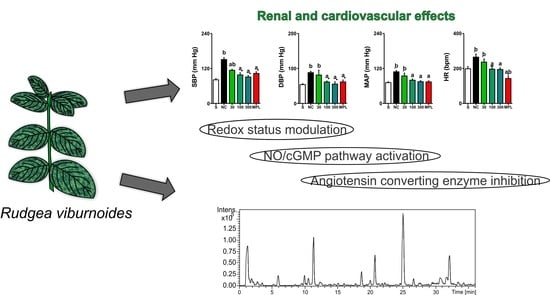
Prolonged Administration of Rudgea viburnoides (Cham.) Benth. Prevents Impairment of Redox Status, Renal Dysfunction, and Cardiovascular Damage in 2K1C-Hypertensive Rats by Inhibiting ACE Activity and NO-GMPC Pathway Activation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drugs and Reagents
2.2. Plant Material and Extraction
2.3. Chemical Analyses by LC-DAD-MS
2.4. Ethnopharmacological Studies
2.4.1. Animals
2.4.2. Acute Toxicity
2.4.3. Hypertension Induction (Goldblatt Model; Two Kidneys, One Clip; 2K1C)
2.4.4. Experimental Design
2.4.5. Renal Function
2.4.6. Electrocardiography
2.4.7. Blood Pressure (BP) Assessment
2.4.8. Biochemical Parameters
2.4.9. Mesenteric Vascular Beds (MVBs) Reactivity
2.4.10. Relative Weight and Histopathological Analysis of the Heart and Left Ventricle Morphometry
2.4.11. Cardiac and Vascular Redox Status
2.4.12. Cyclic Guanosine Monophosphate (cGMP) Determination
2.5. Statistical Analyses
3. Results
3.1. Chemical Constituents from AERV
3.2. Toxicological Evaluations
3.3. Effects on Urinary Volume and Renal Electrolyte Excretion
3.4. Effects on Electrical Cardiac Activity
3.5. Effects on Blood Pressure and Heart Rate
3.6. Effects on Serum Parameters
3.7. Effects on MVBs Reactivity
3.8. Effects on Cardiac Morphology
3.9. Effects on Cardiac and Vascular Redox Status
3.10. Effects on Intracellular cGMP Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO (World Health Organization). High Blood Pressure: A Public Health Priority, World Health Day, Geneva; WHO: Geneva, Switzerland, 2013; pp. 1–13. [Google Scholar]
- Brown, M.J.; Cruickshank, J.K.; Dominiczak, A.; MacGregor, G.A.; Poulter, N.R.; Russell, G.I.; Thom, S.; Williams, B. Better blood pressure control: How to combine drugs. J. Hum. Hypertens. 2003, 17, 81–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Oliveira, G.M.M.; Mendes, M.; Malachias, M.V.B.; Morais, J.; Filho, O.M.; Coelho, A.S.; Capingana, D.P.; Azevedo, V.; Soares, I.; Menete, A.; et al. 2017 Guidelines for Arterial Hypertension Management in Primary Health Care in Portuguese Language Countries. Arq. Bras. Cardiol. 2017, 109, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Lopatowska, P.; Mlodawska, E.; Tomaszuk-Kazberuk, A.; Banach, M.; Malyszko, J. Adhering to the principles of clinical pharmacology—The correct fixed combinations of antihypertensive drugs. Expert Rev. Clin. Pharmacol. 2017, 11, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Balogun, F.O.; Ashafa, A.O.T. A Review of Plants Used in South African Traditional Medicine for the Management and Treatment of Hypertension. Planta Medica 2019, 85, 312–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Moura, R.S.; Resende, Â.C. Cardiovascular and Metabolic Effects of Açaí, an Amazon Plant. J. Cardiovasc. Pharmacol. 2016, 68, 19–26. [Google Scholar] [CrossRef]
- Da Silva, C.S.P.; Proença, C.E.B. Uso e disponibilidade de recursos medicinais no município de Ouro Verde de Goiás, GO, Brasil. Acta Bot. Bras. 2008, 22, 481–492. [Google Scholar] [CrossRef]
