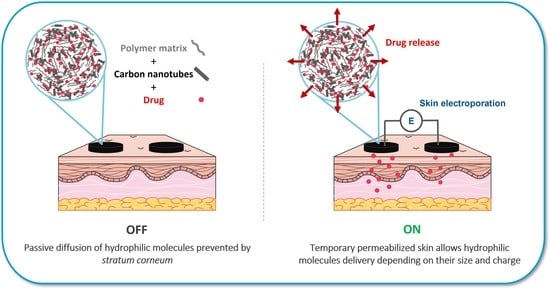
Transdermal Delivery of Macromolecules Using Two-in-One Nanocomposite Device for Skin Electroporation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Platform Preparation
2.2. Electroporation Treatment on Mice Skins
2.3. Mice Skin Surface Fluorescence Imaging
2.4. Histology
2.5. Statistical Analysis
3. Results
3.1. Two-in-One Device Allows Skin Electroporation
3.2. Evaluation of the Skin Permeabilization Transitory Nature
3.3. Influence of the Molecule Size on Delivery
3.4. Influence of the Molecule Charge on Delivery
3.5. Two-in-One Device Allowed Immediate Delivery with Skin Electroporation
3.6. Validation of FD4 as a Model Molecule for Insulin Delivery Using Skin Electroporation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prausnitz, M.R.; Elias, P.M.; Franz, T.J.; Schmuth, M.; Tsai, J.-C.; Menon, G.K.; Holleran, W.M.; Feingold, K.R. Skin Barrier and Transdermal Drug Delivery. In Dermatology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 2176–2185. [Google Scholar]
- Prausnitz, M.R.; Langer, R. Transdermal Drug Delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Engelke, L.; Winter, G.; Hook, S.; Engert, J. Recent Insights into Cutaneous Immunization: How to Vaccinate via the Skin. Vaccine 2015, 33, 4663–4674. [Google Scholar] [CrossRef] [PubMed]
- Ramadon, D.; McCrudden, M.T.C.; Courtenay, A.J.; Donnelly, R.F. Enhancement Strategies for Transdermal Drug Delivery Systems: Current Trends and Applications. Drug Deliv. Transl. Res. 2021, 1–34. [Google Scholar] [CrossRef]
- Neumann, E.; Schaefer-Ridder, M.; Wang, Y.; Hofschneider, P.H. Gene Transfer into Mouse Lyoma Cells by Electroporation in High Electric Fields. EMBO J. 1982, 1, 841–845. [Google Scholar] [CrossRef]
- Mir, L.M.; Orlowski, S. Mechanisms of Electrochemotherapy. Adv. Drug Deliv. Rev. 1999, 35, 107–118. [Google Scholar] [CrossRef]
- André, F.M.; Gehl, J.; Sersa, G.; Préat, V.; Hojman, P.; Eriksen, J.; Golzio, M.; Cemazar, M.; Pavselj, N.; Rols, M.-P.; et al. Efficiency of High- and Low-Voltage Pulse Combinations for Gene Electrotransfer in Muscle, Liver, Tumor, and Skin. Hum. Gene Ther. 2008, 19, 1261–1272. [Google Scholar] [CrossRef] [Green Version]
- Cemazar, M.; Golzio, M.; Sersa, G.; Rols, M.P.; Teissie, J. Electrically-Assisted Nucleic Acids Delivery to Tissues In Vivo: Where Do We Stand? Curr. Pharm. Des. 2006, 12, 3817–3825. [Google Scholar] [CrossRef]
- Pliquett, U.F.; Vanbever, R.; Preat, V.; Weaver, J.C. Local Transport Regions (LTRs) in Human Stratum Corneum Due to Long and Short ‘High Voltage’ Pulses. Bioelectrochem. Bioenerg. 1998, 47, 151–161. [Google Scholar] [CrossRef]
- Denet, A.-R.; Vanbever, R.; Préat, V. Skin Electroporation for Transdermal and Topical Delivery. Adv. Drug Deliv. Rev. 2004, 56, 659–674. [Google Scholar] [CrossRef]
- Weaver, J.C.; Vaughan, T.E.; Chizmadzhev, Y. Theory of Electrical Creation of Aqueous Pathways across Skin Transport Barriers. Adv. Drug Deliv. Rev. 1999, 35, 21–39. [Google Scholar] [CrossRef]
- Chizmadzhev, Y.A.; Zarnitsin, V.G.; Weaver, J.C.; Potts, R.O. Mechanism of Electroinduced Ionic Species Transport through a Multilamellar Lipid System. Biophys. J. 1995, 68, 749–765. [Google Scholar] [CrossRef] [Green Version]
