Development and Evaluation of Amorphous Oral Thin Films Using Solvent-Free Processes: Comparison between 3D Printing and Hot-Melt Extrusion Technologies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
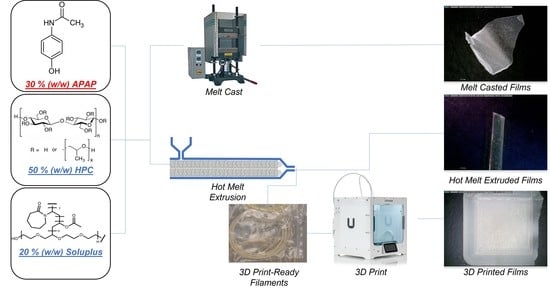
2.2. Preparation of the Oral Dispersible Films
2.2.1. Melt Casting Methods
2.2.2. Melt-Extruded Films
2.2.3. 3D Printed Films
2.3. Assessment of Films Morphology
2.4. Texture Analysis of the Films
2.5. Solid States Analysis
2.5.1. Thermogravimetric Analysis (TGA)
2.5.2. Differential Scanning Calorimetry (DSC)
2.5.3. Powder X-ray Diffraction (PXRD)
2.5.4. Hot-Stage Polarized Light Microscopy (PLM)
2.6. Raman Spectroscopy and Raman Mapping
2.7. Disintegration Studies and In Vitro Drug Release Study of the Films in Simulated Saliva
2.8. In Vitro Drug Release Study
3. Results and Discussion
3.1. Solid States Analyses
3.1.1. Thermogravimetric Analysis (TGA)
3.1.2. Differential Scanning Calorimetry (DSC)
3.1.3. Powder X-ray Diffraction Analysis of the Crystallinity
3.1.4. Hot-Staged Polarized Microscopy (PLM)
3.2. Raman Analysis
3.2.1. Impact of the Preparation Methods on the Films
3.2.2. Appearance of the Films
3.2.3. Amorphization of the APAP
3.2.4. Process Quality
3.3. Texture Analysis
3.4. Film Disintegration
- (1).
- The rate of forming hydrocolloids is faster than the rate of dissolution or erosion. The film will completely form hydrocolloids where diffusion dominated the drug release, then the erosion or the dissolution of the polymeric matrix dominates the drug release.
- (2).
- The rate of forming hydrocolloids is slower than or equal to the rate of dissolution or erosion. Both the diffusion and erosion/dissolution will dominate the drug release till the end.
3.5. Drug Release from the Films
3.5.1. Dissolution in Simulated Saliva
3.5.2. Dissolution in SIF
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schar, M.S.; Omari, T.I.; Fraser, R.J.; Bersten, A.D.; Bihari, S. Disordered Swallowing Associated with Prolonged Oral Endotracheal Intubation in Critical Illness. Intensive Care Med. 2020, 46, 140–142. [Google Scholar] [CrossRef] [PubMed]
- Carnaby-Mann, G.; Crary, M. Pill Swallowing by Adults with Dysphagia. Arch. Otolaryngol. Head Neck Surg. 2005, 131, 970–975. [Google Scholar] [CrossRef] [Green Version]
- He, M.; Zhu, L.; Yang, N.; Li, H.; Yang, Q. Recent Advances of Oral Film as Platform for Drug Delivery. Int. J. Pharm. 2021, 604, 120759. [Google Scholar] [CrossRef] [PubMed]