- Alves, R.; Stehmann, J.; Isaias, R.; Brandão, M. Caracterização botânica e química de Rudgea viburnoides (Cham) Benth., (Rubiaceae). Rev. Bras. Farm. 2004, 14, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Nunes, G.; Da Silva, M.; Resende, U.; De Siqueira, J. Plantas medicinais comercializadas por raizeiros no Centro de Campo Grande, Mato Grosso do Sul. Rev. Bras. Farm. 2003, 13, 83–92. [Google Scholar] [CrossRef]
- Galdino, P.M.; Alexandre, L.N.; Pacheco, L.F.; Junior, R.D.S.L.; de Paula, J.R.; Pedrino, G.R.; Xavier, C.; Ferreira, P.M. Nephroprotective effect of Rudgea viburnoides (Cham.) Benth leaves on gentamicin-induced nephrotoxicity in rats. J. Ethnopharmacol. 2017, 201, 100–107. [Google Scholar] [CrossRef]
- Pucci, L.L.; Cunha, L.C.; Tresvenzol, L.M.; Paula, J.R.D.; Bozinis, M.C.; Freitas, P.C. Avaliação da toxicidade aguda oral e da atividade diurética da Rudgea viburnoides. Lat. Am. J. Pharm. 2010, 29, 30–37. [Google Scholar]
- Almeida, J.M.; Ferreira, A.V.; Oliveira, V.B.; Oliveira, M.; Teixeira, M.M.; Brandão, M.G. Effects of Rudgea viburnoides (Cham.) Benth. (Rubiaceae) Leaves on Metabolic and Inflammatory Dysfunction Induced by High Refined Carbohydrate-Containing Diet in Mice. J. Med. Food 2018, 21, 1266–1275. [Google Scholar] [CrossRef] [PubMed]
- Young, M.C.M.; Araujo, A.R.; da Silva, C.A.; Lopes, M.N.; Trevisan, L.M.V.; Bolzani, V. Triterpenes and Saponins from Rudgea viburnioides. J. Nat. Prod. 1998, 61, 936–938. [Google Scholar] [CrossRef] [PubMed]
- Younis, W.; Schini-Kerth, V.; da Silva, D.B.; Junior, A.G.; Bukhari, I.A.; Assiri, A.M. Role of the NO/cGMP pathway and renin-angiotensin system in the hypotensive and diuretic effects of aqueous soluble fraction from Crataegus songarica K. Koch. J. Ethnopharmacol. 2020, 249, 112400. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. In Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011.
- OECD (Organization for Economic Co-operation and Development). Guidelines for Testing of Chemical, Guideline 425. In Acute Oral Toxicity—Up-and-Down-Procedure (UDP); OECD Publishing: Paris, France, 2007; pp. 1–8. [Google Scholar]
- Malone, M.H.; Robichaud, R.C. A hippocratic screen for pure or crude drug materials. Lloydia 1962, 25, 320–332. [Google Scholar]
- Umar, A.; Imam, G.; Yimin, W.; Kerim, P.; Tohti, I.; Berke, B.; Moore, N. Antihypertensive effects of Ocimum basilicum L. (OBL) on blood pressure in renovascular hypertensive rats. Hypertens. Res. 2010, 33, 727–730. [Google Scholar] [CrossRef] [Green Version]
- Gasparotto-Junior, A.; Boffo, M.A.; Lourenço, E.L.B.; Stefanello, M.E.A.; Kassuya, C.A.L.; Marques, M.C.A. Natriuretic and diuretic effects of Tropaeolum majus (Tropaeolaceae) in rats. J. Ethnopharmacol. 2009, 122, 517–522. [Google Scholar] [CrossRef]
- Santos, R.; Krieger, E.M.; Greene, L.J. An improved fluorometric assay of rat serum and plasma converting enzyme. Hypertension 1985, 7, 244–252. [Google Scholar] [CrossRef] [Green Version]
- McGregor, D.D. The effect of sympathetic nerve stimulation on vasoconstrictor responses in perfused mesenteric blood vessels of the rat. J. Physiol. 1965, 177, 21–30. [Google Scholar] [CrossRef]
