bovine serum albumin binding, antioxidant and anticancer properties of an oxidovanadium(IV) complex with luteolin. J. Inorg. Biochem. 2016, 157, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Tu, L.-Y.; Pi, J.; Jin, H.; Cai, J.-Y.; Deng, S.-P. Synthesis, characterization and anticancer activity of kaempferol-zinc(II) complex. Bioorganic Med. Chem. Lett. 2016, 26, 2730–2734. [Google Scholar] [CrossRef]
- Roy, A.S.; Tripathy, D.R.; Samanta, S.; Ghosh, S.K.; Dasgupta, S. DNA damaging, cell cytotoxicity and serum albumin binding efficacy of the rutin-Cu(II) complex. Mol. BioSyst. 2016, 12, 1687–1701. [Google Scholar] [CrossRef]
- Qian, J.-Z.; Wang, B.C.; Fan, Y.; Tan, J.; Yang, X. QSAR study of flavonoid-metal complexes and their anticancer activities. J. Struct. Chem. 2015, 56, 338–345. [Google Scholar] [CrossRef]
- De Souza, R.F.V.; De Giovani, W.F. Antioxidant properties of complexes of flavonoids with metal ions. Redox Rep. 2004, 9, 97–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, Y.-B.; Yang, N.; Liu, W.-S.; Tang, N. Synthesis, characterization and DNA-binding properties of La(III) complex of chrysin. J. Inorg. Biochem. 2003, 97, 258–264. [Google Scholar] [CrossRef]
- Schmidlehner, M.; Flocke, L.S.; Roller, A.; Hejl, M.; Jakupec, M.A.; Kandioller, W.; Keppler, B.K. Cytotoxicity and preliminary mode of action studies of novel 2-aryl-4-thiopyrone-based organometallics. Dalton Trans. 2015, 45, 724–733. [Google Scholar] [CrossRef]
- Kurzwernhart, A.; Mokesch, S.; Klapproth, E.; Adib-Ravazi, M.S.; Jakupec, M.A.; Hartinger, C.G.; Kandioller, W.; Keppler, B.K. Flavonoid-Based Organometallics with Different Metal Centers—Investigations of the Effects on Reactivity and Cytotoxicity. Eur. J. Inorg. Chem. 2015, 2016, 240–246. [Google Scholar] [CrossRef]
- Kurzwernhart, A.; Kandioller, W.; Bächler, S.; Bartel, C.; Martic, S.; Buczkowska, M.; Mühlgassner, G.; Jakupec, M.A.; Kraatz, H.-B.; Bednarski, P.J.; et al. Structure–Activity Relationships of Targeted RuII(η6-p-Cymene) Anticancer Complexes with Flavonol-Derived Ligands. J. Med. Chem. 2012, 55, 10512–10522. [Google Scholar] [CrossRef] [PubMed]
- Kurzwernhart, A.; Kandioller, W.; Bartel, C.; Bächler, S.; Trondl, R.; Mühlgassner, G.; Jakupec, M.A.; Arion, V.B.; Marko, D.; Keppler, B.K.; et al. Targeting the DNA-topoisomerase complex in a double-strike approach with a topoisomerase inhibiting moiety and covalent DNA binder. Chem. Commun. 2012, 48, 4839–4841. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, M.B.; Kurzwernhart, A.; Roller, A.; Kandioller, W.; Keppler, B.K.; Hartinger, C.G. Rhodium(Cp*) Compounds with Flavone-derived Ligand Systems: Synthesis and Characterization. Z. Anorg. Allg. Chem. 2013, 639, 1648–1654. [Google Scholar] [CrossRef]
- Tabrizi, L.; Nguyen, T.L.A.; Dao, D.Q. Experimental and theoretical investigation of cyclometalated phenylpyridine iridium(iii) complex based on flavonol and ibuprofen ligands as potent antioxidant. RSC Adv. 2019, 9, 17220–17237. [Google Scholar] [CrossRef] [Green Version]