- Lucci, N.; McConnell, C.; Biddle, C. Understanding Normal and Abnormal Swallowing: Patient Safety Considerations for the Perianesthetic Nurse. J. PeriAnesthesia Nurs. 2018, 33, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Vaezi, M.F. Dysphagia in the Elderly. Gastroenterol. Hepatol. 2013, 9, 784. [Google Scholar]
- Shah, R.B.; Collier, J.S.; Sayeed, V.A.; Bryant, A.; Habib, M.J.; Khan, M.A. Tablet Splitting of a Narrow Therapeutic Index Drug: A Case with Levothyroxine Sodium. AAPS PharmSciTech 2010, 11, 1359–1367. [Google Scholar] [CrossRef] [Green Version]
- Zhao, N.; Zidan, A.; Tawakkul, M.; Sayeed, V.A.; Khan, M. Tablet Splitting: Product Quality Assessment of Metoprolol Succinate Extended Release Tablets. Int. J. Pharm. 2010, 401, 25–31. [Google Scholar] [CrossRef]
- Martin, L.R.; Williams, S.L.; Haskard, K.B.; DiMatteo, M.R. The Challenge of Patient Adherence. Ther. Clin. Risk Manag. 2005, 1, 189–199. [Google Scholar]
- Yir-Erong, B.; Bayor, M.T.; Ayensu, I.; Gbedema, S.Y.; Boateng, J.S. Oral Thin Films as a Remedy for Noncompliance in Pediatric and Geriatric Patients. Ther. Deliv. 2019, 10, 443–464. [Google Scholar] [CrossRef]
- Pimparade, M.B.; Vo, A.; Maurya, A.S.; Bae, J.; Morott, J.T.; Feng, X.; Kim, D.W.; Kulkarni, V.I.; Tiwari, R.; Vanaja, K.; et al. Development and Evaluation of an Oral Fast Disintegrating Anti-Allergic Film Using Hot-Melt Extrusion Technology. Eur. J. Pharm. Biopharm. 2017, 119, 81–90. [Google Scholar] [CrossRef]
- Akil, A.; Agashe, H.; Dezzutti, C.S.; Moncla, B.J.; Hillier, S.L.; Devlin, B.; Shi, Y.; Uranker, K.; Rohan, L.C. Formulation and Characterization of Polymeric Films Containing Combinations of Antiretrovirals (ARVs) for HIV Prevention. Pharm. Res. 2015, 32, 458–468. [Google Scholar] [CrossRef] [Green Version]
- Castro, P.M.; Sousa, F.; Magalhães, R.; Ruiz-Henestrosa, V.M.P.; Pilosof, A.M.R.; Madureira, A.R.; Sarmento, B.; Pintado, M.E. Incorporation of Beads into Oral Films for Buccal and Oral Delivery of Bioactive Molecules. Carbohydr. Polym. 2018, 194, 411–421. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.; Niu, M.; Hu, F.; Lu, Y.; Qi, J.; Yin, Z.; Wu, W. Integrity and Stability of Oral Liposomes Containing Bile Salts Studied in Simulated and Ex Vivo Gastrointestinal Media. Int. J. Pharm. 2013, 441, 693–700. [Google Scholar] [CrossRef]
- FDA. Onsolis (Fentanyl Buccal Soluble Film) Information. Available online: https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/onsolis-fentanyl-buccal-soluble-film-information (accessed on 21 September 2021).
- FDA; CDER. Highlights of Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/022266s017s018lbl.pdf (accessed on 22 September 2021).