- Gao, R.; Yuan, Z.; Zhao, Z.; Gao, X. Mechanism of pyrogallol autoxidation and determination of superoxide dismutase enzyme activity. Bioelectrochem. Bioenerg. 1998, 45, 41–45. [Google Scholar] [CrossRef]
- Jiang, Z.-Y.; Hunt, J.V.; Wolff, S.P. Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoprotein. Anal. Biochem. 1992, 202, 384–389. [Google Scholar] [CrossRef]
- Beers, R.F.; Sizer, I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Estancial, C.S.; Rodrigues, R.L.; De Nucci, G.; Antunes, E.; Mónica, F.Z. Pharmacological characterization of the relaxation induced by the soluble guanylate cyclase activator, BAY 60-2770 in rabbit corpus cavernosum. BJU Int. 2015, 116, 657–664. [Google Scholar] [CrossRef] [Green Version]
- da Silva, D.; Okano, L.T.; Lopes, N.P.; De Oliveira, D.C. Flavanone glycosides from Bidens gardneri Bak. (Asteraceae). Phytochemistry 2013, 96, 418–422. [Google Scholar] [CrossRef]
- Clifford, M.N.; Johnston, K.L.; Knight, S.; Kuhnert, N. Hierarchical Scheme for LC-MSnIdentification of Chlorogenic Acids. J. Agric. Food Chem. 2003, 51, 2900–2911. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, Q.; Yang, Q.; Yan, X.; Feng, S.; Wang, Z. Comparison of Anthraquinones, Iridoid Glycosides and Triterpenoids in Morinda officinalis and Morinda citrifolia Using UPLC/Q-TOF-MS and Multivariate Statistical Analysis. Molecules 2019, 25, 160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Wei, J.; Yang, M. Simultaneous Analysis of Iridoid Glycosides and Anthraquinones in Morinda officinalis Using UPLC-QqQ-MS/MS and UPLC-Q/TOF-MSE. Molecules 2018, 23, 1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.-M.; Zhang, X.-L.; Xue, X.-Y.; Zhang, F.-F.; Xu, Q.; Liang, X.-M. Structural characterization of iridoid glucosides by ultra-performance liquid chromatography/electrospray ionization quadrupole time-of-flight tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2008, 22, 1941–1954. [Google Scholar] [CrossRef]
- de Villiers, A.; Venter, P.; Pasch, H. Recent advances and trends in the liquid-chromatography–mass spectrometry analysis of flavonoids. J. Chromatogr. A 2016, 1430, 16–78. [Google Scholar] [CrossRef]
- Abe, F.; Yamauchi, T. Trachelospermum. IV. Glycosides of 19.ALPHA.-hydroxyoleanane-type triterpenoids from Trachelospermum asiaticum. Chem. Pharm. Bull. 1987, 35, 1833–1838. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; He, X.; Zhao, Y.; Sun, Y.; Chen, X.; Cun, Y.; Huang, L.; Bai, Y.; Zheng, X. Phytochemistry, Pharmacology and Traditional Uses of Plants from the Genus Trachelospermum L. Molecules 2017, 22, 1406. [Google Scholar] [CrossRef]
- Kanwal, N.; Siddiqui, A.J.; Haq, F.U.; El-Seedi, H.R.; Musharraf, S.G. Two-stage mass spectrometry approach for the analysis of triterpenoid glycosides in Fagonia indica. RSC Adv. 2018, 8, 41023–41031. [Google Scholar] [CrossRef] [Green Version]
- Goldblatt, H. Studies on experimental hypertension: III. The production of persistent hypertension in monkeys (macaque) by renal ischemia. J. Exp. Med. 1937, 65, 671–675. [Google Scholar] [CrossRef] [Green Version]
- Samadian, F.; Dalili, N.; Jamalian, A. New Insights into Pathophysiology, Diagnosis, and Treatment of Renovascular Hypertension. Iran. J. Kidney Dis. 2017, 11, 79–89. [Google Scholar]