- Kasprzak, M.M.; Erxleben, A.; Ochocki, J. Properties and applications of flavonoid metal complexes. RSC Adv. 2015, 5, 45853–45877. [Google Scholar] [CrossRef]
- Mercer, L.D.; Kelly, B.L.; Horne, M.K.; Beart, P.M. Dietary polyphenols protect dopamine neurons from oxidative insults and apoptosis: Investigations in primary rat mesencephalic cultures. Biochem. Pharmacol. 2005, 69, 339–345. [Google Scholar] [CrossRef]
- Gresa-Arribas, N.; Serratosa, J.; Saura, J.; Solà, C. Inhibition of CCAAT/enhancer binding protein δ expression by chrysin in microglial cells results in anti-inflammatory and neuroprotective effects. J. Neurochem. 2010, 115, 526–536. [Google Scholar] [CrossRef]
- Mansour, S.Z.; Moawed, F.S.; Elmarkaby, S.M. Protective effect of 5, 7-dihydroxyflavone on brain of rats exposed to acrylamide or γ-radiation. J. Photochem. Photobiol. B Biol. 2017, 175, 149–155. [Google Scholar] [CrossRef]
- Phan, T.; Yu, X.-M.; Kunnimalaiyaan, M.; Chen, H. Antiproliferative Effect of Chrysin on Anaplastic Thyroid Cancer. J. Surg. Res. 2011, 170, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Zahirović, A.; Kahrović, E.; Cindrić, M.; Pavelić, S.K.; Hukić, M.; Harej, A.; Turkušić, E. Heteroleptic ruthenium bioflavonoid complexes: From synthesis to in vitro biological activity. J. Coord. Chem. 2017, 70, 4030–4053. [Google Scholar] [CrossRef]
- Munteanu, A.-C.; Notaro, A.; Jakubaszek, M.; Cowell, J.; Tharaud, M.; Goud, B.; Uivarosi, V.; Gasser, G. Synthesis, Characterization, Cytotoxic Activity, and Metabolic Studies of Ruthenium(II) Polypyridyl Complexes Containing Flavonoid Ligands. Inorg. Chem. 2020, 59, 4424–4434. [Google Scholar] [CrossRef]
- Roy, S.; Sil, A.; Chakraborty, T. Potentiating apoptosis and modulation of p53, Bcl2, and Bax by a novel chrysin ruthenium complex for effective chemotherapeutic efficacy against breast cancer. J. Cell. Physiol. 2018, 234, 4888–4909. [Google Scholar] [CrossRef] [PubMed]
- Ravishankar, D.; Salamah, M.; Attina, A.; Pothi, R.; Vallance, T.; Javed, M.; Williams, H.F.; Alzahrani, E.M.S.; Kabova, E.; Vaiyapuri, R.; et al. Ruthenium-conjugated chrysin analogues modulate platelet activity, thrombus formation and haemostasis with enhanced efficacy. Sci. Rep. 2017, 7, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, S.; Singh, J.D. Synthesis and preliminary biological evaluation for the anticancer activity of organochalcogen (S/Se) tethered chrysin based organometallic RuII(η6–p–cymene) complexes. J. Biomol. Struct. Dyn. 2019, 37, 3337–3353. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Wang, J.; Yu, L.; Zhao, L.; Lu, N.; You, Q.; Guo, Q.; Li, Z. Synthesis and biological evaluation of 7-O-modified oroxylin A derivatives. Bioorganic Med. Chem. Lett. 2012, 22, 1118–1121. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Wang, W.; Cheng, H.; Pan, S.; Ren, J. Synthesis and cytotoxicity of novel chrysin derivatives. Med. Chem. Res. 2010, 20, 838–846. [Google Scholar] [CrossRef]