- Caughman, C.Y.; Factor, S. A Critical Review of Apomorphine Hydrochloride Sublingual Film for the Treatment of Parkinson’s Disease ‘OFF’ Episodes. Expert Rev. Neurother. 2020, 21, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Marimuthu, P.; Schätzlein, A.G. Biological Barriers: Transdermal, Oral, Mucosal, Blood Brain Barrier, and the Blood Eye Barrier. Fundam. Pharm. Nanosci. 2013, 301–336. [Google Scholar] [CrossRef]
- Thakkar, R.; Thakkar, R.; Pillai, A.; Ashour, E.A.; Repka, M.A. Systematic Screening of Pharmaceutical Polymers for Hot Melt Extrusion Processing: A Comprehensive Review. Int. J. Pharm. 2020, 576, 118989. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Chen, H.; Huang, Z.; Zhu, J.; Wan, F.; Peng, T.; Pan, X.; Huang, Y.; Wu, C. Taste-Masking and Colloidal-Stable Cubosomes Loaded with Cefpodoxime Proxetil for Pediatric Oral Delivery. Int. J. Pharm. 2020, 575, 118875. [Google Scholar] [CrossRef]
- Domokos, A.; Balogh, A.; Dénes, D.; Nyerges, G.; Ződi, L.; Farkas, B.; Marosi, G.; Nagy, Z.K. Continuous Manufacturing of Orally Dissolving Webs Containing a Poorly Soluble Drug via Electrospinning. Eur. J. Pharm. Sci. 2019, 130, 91–99. [Google Scholar] [CrossRef]
- Bülbül, E.Ö.; Mesut, B.; Cevher, E.; Öztaş, E.; Özsoy, Y. Product Transfer from Lab-Scale to Pilot-Scale of Quetiapine Fumarate Orodispersible Films Using Quality by Design Approach. J. Drug Deliv. Sci. Technol. 2019, 54, 101358. [Google Scholar] [CrossRef]
- Speer, I.; Lenhart, V.; Preis, M.; Breitkreutz, J. Prolonged Release from Orodispersible Films by Incorporation of Diclofenac-Loaded Micropellets. Int. J. Pharm. 2019, 554, 149–160. [Google Scholar] [CrossRef]
- Zhou, Y.; Horne, D.; Steele, T.W.J. Tuning Drug Release via Twin Screw Extrusion in Polyester Films. J. Pharm. Sci. 2019, 108, 2430–2437. [Google Scholar] [CrossRef]
- Eleftheriadis, G.K.; Monou, P.K.; Bouropoulos, N.; Boetker, J.; Rantanen, J.; Jacobsen, J.; Vizirianakis, I.S.; Fatouros, D.G. Fabrication of Mucoadhesive Buccal Films for Local Administration of Ketoprofen and Lidocaine Hydrochloride by Combining Fused Deposition Modeling and Inkjet Printing. J. Pharm. Sci. 2020, 109, 2757–2766. [Google Scholar] [CrossRef]
- Drašković, M.; Turković, E.; Vasiljević, I.; Trifković, K.; Cvijić, S.; Vasiljević, D.; Parojčić, J. Comprehensive Evaluation of Formulation Factors Affecting Critical Quality Attributes of Casted Orally Disintegrating Films. J. Drug Deliv. Sci. Technol. 2020, 56, 101614. [Google Scholar] [CrossRef]
- Bharti, K.; Mittal, P.; Mishra, B. Formulation and Characterization of Fast Dissolving Oral Films Containing Buspirone Hydrochloride Nanoparticles Using Design of Experiment. J. Drug Deliv. Sci. Technol. 2019, 49, 420–432. [Google Scholar] [CrossRef]
- Speer, I.; Preis, M.; Breitkreutz, J. Prolonged Drug Release Properties for Orodispersible Films by Combining Hot-Melt Extrusion and Solvent Casting Methods. Eur. J. Pharm. Biopharm. 2018, 129, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Feng, X.; Patil, H.; Tiwari, R.V.; Repka, M.A. Coupling 3D Printing with Hot-Melt Extrusion to Produce Controlled-Release Tablets. Int. J. Pharm. 2017, 519, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Vo, A.Q.; Feng, X.; Bandari, S.; Repka, M.A. Pharmaceutical Additive Manufacturing: A Novel Tool for Complex and Personalized Drug Delivery Systems. AAPS PharmSciTech 2018, 19, 3388–3402. [Google Scholar] [CrossRef] [PubMed]