- Messerli, F.H.; Rimoldi, S.F.; Bangalore, S. The Transition from Hypertension to Heart Failure: Contemporary update. JACC Heart Fail. 2017, 5, 543–551. [Google Scholar] [CrossRef]
- Bastard, J.-P.; Fellahi, S.; Regeniter, A.; Capeau, J.; Ronco, P.; Plaisier, E. Aside from acute renal failure cases, are urinary markers of glomerular and tubular function useful in clinical practice? Clin. Biochem. 2019, 65, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Tse, G.; Korantzopoulos, P.; Letsas, K.P.; Ali-Hasan-Al-Saegh, S.; Kamel, H.; Li, G.; Lip, G.Y.; Liu, T. P-Wave Indices and Risk of Ischemic Stroke: A Systematic Review and Meta-Analysis. Stroke 2017, 48, 2066–2072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Postema, P.G.; Wilde, A.A.M. The Measurement of the QT Interval. Curr. Cardiol. Rev. 2014, 10, 287–294. [Google Scholar] [CrossRef]
- Kunisek, J.; Zaputović, L.; Cubranic, Z.; Kunisek, L.; Butorac, M.Z.; Lukin-Eskinja, K.; Karlavaris, R. Influence of the left ventricular types on QT intervals in hypertensive patients. Anatol. J. Cardiol. 2015, 15, 33–39. [Google Scholar] [CrossRef]
- Panza, J.A.; Quyyumi, A.A.; Brush, J.E.; Epstein, S.E. Abnormal Endothelium-Dependent Vascular Relaxation in Patients with Essential Hypertension. N. Engl. J. Med. 1990, 323, 22–27. [Google Scholar] [CrossRef]
- Panza, J.A.; García, C.E.; Kilcoyne, C.M.; Quyyumi, A.A.; Cannon, R.O. Impaired Endothelium-Dependent Vasodilation in Patients with Essential Hypertension: Evidence that nitric oxide abnormality is not localized to a single signal transduction pathway. Circulation 1995, 91, 1732–1738. [Google Scholar] [CrossRef] [PubMed]
- Sander, M.; Chavoshan, B.; Victor, R.G. A Large Blood Pressure–Raising Effect of Nitric Oxide Synthase Inhibition in Humans. Hypertension 1999, 33, 937–942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doughan, A.K.; Harrison, D.G.; Dikalov, S.I. Molecular Mechanisms of Angiotensin II–Mediated Mitochondrial dysfunction: Linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ. Res. 2008, 102, 488–496. [Google Scholar] [CrossRef] [Green Version]
- Harrison, D.G.; Gongora, M.C. Oxidative Stress and Hypertension. Med. Clin. N. Am. 2009, 93, 621–635. [Google Scholar] [CrossRef] [PubMed]
- Widlansky, M.; Gokce, N.; Keaney, J.; Vita, J. The clinical implications of endothelial dysfunction. J. Am. Coll. Cardiol. 2003, 42, 1149–1160. [Google Scholar] [CrossRef] [Green Version]
- Hanif, K.; Bid, H.K.; Konwar, R. Reinventing the ACE inhibitors: Some old and new implications of ACE inhibition. Hypertens. Res. 2009, 33, 11–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hocht, C.; Bertera, F.M.; Del Mauro, J.S.; Plantamura, Y.S.; Taira, C.A.; Polizio, A.H. What is the Real Efficacy of Beta-Blockers for the Treatment of Essential Hypertension? Curr. Pharm. Des. 2017, 23, 4658–4677. [Google Scholar] [CrossRef] [PubMed]
- Bondonno, C.P.; Croft, K.D.; Ward, N.; Considine, M.J.; Hodgson, J.M. Dietary flavonoids and nitrate: Effects on nitric oxide and vascular function. Nutr. Rev. 2015, 73, 216–235. [Google Scholar] [CrossRef]