- Shin, J.-S.; Kim, K.-S.; Kim, M.-B.; Jeong, J.-H.; Kim, B.-K. Synthesis and hypoglycemic effect of chrysin derivatives. Bioorganic Med. Chem. Lett. 1999, 9, 869–874. [Google Scholar] [CrossRef]
- Cheng, N.; Yi, W.-B.; Wang, Q.-Q.; Peng, S.-M.; Zou, X.-Q. Synthesis and α-glucosidase inhibitory activity of chrysin, diosmetin, apigenin, and luteolin derivatives. Chin. Chem. Lett. 2014, 25, 1094–1098. [Google Scholar] [CrossRef]
- Mistry, B.M.; Patel, R.V.; Keum, Y.-S.; Kim, D.H. Chrysin–benzothiazole conjugates as antioxidant and anticancer agents. Bioorganic Med. Chem. Lett. 2015, 25, 5561–5565. [Google Scholar] [CrossRef] [PubMed]
- Babu, K.S.; Babu, T.H.; Srinivas, P.; Kishore, K.H.; Murthy, U.; Rao, J.M. Synthesis and biological evaluation of novel C (7) modified chrysin analogues as antibacterial agents. Bioorganic Med. Chem. Lett. 2006, 16, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.-Q.; Peng, S.-M.; Hu, C.-P.; Tan, L.-F.; Yuan, Q.; Deng, H.-W.; Li, Y.-J. Synthesis, characterization and vasculoprotective effects of nitric oxide-donating derivatives of chrysin. Bioorganic Med. Chem. 2010, 18, 3020–3025. [Google Scholar] [CrossRef]
- Lapkin, A.; Adou, E.; Mlambo, B.N.; Chemat, S.; Suberu, J.; Collis, A.E.; Clark, A.; Barker, G. Integrating medicinal plants extraction into a high-value biorefinery: An example of Artemisia annua L. C. R. Chim. 2014, 17, 232–241. [Google Scholar] [CrossRef]
- Valdez-Calderón, A.; González-Montiel, S.; Martínez-Otero, D.; Martínez-Torres, A.; Vásquez-Pérez, J.M.; Molina-Vera, C.; Torres-Valencia, J.M.; Alvarado-Rodríguez, J.G.; Cruz-Borbolla, J. Synthesis, structural study and biological activity of new derivatives of chrysin containing a 2-mercaptopyridyl or 5-(trifluoromethyl)-2-mercaptopyridyl fragments. J. Mol. Struct. 2016, 1110, 196–207. [Google Scholar] [CrossRef]
- Zelonka, M.C.; Baird, R.A. Benzene Complexes of Ruthenium(II). Can. J. Chem. 1972, 50, 3063–3072. [Google Scholar] [CrossRef]
- Bennett, M.A.; Smith, A.K. Arene ruthenium(II) complexes formed by dehydrogenation of cyclohexadienes with ruthenium(III) trichloride. J. Chem. Soc. Dalton Trans. 1974, 233–241. [Google Scholar] [CrossRef]
- White, C.; Yates, A.; Maitlis, P.M. (eta5-pentamethylcyclopentadienyl)-rhodium and -iridium compounds. Inorg. Synth. 1992, 29, 228–234. [Google Scholar]
- Bruker. SAINT v8.37, APEX3 v2016.1.0; Bruker AXS: Madison, WI, USA, 2016. [Google Scholar]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. SADABS. J. Appl. Crystallogr. 2015, 48, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farrugia, L.J. WinGXandORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELX-2014, Program for Crystal Structure Refinement; University of Göttingen: Göttingen, Germany, 2014. [Google Scholar]