- Elbl, J.; Gajdziok, J.; Kolarczyk, J. 3D Printing of Multilayered Orodispersible Films with In-Process Drying. Int. J. Pharm. 2020, 575, 118883. [Google Scholar] [CrossRef] [PubMed]
- Marques, M.R.C.; Loebenberg, R.; Almukainzi, M. Simulated Biological Fluids with Possible Application in Dissolution Testing. Dissolution Technol. 2011, 18, 15–28. [Google Scholar] [CrossRef]
- Gupta, S.S.; Solanki, N.; Serajuddin, A.T.M.M. Investigation of Thermal and Viscoelastic Properties of Polymers Relevant to Hot Melt Extrusion, IV: AffinisolTM HPMC HME Polymers. AAPS PharmSciTech 2016, 17, 148–157. [Google Scholar] [CrossRef]
- Zhang, J.; Thakkar, R.; Zhang, Y.; Maniruzzaman, M. Microwave Induced Dielectric Heating for the On-Demand Development of Indomethacin Amorphous Solid Dispersion Tablets. J. Drug Deliv. Sci. Technol. 2020, 61, 102109. [Google Scholar] [CrossRef]
- Kauffman, J.F.; Batykefer, L.M.; Tuschel, D.D. Raman Detected Differential Scanning Calorimetry of Polymorphic Transformations in Acetaminophen. J. Pharm. Biomed. Anal. 2008, 48, 1310–1315. [Google Scholar] [CrossRef] [PubMed]
- Nanubolu, J.B.; Burley, J.C. Investigating the Recrystallization Behavior of Amorphous Paracetamol by Variable Temperature Raman Studies and Surface Raman Mapping. Mol. Pharm. 2012, 9, 1544–1558. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Zhang, J.; Repka, M.; Chen, R. Characterization of the Solid Physical State of API and Its Distribution in Pharmaceutical Hot Melt Extrudates Using Terahertz Raman Imaging. AAPS PharmSciTech 2019, 20, 62. [Google Scholar] [CrossRef] [PubMed]
- Bala, R.; Pawar, P.; Khanna, S.; Arora, S. Orally Dissolving Strips: A New Approach to Oral Drug Delivery System. Int. J. Pharm. Investig. 2013, 3, 67–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kushch, V.I.; Dub, S.N.; Litvin, P.M. Determination of the Young Modulus from Elastic Section of the Berkovich Indenter Loading Curve. J. Superhard Mater. 2007, 29, 228–234. [Google Scholar] [CrossRef]
- Higuchi, T. Rate of Release of Medicaments from Ointment Bases Containing Drugs in Suspension. J. Pharm. Sci. 1961, 50, 874–875. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A Simple Equation for Description of Solute Release II. Fickian and Anomalous Release from Swellable Devices. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Peppas, N.A.; Sahlin, J.J. A Simple Equation for the Description of Solute Release. III. Coupling of Diffusion and Relaxation. Int. J. Pharm. 1989, 57, 169–172. [Google Scholar] [CrossRef]
- Bruschi, M.L. Mathematical Models of Drug Release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Woodhead Publishing: Cambridge, UK, 2015; pp. 63–86. [Google Scholar] [CrossRef]
- Paul, D.R. Elaborations on the Higuchi Model for Drug Delivery. Int. J. Pharm. 2011, 418, 13–17. [Google Scholar] [CrossRef]
- Higuchi, T. Mechanism of Sustained-action Medication. Theoretical Analysis of Rate of Release of Solid Drugs Dispersed in Solid Matrices. J. Pharm. Sci. 1963, 52, 1145–1149. [Google Scholar] [CrossRef]
- Brannon-Peppas, L.; Peppas, N.A. Equilibrium Swelling Behavior of PH-Sensitive Hydrogels. Chem. Eng. Sci. 1991, 46, 715–722. [Google Scholar] [CrossRef]