- Pinheiro, L.C.; Tanus-Santos, J.E.; Castro, M.M. The potential of stimulating nitric oxide formation in the treatment of hypertension. Expert Opin. Ther. Targets 2017, 21, 543–556. [Google Scholar] [CrossRef]
- Montenegro, M.F.; Amaral, J.H.; Pinheiro, L.C.; Sakamoto, E.K.; Ferreira, G.C.; Reis, R.I.; Marçal, D.M.; Pereira, R.P.; Tanus-Santos, J.E. Sodium nitrite downregulates vascular NADPH oxidase and exerts antihypertensive effects in hypertension. Free. Radic. Biol. Med. 2011, 51, 144–152. [Google Scholar] [CrossRef] [Green Version]
- Hügel, H.M.; Jackson, N.; May, B.; Zhang, A.L.; Xue, C.C. Polyphenol protection and treatment of hypertension. Phytomedicine 2016, 23, 220–231. [Google Scholar] [CrossRef]
- Siasos, G.; Tousoulis, D.; Tsigkou, V.; Kokkou, E.; Oikonomou, E.; Vavuranakis, M.; Basdra, E.; Papavassiliou, A.; Stefanadis, C. Flavonoids in Atherosclerosis: An Overview of Their Mechanisms of Action. Curr. Med. Chem. 2013, 20, 2641–2660. [Google Scholar] [CrossRef]
- Khoo, N.K.; White, C.R.; Pozzo-Miller, L.; Zhou, F.; Constance, C.; Inoue, T.; Patel, R.; Parks, D.A. Dietary flavonoid quercetin stimulates vasorelaxation in aortic vessels. Free. Radic. Biol. Med. 2010, 49, 339–347. [Google Scholar] [CrossRef] [Green Version]
- Yao, L.H.; Jiang, Y.M.; Shi, J.; Datta, N.; Singanusong, R.; Chen, S.S. Flavonoids in Food and Their Health Benefits. Plant Foods Hum. Nutr. 2004, 59, 113–122. [Google Scholar] [CrossRef]
- Chaudhry, M.A.; Mushtaq, M.N.; Bukhari, I.A.; Assiri, A.M. Ipomoea hederacea Jacq.: A plant with promising antihypertensive and cardio-protective effects. J. Ethnopharmacol. 2021, 268, 113584. [Google Scholar] [CrossRef]
- Wang, C.; Pan, Y.; Zhang, Q.-Y.; Wang, F.-M.; Kong, L.-D. Quercetin and Allopurinol Ameliorate Kidney Injury in STZ-Treated Rats with Regulation of Renal NLRP3 Inflammasome Activation and Lipid Accumulation. PLoS ONE 2012, 7, e38285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Hiwatashi, K.; Shirakawa, H.; Hori, K.; Yoshiki, Y.; Suzuki, N.; Hokari, M.; Komai, M.; Takahashi, S. Reduction of Blood Pressure by Soybean Saponins, Renin Inhibitors from Soybean, in Spontaneously Hypertensive Rats. Biosci. Biotechnol. Biochem. 2010, 74, 2310–2312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhiouani, H.; Settaf, A.; Lyoussi, B.; Cherrah, Y.; Lacaille-Dubois, M.-A.; Hassar, M. Effects of saponins from Herniaria glabra on blood pressure and renal function in spontaneously hypertensive rats. Therapie 2000, 54, 735–739. [Google Scholar]






| Peak | RT (min) | UV(nm) | MF | Positive Ion Mode (m/z) | Negative Ion Mode (m/z) | Compound | Metabolite Class | % | |
|---|---|---|---|---|---|---|---|---|---|
| MS [M+H]+ | MS [M-H]− | MS/MS | |||||||
| 1 | 1.1 | - | C7H12O6 | 193.0696 | 191.0534 | 191 (bp), 173 | Quinic acid | Carboxylic acid | 18.34 |
| 2 | 1.1 | - | C12H22O11 | - | 341.1095 | 191(bp) | di-O-hexoside | Primary metabolite | 4.36 |
| 3 | 1.5 | - | C6H8O7 | 193.0367 | 191.0209 | - | Citric acid | Carboxylic acid | 1.31 |
| 4 | 2.7 | - | C16H20O10 | 373.1113 | 371.0991 | 191, 173 (bp) | Glycosylated iridoid | Iridoid | 0.31 |