- Zanda, E.; Busto, N.; Biancalana, L.; Zacchini, S.; Biver, T.; Garcia, B.; Marchetti, F. Anticancer and antibacterial potential of robust Ruthenium(II) arene complexes regulated by choice of α-diimine and halide ligands. Chem. Interact. 2021, 344, 109522. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Moon, B.-H.; Lee, E.; Lee, Y.; Yoon, Y.; Ahn, J.-H.; Lim, Y. 1H and 13C-NMR data of hydroxyflavone derivatives. Magn. Reson. Chem. 2007, 45, 674–679. [Google Scholar] [CrossRef]
- Nguyen, T.K.P.; Nguyen, K.P.P.; Kamounah, F.S.; Zhang, W.; Hansen, P.E. NMR of a series of novel hydroxyflavothiones. Magn. Reson. Chem. 2009, 47, 1043–1054. [Google Scholar] [CrossRef]
- Uivarosi, V.; Badea, M.; Olar, R.; Drǎghici, C.; Bǎrbuceanu, F. Synthesis and Characterization of Some New Complexes of Magnesium (II) and Zinc (II) with the Natural Flavonoid Primuletin. Molecules 2013, 18, 7631–7645. [Google Scholar] [CrossRef] [Green Version]
- Parveen, S.; Hanif, M.; Movassaghi, S.; Sullivan, M.P.; Kubanik, M.; Shaheen, M.A.; Söhnel, T.; Jamieson, S.M.F.; Hartinger, C.G. Cationic Ru(η6-p-cymene) Complexes of 3-Hydroxy-4-pyr(id)ones—Lipophilic Triphenylphosphine as Co-Ligand Is Key to Highly Stable and Cytotoxic Anticancer Agents. Eur. J. Inorg. Chem. 2016, 2017, 1721–1727. [Google Scholar] [CrossRef] [Green Version]
- Biancalana, L.; Pampaloni, G.; Zacchini, S.; Marchetti, F. Synthesis, characterization and behavior in water/DMSO solution of Ru(II) arene complexes with bioactive carboxylates. J. Organomet. Chem. 2018, 869, 201–211. [Google Scholar] [CrossRef]
- Patra, M.; Joshi, T.; Pierroz, V.; Ingram, K.; Kaiser, M.; Ferrari, P.-D.D.S.; Spingler, P.-D.D.B.; Keiser, J.; Gasser, G. DMSO-Mediated Ligand Dissociation: Renaissance for Biological Activity of N-Heterocyclic-[Ru(η6-arene)Cl2] Drug Candidates. Chem. A Eur. J. 2013, 19, 14768–14772. [Google Scholar] [CrossRef]
- Dömötör, O.; Aicher, S.; Schmidlehner, M.; Novak, M.S.; Roller, A.; Jakupec, M.; Kandioller, W.; Hartinger, C.; Keppler, B.; Enyedy, A. Antitumor pentamethylcyclopentadienyl rhodium complexes of maltol and allomaltol: Synthesis, solution speciation and bioactivity. J. Inorg. Biochem. 2014, 134, 57–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisen, M.S.; Haskel, A.; Chen, H.; Olmstead, M.M.; Smith, D.P.; Maestre, M.F.; Fish, R.H. Aqueous Organometallic Chemistry: Structure and Dynamics in the Formation of (η.5-Pentamethylcyclopentadienyl)rhodium Aqua Complexes as a Function of pH. Organometallics 1995, 14, 2806–2812. [Google Scholar] [CrossRef]
- Ogo, S.; Makihara, N.; Watanabe, Y. pH-dependent transfer hydrogenation of water-soluble carbonyl compounds with [Cp*IrIII(H2O)3]2+ (Cp* = η5C5Me5) as a catalyst precursor and HCOONa as a hydrogen donor in water. Organometallics 1999, 18, 5470–5474. [Google Scholar] [CrossRef]