- Siepmann, J.; Peppas, N.A. Modeling of Drug Release from Delivery Systems Based on Hydroxypropyl Methylcellulose (HPMC). Adv. Drug Deliv. Rev. 2012, 64, 163–174. [Google Scholar] [CrossRef]
- Peppas, N.A.; Narasimhan, B. Mathematical Models in Drug Delivery: How Modeling Has Shaped the Way We Design New Drug Delivery Systems. J. Control. Release 2014, 190, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, W.; Vo, A.Q.; Feng, X.; Ye, X.; Kim, D.W.; Repka, M.A. Hydroxypropyl Methylcellulose-Based Controlled Release Dosage by Melt Extrusion and 3D Printing: Structure and Drug Release Correlation. Carbohydr. Polym. 2017, 177, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, J.; Siepmann, F. Modeling of Diffusion Controlled Drug Delivery. J. Control. Release 2012, 161, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, J.; Göpferich, A. Mathematical Modeling of Bioerodible, Polymeric Drug Delivery Systems. Adv. Drug Deliv. Rev. 2001, 48, 229–247. [Google Scholar] [CrossRef]













| Formulation | MC-F | HME-F | 3DP-F | ||||||
|---|---|---|---|---|---|---|---|---|---|
| W (mg) | T (mm) | ρ (kg/m3) | W (mg) | T (mm) | ρ (kg/m3) | W (mg) | T (mm) | ρ (kg/m3) | |
| Average | 17.33 | 0.21 | 0.84 | 19.33 | 0.24 | 0.92 | 27.67 | 0.26 | 1.06 |
| SD. | 2.08 | 0.03 | 0.02 | 4.93 | 0.06 | 0.03 | 0.58 | 0.00 | 0.02 |
| RSD % | 12.01 | 12.18 | 1.94 | 25.51 | 26.10 | 2.91 | 2.09 | 0.00 | 2.09 |
| Films | First-Order | Higuchi | Korsmeyer–Peppas | Peppas–Sahlin | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| k1 | R2 | kH | R2 | kKP | n | R2 | k1 | k2 | m | R2 | |
| MC-F | 0.009 | 0.4642 | 4.766 | 0.8216 | 6.850 | 0.394 | 0.8504 | 3.233 | −0.077 | 0.817 | 0.9560 |
| HME-F | 0.014 | 0.8217 | 5.313 | 0.9510 | 4.733 | 0.541 | 0.9536 | 2.196 | −0.042 | 1.007 | 0.9958 |
| 3DP-F | 0.046 | 0.7650 | 12.133 | 0.8977 | 16.607 | 0.388 | 0.9243 | 9.243 | −0.356 | 0.819 | 0.9880 |
| Films | First-Order | Higuchi | Korsmeyer–Peppas | Peppas–Sahlin | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| k1 | R2 | kH | R2 | kKP | n | R2 | k1 | k2 | m | R2 | |
| MC-F | 0.024 | 0.5600 | 8.182 | 0.7610 | 16.383 | 0.319 | 0.8701 | 9.062 | −0.311 | 0.660 | 0.9798 |
| HME-F | 0.006 | 0.7584 | 3.586 | 0.8765 | 3.101 | 0.537 | 0.8789 | 1.289 | −0.013 | 0.928 | 0.9740 |
| 3DP-F | 0.009 | 0.7176 | 5.322 | 0.9180 | 6.543 | 0.447 | 0.9245 | 3.779 | −0.079 | 0.731 | 0.9803 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Lu, A.; Thakkar, R.; Zhang, Y.; Maniruzzaman, M. Development and Evaluation of Amorphous Oral Thin Films Using Solvent-Free Processes: Comparison between 3D Printing and Hot-Melt Extrusion Technologies. Pharmaceutics 2021, 13, 1613. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics13101613
Zhang J, Lu A, Thakkar R, Zhang Y, Maniruzzaman M. Development and Evaluation of Amorphous Oral Thin Films Using Solvent-Free Processes: Comparison between 3D Printing and Hot-Melt Extrusion Technologies. Pharmaceutics. 2021; 13(10):1613. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics13101613
Chicago/Turabian StyleZhang, Jiaxiang, Anqi Lu, Rishi Thakkar, Yu Zhang, and Mohammed Maniruzzaman. 2021. "Development and Evaluation of Amorphous Oral Thin Films Using Solvent-Free Processes: Comparison between 3D Printing and Hot-Melt Extrusion Technologies" Pharmaceutics 13, no. 10: 1613. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics13101613