| 5 | 5.9 | 295 sh, 325 | C16H18O9 | 355.1006 | 353.0873 | 191 (bp), 179 | 3-O-E-caffeoylquinic acid st | Chlorogenic acid | 3.66 |
| 6 | 9.9 | - | C18H24O12 | - | 431.1195 | 251, 165 (bp) | Asperulosidic acid | Iridoid | 4.67 |
| 7 | 10.5 | 295 sh, 325 | C16H18O9 | 355.1021 | 353.0873 | 191 (bp), 179 | 5-O-E-caffeoylquinic acid st | Chlorogenic acid | 2.48 |
| 8 | 11.3 | - | C18H22O11 | 415.1223 | 413.1084 | 191, 147 (bp) | Asperuloside | Iridoid | 11.98 |
| 9 | 18.7 | 265, 355 | C27H30O16 | 611.1595 | 609.1472 | 300 (bp), 301, 271, 255, 243 | O-hexosyl-deoxyhexosyl quercetin | Flavonol | 3.87 |
| 10 | 20.0 | - | C24H42O11 | 507.2822 | 505.2655 | 191 (bp) | Unknown | - | 1.81 |
| 11 | 20.8 | 265, 346 | C27H30O15 | 595.1644 | 593.1521 | 327, 285 (bp), 284, 255, 277, 162 | O-hexosyl-deoxyhexosyl kaempferol | Flavonol | 9.26 |
| 12 | 25.0 | - | C36H58O12 | 683.3967 | 681.3823 | 519 (bp), 501, 407, 207 | Trachelosperoside B-1 or E-1 | Glycosylated triterpene | 14.40 |
| 13 | 25.1 | - | C36H58O12 | 683.3981 | 681.3832 | 519 (bp), 501, 489, 457, 407, 207 | Trachelosperoside B-1 or E-1 | Glycosylated triterpene | 12.84 |
| 14 | 26.6 | - | C36H58O12 | 667.4027 | 665.3757 | 503 (bp), 441, 409 | Glycosylated triterpene (Arjunoglucoside I) | Glycosylated triterpene | 2.25 |
| 15 | 32.1 | - | C53H84O23 | 1089.5298 | 1087.5303 | 925 (bp), 793, 631, 613, 569, 469, 455, 353, 161 | O-glucoronyl-hexosyl-pentosyl O-hexosyl triterpene | Triperpenoid saponin | 2.09 |
| 16 | 32.2 | - | C48H76O19 | 957.5026 | 955.4914 | 793 (bp), 749, 731, 631, 613, 569, 551, 455, 337, 179 | O-glucoronyl-hexosyl O-hexosyl triterpene | Triperpenoid saponin | 6.37 |
| Group | Urinary Volume (mL/100 g/8 h) | ElNa+ (µEq/min/100 g) | Elk+ (µEq/min/100 g) | ElCa2+ (µEq/min/100 g) | ElCl− (µEq/min/100 g) | pH | Density |
|---|---|---|---|---|---|---|---|
| Sham | 3.771 ± 0.188 | 1.694 ± 0.108 | 0.571 ± 0.029 | 0.021 ± 0.001 | 1.902 ± 0.113 | 8.5 ± 0.04 | 1015 ± 0.84 |
| C- | 4.517 ± 0.219 | 0.826 ± 0.006 b | 0.546 ± 0.010 | 0.012 ± 0.002 b | 0.981± 0.016 b | 8.3 ± 0.02 | 1010 ± 0.28 b |
| AERV 30 (mg/kg) | 3.665 ± 0.021 | 0.887 ± 0.087 b | 0.615 ± 0.032 | 0.007 ± 0.001 b | 1.021 ± 0.029 b | 8.7 ± 0.13 | 1015 ± 0.42 |
| AERV (100 mg/kg) | 3.831 ± 0.445 | 0.878 ± 0.057 b | 0.614 ± 0.045 | 0.012 ± 0.001 b | 1.026 ± 0.074 b | 8.7 ± 0.15 | 1015 ± 0.42 |
| AERV (300 mg/kg) | 3.279 ± 0.129 | 1.473 ± 0.054 a | 0.554 ±0.018 | 0.029 ± 0.001 a | 1.653 ± 0.025 a | 8.6 ± 0.05 | 1016 ± 1.52 |
| MPL (25 mg/kg) | 4.378 ± 0.223 | 1.651 ± 0.082 a | 0.520 ± 0.069 | 0.022 ± 0.003 a | 1.791 ± 0.038 a | 8.1 ± 0.17 | 1014 ± 1.15 |
| Group | Urinary Volume (mL/100 g/8 h) | ElNa+ (µEq/min/100 g) | Elk+ (µEq/min/100 g) | ElCa2+ (µEq/min/100 g) | ElCl− (µEq/min/100 g) | pH | Density |
|---|---|---|---|---|---|---|---|
| Sham | 5.272 ± 0.224 | 1.207 ± 0.087 | 0.591 ± 0.031 | 0.011 ± 0.001 | 1.348 ± 0.194 | 7.8 ± 0.06 | 1007 ± 0.42 |
| C- | 3.862 ± 0.613 b | 1.261 ± 0.124 | 0.597 ± 0.034 | 0.013 ± 0.003 | 1.344 ± 0.127 | 7.7 ± 0.05 | 1013 ± 0.57 b |
| AERV 30 (mg/kg) | 3.661 ± 0.208 b | 1.178 ± 0.026 | 0.570 ± 0.011 | 0.009 ± 0.001 | 1.319 ± 0.122 | 7.8 ± 0.07 | 1017 ± 1.11 b |