- Kandioller, W.; Hartinger, C.G.; Nazarov, A.A.; Bartel, C.; Skocic, M.; Jakupec, M.; Arion, V.B.; Keppler, B. Maltol-Derived Ruthenium-Cymene Complexes with Tumor Inhibiting Properties: The Impact of Ligand-Metal Bond Stability on Anticancer Activity In Vitro. Chem. A Eur. J. 2009, 15, 12283–12291. [Google Scholar] [CrossRef] [PubMed]
- Turel, I.; Kljun, J.; Perdih, F.; Morozova, E.; Bakulev, V.; Kasyanenko, N.; Byl, J.A.W.; Osheroff, N. First Ruthenium Organometallic Complex of Antibacterial Agent Ofloxacin. Crystal Structure and Interactions with DNA. Inorg. Chem. 2010, 49, 10750–10752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peacock, A.; Melchart, M.; Deeth, R.; Habtemariam, A.; Parsons, S.; Sadler, P.J. Osmium(II) and Ruthenium(II) Arene Maltolato Complexes: Rapid Hydrolysis and Nucleobase Binding. Chem. A Eur. J. 2007, 13, 2601–2613. [Google Scholar] [CrossRef]
- Bíró, L.; Farkas, E.; Buglyó, P. Hydrolytic behaviour and chloride ion binding capability of [Ru(η6-p-cym)(H2O)3]2+: A solution equilibrium study. Dalton Trans. 2012, 41, 285–291. [Google Scholar] [CrossRef]
- Nutton, A.; Bailey, P.M.; Maitlis, P.M. Pentamethylcyclopentadienyl-rhodium and -iridium Complexes. Part 29. Syntheses and X-Ray Structure Determinations of [(Rh(C5Me5)}2-(OH)3]OH-11H2O and [{Ir(C5Me5)}2(OH)3]O2CMe.14H2O and Related Complexes. J. Chem. Soc. Dalton Trans. 1981, 9, 1997–2002. [Google Scholar] [CrossRef]
- Po, H.N.; Senozan, N.M. The Henderson-Hasselbalch Equation: Its History and Limitations. J. Chem. Educ. 2001, 78, 1499–1503. [Google Scholar] [CrossRef]
- Ang, K.-P. A Spectrophotometric Method for the Determination of Overlapping Ionization Constants. J. Phys. Chem. 1958, 62, 1109–1112. [Google Scholar] [CrossRef]
- Castro, G.; Ferretti, F.; Blanco, S. Determination of the overlapping pKa values of chrysin using UV–vis spectroscopy and ab initio methods. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2005, 62, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Um, I.-H.; Lee, E.-J.; Jeon, S.-E. Effect of solvent on reactivity and basicity: Aminolyses of p-nitrophenyl acetate in H2O and in DMSO. J. Phys. Org. Chem. 2002, 15, 561–565. [Google Scholar] [CrossRef]
- Martinez-Alonso, M.; Busto, N.; Jalon, F.A.; Manzano, B.R.; Leal, J.M.; Rodriguez, A.M.; Garcia, B.; Espino, G. Derivation of Structure—Activity Relationships from the Anticancer Properties of Ruthenium(II) Arene Complexes with 2-Aryldiazole Ligands. Inorg. Chem. 2014, 53, 11274–11288. [Google Scholar] [CrossRef] [PubMed]
- Mészáros, J.P.; Dömötör, O.; Hackl, C.M.; Roller, A.; Keppler, B.K.; Kandioller, W.; Enyedy, E.A.A. Structural and solution equilibrium studies on half-sandwich organorhodium complexes of (N,N) donor bidentate ligands. New J. Chem. 2018, 42, 11174–11184. [Google Scholar] [CrossRef] [Green Version]