- Pavšelj, N.; Préat, V.; Miklavčič, D. A Numerical Model of Skin Electropermeabilization Based on In Vivo Experiments. Ann. Biomed. Eng. 2007, 35, 2138–2144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavšelj, N.; Miklavčič, D. Resistive Heating and Electropermeabilization of Skin Tissue during in Vivo Electroporation: A Coupled Nonlinear Finite Element Model. Int. J. Heat Mass Transf. 2011, 54, 2294–2302. [Google Scholar] [CrossRef]
- Lombry, C.; Dujardin, N.; Preat, V. Transdermal Delivery of Macromolecules Using Skin Electroporation. Pharm. Res. 2000, 17, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Vanbever, R. Transdermal Delivery of Fentanyl: Rapid Onset of Analgesia Using Skin Electroporation. J. Control. Release 1998, 50, 225–235. [Google Scholar] [CrossRef]
- Blagus, T.; Markelc, B.; Cemazar, M.; Kosjek, T.; Preat, V.; Miklavcic, D.; Sersa, G. In Vivo Real-Time Monitoring System of Electroporation Mediated Control of Transdermal and Topical Drug Delivery. J. Control. Release 2013, 172, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, Y.; Ye, B. An Efficient Electrochemical Glucose Sensor Based on Porous Nickel-Based Metal Organic Framework/Carbon Nanotubes Composite (Ni-MOF/CNTs). J. Alloys Compd. 2018, 767, 651–656. [Google Scholar] [CrossRef]
- Hong, G.; Shuo, D.; Antaris, A.L.; Chen, C.; Zhang, B.; Zhao, S.; Atochin, D.N.; Huang, P.L.; Andreasson, K.I.; Kuo, C.J.; et al. Through-Skull Fluorescence Imaging of the Brain in a New near-Infrared Window. Nat. Photonics 2014, 8, 723–730. [Google Scholar] [CrossRef] [Green Version]
- Wells, C.; Vollin-Bringel, O.; Fiegel, V.; Harlepp, S.; Van der Schueren, B.; Bégin-Colin, S.; Bégin, D.; Mertz, D. Engineering of Mesoporous Silica Coated Carbon-Based Materials Optimized for an Ultrahigh Doxorubicin Payload and a Drug Release Activated by PH, T, and NIR-Light. Adv. Funct. Mater. 2018, 28, 1706996. [Google Scholar] [CrossRef]
- Béduer, A.; Seichepine, F.; Flahaut, E.; Loubinoux, I.; Vaysse, L.; Vieu, C. Elucidation of the Role of Carbon Nanotube Patterns on the Development of Cultured Neuronal Cells. Langmuir 2012, 28, 17363–17371. [Google Scholar] [CrossRef]
- Correa-Duarte, M.A.; Wagner, N.; Rojas-Chapana, J.; Morsczeck, C.; Thie, M.; Giersig, M. Fabrication and Biocompatibility of Carbon Nanotube-Based 3D Networks as Scaffolds for Cell Seeding and Growth. Nano Lett. 2004, 4, 2233–2236. [Google Scholar] [CrossRef]
- Kostarelos, K. The Long and Short of Carbon Nanotube Toxicity. Nat. Biotechnol. 2008, 26, 774–776. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.; Flahaut, E.; Golzio, M. Overview of Carbon Nanotubes for Biomedical Applications. Materials 2019, 12, 624. [Google Scholar] [CrossRef] [Green Version]
- Guillet, J.-F.; Flahaut, E.; Golzio, M. A Hydrogel/Carbon-Nanotube Needle-Free Device for Electrostimulated Skin Drug Delivery. ChemPhysChem 2017, 18, 2715–2723. [Google Scholar] [CrossRef] [Green Version]