| AERV (100 mg/kg) | 2.939 ± 0.396 b | 0.906 ± 0.096 | 0.438 ± 0.022 | 0.009 ± 0.001 | 0.971 ± 0.106 | 7.8 ± 0.11 | 1017 ± 1.83 b |
| AERV (300 mg/kg) | 3.513 ± 0.334 b | 0.702 ± 0.268 | 0.396 ± 0.099 | 0.008 ± 0.004 | 0.803 ± 0.314 | 7.6 ± 0.14 | 1006 ± 2.30 a |
| MPL (25 mg/kg) | 4.043 ± 0.333 | 1.181 ± 0.140 | 0.540 ± 0.078 | 0.009 ± 0.006 | 1.295 ± 0.182 | 7.7 ± 0.02 | 1008 ± 0.57 a |
| Group | Urinary Volume (mL/100 g/8 h) | ElNa+ (µEq/min/100 g) | Elk+ (µEq/min/100 g) | ElCa2+ (µEq/min/100 g) | ElCl− (µEq/min/100 g) | pH | Density |
|---|---|---|---|---|---|---|---|
| Sham | 4.319 ± 0.399 | 1.363 ± 0.101 | 0.635 ± 0.057 | 0.034 ± 0.002 | 1.853 ± 0.124 | 7.8 ± 0.02 | 1016 ± 0.96 |
| C- | 3.389 ± 0.032 b | 0.846 ± 0.049 b | 0.809 ± 0.101 | 0.017 ± 0.003 b | 1.376 ± 0.096 b | 7.8 ± 0.14 | 1018 ± 0.80 |
| AERV 30 (mg/kg) | 3.139 ± 0.186 b | 0.893 ± 0.028 b | 0.641 ± 0.049 | 0.018 ± 0.002 b | 1.340 ± 0.016 b | 7.7 ± 0.20 | 1018 ± 1.23 |
| AERV (100 mg/kg) | 2.794 ± 0.407 b | 1.193 ± 0.141 | 0.658 ± 0.076 | 0.012 ± 0.002 b | 1.003 ± 0.153 b | 7.6 ± 0.16 | 1015 ± 0.73 |
| AERV (300 mg/kg) | 4.350 ± 0.164 a | 1.319 ± 0.023 a | 0.796 ± 0.129 | 0.040 ± 0.009 a | 1.807 ± 0.082 a | 7.7 ± 0.02 | 1016 ± 0.28 |
| MPL (25 mg/kg) | 3.975 ± 0.050 a | 1.169 ± 0.118 | 0.652 ± 0.009 | 0.009 ± 0.002 b | 1.190 ± 0.134 b | 7.6 ± 0.08 | 1017 ± 1.15 |
| Group | Urinary Volume (mL/100 g/8 h) | ElNa+ (µEq/min/100 g) | Elk+ (µEq/min/100 g) | ElCa2+ (µEq/min/100g) | ElCl− (µEq/min/100 g) | pH | Density |
|---|---|---|---|---|---|---|---|
| Sham | 5.111 ± 0.117 | 1.319 ± 0.144 | 0.667 ± 0.030 | 0.020 ± 0.001 | 1.486 ± 0.041 | 7.2 ± 0.12 | 1012 ± 0.55 |
| C- | 3.209 ± 0.422 b | 1.157 ± 0.182 | 0.419 ± 0.069 b | 0.007 ± 0.004 b | 1.279 ± 0.196 | 7.3 ± 0.12 | 1018 ± 1.15 b |
| AERV 30 (mg/kg) | 3.143 ± 0.040 b | 1.192 ± 0.011 | 0.523 ± 0.015 | 0.027 ± 0.001 a | 1.422 ± 0.015 | 7.6 ± 0.17 | 1022 ± 0.73 b |
| AERV (100 mg/kg) | 3.712 ± 0.099 b | 1.295 ± 0.028 | 0.565 ± 0.013 | 0.018 ± 0.002 a | 1.417 ± 0.052 | 7.6 ± 0.19 | 1017 ± 1.11 b |
| AERV (300 mg/kg) | 4.387 ± 0.264 a | 0.899 ± 0.169 | 0.781 ± 0.013 a | 0.041 ± 0.002 a | 1.344 ± 0.060 | 7.3 ± 0.11 | 1012 ± 1.73 a |
| MPL (25 mg/kg) | 3.062 ± 0.022 b | 0.880 ± 0.182 | 0.495 ± 0.099 | 0.009 ± 0.003 b | 0.892 ± 0.213 b | 7.3 ± 0.34 | 1019 ± 0.28 b |
| Group | Urinary Volume (mL/100 g/8 h) | ElNa+ (µEq/min/100 g) | Elk+ (µEq/min/100 g) | ElCa2+ (µEq/min/100 g) | ElCl− (µEq/min/100 g) | pH | Density |
|---|---|---|---|---|---|---|---|
| Sham | 4.196 ± 0.098 | 1.348 ± 0.086 | 0.639 ± 0.026 | 0.063 ± 0.005 | 1.532 ± 0.081 | 7.4 ± 0.17 | 1015 ± 0.91 |
| C- | 3.350 ± 0.064 b | 0.860 ± 0.022 b | 0.680 ± 0.010 | 0.020 ± 0.001 b | 1.039 ± 0.027 b | 7.6 ± 0.18 | 1019 ± 0.57 b |
| AERV 30 (mg/kg) | 3.504 ± 0.142 b | 1.311 ± 0.102 | 0.681 ± 0.045 | 0.022 ± 0.003 b | 1.527 ± 0.085 a | 7.7 ± 0.17 | 1018 ± 0.42 b |
| AERV (100 mg/kg) | 3.449 ± 0.125 b | 1.296 ± 0.071 | 0.608 ± 0.001 | 0.015 ± 0.001 b | 1.413 ± 0.077 a | 7.6 ± 0.14 | 1014 ± 0.55 a |
| AERV (300 mg/kg) | 3.927 ± 0.192 a | 1.289 ± 0.079 | 0.667 ± 0.026 | 0.037 ± 0.016 | 1.465 ± 0.098 a | 7.5 ± 0.12 a | 1015 ± 0.57 a |