- Dehkhodaei, M.; Sahihi, M.; Rudbari, H.A.; Momenbeik, F. DNA and HSA interaction of Vanadium (IV), Copper (II), and Zinc (II) complexes derived from an asymmetric bidentate Schiff-base ligand: Multi spectroscopic, viscosity measurements, molecular docking, and ONIOM studies. J. Biol. Inorg. Chem. 2017, 23, 181–192. [Google Scholar] [CrossRef]
- Liu, H.-K.; Sadler, P.J. Metal Complexes as DNA Intercalators. Accounts Chem. Res. 2011, 44, 349–359. [Google Scholar] [CrossRef]
- Rubio-Antolin, A.R. Interacción de ARN Con Doxorrubicina: Influencia del Ligando y del Metal en la Actividad Biológica de Complejos Organometálicos. Ph.D. Thesis, University of Burgos, Burgos, Spain, 2020. [Google Scholar] [CrossRef]
- Neissa, J.; Pérez-Arnaiz, C.; Porto, V.; Busto, N.; Borrajo, E.; Leal, J.M.; López-Quintela, M.A.; García, B.; Dominguez, F. Interaction of silver atomic quantum clusters with living organisms: Bactericidal effect of Ag3 clusters mediated by disruption of topoisomerase–DNA complexes. Chem. Sci. 2015, 6, 6717–6724. [Google Scholar] [CrossRef]
- Moreira, J.D.V.; Schwartz, L.; Jolicoeur, M. Targeting Mitochondrial Singlet Oxygen Dynamics Offers New Perspectives for Effective Metabolic Therapies of Cancer. Front. Oncol. 2020, 10, 573399. [Google Scholar] [CrossRef] [PubMed]
- Habas, K.; Soldevila-Barreda, J.J.; Azmanova, M.; Rafols, L.; Pitto-Barry, A.; Anderson, D.; Barry, N.P.E. Evaluation of the Toxicity of Two Electron-Deficient Half-Sandwich Complexes against Human Lymphocytes from Healthy Individuals. ChemMedChem 2021, 16, 624–629. [Google Scholar] [CrossRef]
- Li, J.; Tian, Z.; Xu, Z.; Zhang, S.; Feng, Y.; Zhang, L.; Liu, Z. Highly potent half-sandwich iridium and ruthenium complexes as lysosome-targeted imaging and anticancer agents. Dalton Trans. 2018, 47, 15772–15782. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, Y.; Zhang, S.; Jia, X.; Zhong, G.; Yang, Y.; Du, Q.; Li, J.; Liu, Z. Novel half-sandwich iridium OˆC (carbene)-Complexes: In vitro and in vivo tumor growth suppression and pro-apoptosis via ROS-mediated cross-talk between mitochondria and lysosomes. Cancer Lett. 2019, 447, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.G.; Chang, T.-S.; Jeong, W.; Kang, D. Methods for detection and measurement of hydrogen peroxide inside and outside of cells. Mol. Cells 2010, 29, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tian, Z.; Ge, X.; Xu, Z.; Feng, Y.; Liu, Z. Design, synthesis, and evaluation of fluorine and Naphthyridine–Based half-sandwich organoiridium/ruthenium complexes with bioimaging and anticancer activity. Eur. J. Med. Chem. 2019, 163, 830–839. [Google Scholar] [CrossRef]
- Chen, C.; Xu, C.; Li, T.; Lu, S.; Luo, F.; Wang, H. Novel NHC-coordinated ruthenium(II) arene complexes achieve synergistic efficacy as safe and effective anticancer therapeutics. Eur. J. Med. Chem. 2020, 203, 112605. [Google Scholar] [CrossRef]
- Sullivan, M.P.; Holtkamp, H.U.; Hartinger, C.G. Antitumor Metallodrugs that Target Proteins. Met.-Drugs Dev. Action Anticancer Agents 2018, 18, 351–386. [Google Scholar]
- Pérez-Arnaiz, C.; Leal, J.; Busto, N.; Carrión, M.C.; Rubio, A.R.; Ortiz, I.; Barone, G.; de Greñu, B.D.; Santolaya, J.; Leal, J.M.; et al. Role of Seroalbumin in the Cytotoxicity of cis-Dichloro Pt(II) Complexes with (N^N)-Donor Ligands Bearing Functionalized Tails. Inorg. Chem. 2018, 57, 6124–6134. [Google Scholar] [CrossRef] [Green Version]

















| Complex | SW480 | A549 | Complex | SW480 | A549 |
|---|---|---|---|---|---|
| L1-Ru | 95.7 ± 5.2 | 159.9 ± 1.2 | L3-Ru | 117.3 ± 3.2 | 114.9 ± 1.2 |
| L1-Rh | 33.5 ± 1.1 | 134.4 ± 1.1 | L3-Rh | >200 | 57.3 ± 1.1 |
| L1-Ir | 51.9 ± 1.6 | >200 | L3-Ir | >200 | 36.8 ± 1.2 |
| HL1 | >200 | >200 | HL3 | >200 | 63.7 ± 1.2 |
| L2-Ru | 134.1 ± 9.2 | 122.6 ± 1.2 | L4-Ru | 28.5 ± 1.3 | 31.1 ± 1.6 |
| L2-Rh | 124.2 ± 1.1 | 97.4 ± 1.2 | L4-Rh | 31.3 ± 1.3 | 35.3 ± 1.0 |
| L2-Ir | 119.8 ± 1.1 | >200 | L4-Ir | 15.9 ± 1.3 | 18.9 ± 1.1 |
| HL2 | >200 | >200 | HL4 | 20.8 ± 2.0 | 23.8 ± 2.1 |
| CDDP1 | 46.7 ± 1.4 | 37.6 ± 1.3 |
| Complex | IMR-90 | SI | A2780 | A2780cis | RF |
|---|---|---|---|---|---|
| L4-Ru | 54.5 ± 1.1 | 1.8 | 14.9 ± 1.8 | 8.4 ± 0.4 | 0.6 |
| L4-Rh | 55.6 ± 1.0 | 1.6 | 23.3 ± 2.1 | 4.9 ± 0.2 | 0.2 |
| L4-Ir | 33.8 ± 1.2 | 1.8 | 22.7 ± 3.0 | 23.5 ± 1.8 | 1.0 |
| HL4 | 5.1 ± 1.3 | 0.2 | 8.8 ± 0.6 | 13.0 ± 1.6 | 1.5 |
| CDDP1 | 57.7 ± 1.2 | 1.5 | 7.0 ± 0.7 | 60.1 ± 4.3 | 8.6 |
| Complex | pKa | pKa1 | pKa2 |
|---|---|---|---|
| L4-Ru | 9.56 ± 0.08 | >12 | |
| L4-Rh | 9.67 ± 0.03 | >11 | |
| L4-Ir | 9.02 ± 0.01 | 11.41 ± 0.04 | |
| HL4 | 8.59 ± 0.04 | >11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubio, A.R.; González, R.; Busto, N.; Vaquero, M.; Iglesias, A.L.; Jalón, F.A.; Espino, G.; Rodríguez, A.M.; García, B.; Manzano, B.R. Anticancer Activity of Half-Sandwich Ru, Rh and Ir Complexes with Chrysin Derived Ligands: Strong Effect of the Side Chain in the Ligand and Influence of the Metal. Pharmaceutics 2021, 13, 1540. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics13101540
Rubio AR, González R, Busto N, Vaquero M, Iglesias AL, Jalón FA, Espino G, Rodríguez AM, García B, Manzano BR. Anticancer Activity of Half-Sandwich Ru, Rh and Ir Complexes with Chrysin Derived Ligands: Strong Effect of the Side Chain in the Ligand and Influence of the Metal. Pharmaceutics. 2021; 13(10):1540. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics13101540
Chicago/Turabian StyleRubio, Ana R., Rocío González, Natalia Busto, Mónica Vaquero, Ana L. Iglesias, Félix A. Jalón, Gustavo Espino, Ana M. Rodríguez, Begoña García, and Blanca R. Manzano. 2021. "Anticancer Activity of Half-Sandwich Ru, Rh and Ir Complexes with Chrysin Derived Ligands: Strong Effect of the Side Chain in the Ligand and Influence of the Metal" Pharmaceutics 13, no. 10: 1540. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics13101540