| MPL (25 mg/kg) | 3.433 ± 0.215 b | 1.274 ± 0.159 | 0.678 ± 0.106 | 0.016 ± 0.003 b | 1.165 ± 0.171 | 7.3 ± 0.11 | 1017 ± 2.30 |
| Group | Na+ (mmol/L) | K+ (mmol/L) | Urea (mg/dL) | Creatinine (mg/dL) | ACE Activity (mmol/min/mL) |
|---|---|---|---|---|---|
| Sham | 126.5 ± 1.21 | 5.81 ± 0.22 | 57.32 ± 0.93 | 0.32 ± 0.02 | 85 ± 10.10 |
| C- | 130.2 ± 1.86 | 6.15 ± 0.35 | 85.80 ± 2.75 b | 0.67 ± 0.03 b | 155 ± 13.22 b |
| AERV (30 mg/kg) | 131.7 ± 1.99 | 6.22 ± 0.12 | 77.08 ± 1.56 b | 0.59 ± 0.02 b | 121 ± 9.11 b |
| AERV (100 mg/kg) | 129.9 ± 1.23 | 6.13 ± 0.51 | 56.63 ± 1.17 | 0.33 ± 0.01 | 98 ± 10.24 a |
| AERV (300 mg/kg) | 126.0 ± 2.03 | 5.77 ± 0.22 | 57.36 ± 1.23 | 0.35 ± 0.03 | 90 ± 12.11 a |
| MPL (25 mg/kg) | 129.1 ± 1.92 | 5.41 ± 0.21 | 58.22 ± 3.21 | 0.37 ± 0.05 | 100 ± 11.08 a |
| Parameter | Sham | C- | AERV (30 mg/kg) | AERV (100 mg/kg) | AERV (300 mg/kg) | MPL (25 mg/kg) |
|---|---|---|---|---|---|---|
| Heart | ||||||
| SOD | 40.57 ± 1.61 | 30.54 ± 0.61 b | 34.89 ± 1.49 b | 38.03 ± 1.23 a | 40.46 ± 1.40 a | 34.84 ± 1.84 b |
| Cat | 45.10 ± 4.33 | 25 ± 5.15 b | 52 ± 6.68 a | 45 ± 3.61 a | 55 ± 9.21 a | 28 ± 75.35 b |
| LPO | 16.48 ± 0.97 | 25.17 ± 0.35 b | 18.10 ± 0.65 | 17.99 ± 0.73 | 17.42 ± 0.59 a | 17.93 ± 0.37 |
| Aorta | ||||||
| SOD | 71.73 ± 5.00 | 62.37 ± 2.55 | 67.48 ± 1.36 | 68.61 ± 1.37 | 69.64 ± 1.73 | 62.83 ± 1.53 |
| Cat | 61 ± 3.79 | 41 ± 4.58 b | 52 ± 5.79 | 58 ± 4.38 a | 77 ± 4.25 a | 49 ± 4.31 b |
| LPO | 6.90 ± 0.53 | 8.54 ± 0.38 b | 7.61 ± 0.41 | 7.29 ± 0.41 | 6.91 ± 0.47 a | 7.22 ± 0.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paulin, F.V.; Palozi, R.A.C.; Lorençone, B.R.; Macedo, A.L.; Guarnier, L.P.; Tirloni, C.A.S.; Romão, P.V.M.; Gasparotto Junior, A.; Silva, D.B. Prolonged Administration of Rudgea viburnoides (Cham.) Benth. Prevents Impairment of Redox Status, Renal Dysfunction, and Cardiovascular Damage in 2K1C-Hypertensive Rats by Inhibiting ACE Activity and NO-GMPC Pathway Activation. Pharmaceutics 2021, 13, 1579. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics13101579
Paulin FV, Palozi RAC, Lorençone BR, Macedo AL, Guarnier LP, Tirloni CAS, Romão PVM, Gasparotto Junior A, Silva DB. Prolonged Administration of Rudgea viburnoides (Cham.) Benth. Prevents Impairment of Redox Status, Renal Dysfunction, and Cardiovascular Damage in 2K1C-Hypertensive Rats by Inhibiting ACE Activity and NO-GMPC Pathway Activation. Pharmaceutics. 2021; 13(10):1579. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics13101579
Chicago/Turabian StylePaulin, Fernanda Viana, Rhanany Alan Calloi Palozi, Bethânia Rosa Lorençone, Arthur Ladeira Macedo, Lucas Pires Guarnier, Cleide Adriane Signor Tirloni, Paulo Vitor Moreira Romão, Arquimedes Gasparotto Junior, and Denise Brentan Silva. 2021. "Prolonged Administration of Rudgea viburnoides (Cham.) Benth. Prevents Impairment of Redox Status, Renal Dysfunction, and Cardiovascular Damage in 2K1C-Hypertensive Rats by Inhibiting ACE Activity and NO-GMPC Pathway Activation" Pharmaceutics 13, no. 10: 1579. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics13101579