- Guillet, J.-F.; Valdez-Nava, Z.; Golzio, M.; Flahaut, E. Electrical Properties of Double-Wall Carbon Nanotubes Nanocomposite Hydrogels. Carbon 2019, 146, 542–548. [Google Scholar] [CrossRef] [Green Version]
- Flahaut, E.; Bacsa, R.; Peigney, A.; Laurent, C. Gram-Scale CCVD Synthesis of Double-Walled Carbon Nanotubes. Chem. Commun. 2003, 12, 1442–1443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourdiol, F.; Mouchet, F.; Perrault, A.; Fourquaux, I.; Datas, L.; Gancet, C.; Boutonnet, J.-C.; Pinelli, E.; Gauthier, L.; Flahaut, E. Biocompatible Polymer-Assisted Dispersion of Multi Walled Carbon Nanotubes in Water, Application to the Investigation of Their Ecotoxicity Using Xenopus Laevis Amphibian Larvae. Carbon 2013, 54, 175–191. [Google Scholar] [CrossRef] [Green Version]
- Leclerc, E.A.; Huchenq, A.; Kezic, S.; Serre, G.; Jonca, N. Mice Deficient for the Epidermal Dermokine and Isoforms Display Transient Cornification Defects. J. Cell Sci. 2014, 127, 2862–2872. [Google Scholar] [CrossRef] [Green Version]
- Pedron-Mazoyer, S.; Plouët, J.; Hellaudais, L.; Teissie, J.; Golzio, M. New Anti Angiogenesis Developments through Electro-Immunization: Optimization by in Vivo Optical Imaging of Intradermal Electrogenetransfer. Biochim. Biophys. Acta BBA-Gen. Subj. 2007, 1770, 137–142. [Google Scholar] [CrossRef]
- Dujardin, N. In Vivo Assessment of Skin Electroporation Using Square Wave Pulses. J. Control. Release 2002, 79, 219–227. [Google Scholar] [CrossRef]
- Pliquett, U.; Langer, R.; Weaver, J.C. Changes in the Passive Electrical Properties of Human Stratum Corneum Due to Electroporation. Biochim. Biophys. Acta BBA-Biomembr. 1995, 1239, 111–121. [Google Scholar] [CrossRef] [Green Version]
- Atkins, R.M.; Fawcett, T.J.; Gilbert, R.; Hoff, A.M.; Connolly, R.; Brown, D.W.; Jaroszeski, M.J. Real-Time Impedance Feedback to Enhance Cutaneous Gene Electrotransfer in a Murine Skin Model. Bioelectrochemistry 2021, 142, 107885. [Google Scholar] [CrossRef]
- Regnier, V.; De Morre, N.; Jadoul, A.; Préat, V. Mechanisms of a Phosphorothioate Oligonucleotide Delivery by Skin Electroporation. Int. J. Pharm. 1999, 184, 147–156. [Google Scholar] [CrossRef]
- Zorec, B.; Becker, S.; Reberšek, M.; Miklavčič, D.; Pavšelj, N. Skin Electroporation for Transdermal Drug Delivery: The Influence of the Order of Different Square Wave Electric Pulses. Int. J. Pharm. 2013, 457, 214–223. [Google Scholar] [CrossRef] [PubMed]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simon, J.; Jouanmiqueou, B.; Rols, M.-P.; Flahaut, E.; Golzio, M. Transdermal Delivery of Macromolecules Using Two-in-One Nanocomposite Device for Skin Electroporation. Pharmaceutics 2021, 13, 1805. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics13111805
Simon J, Jouanmiqueou B, Rols M-P, Flahaut E, Golzio M. Transdermal Delivery of Macromolecules Using Two-in-One Nanocomposite Device for Skin Electroporation. Pharmaceutics. 2021; 13(11):1805. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics13111805
Chicago/Turabian StyleSimon, Juliette, Bastien Jouanmiqueou, Marie-Pierre Rols, Emmanuel Flahaut, and Muriel Golzio. 2021. "Transdermal Delivery of Macromolecules Using Two-in-One Nanocomposite Device for Skin Electroporation" Pharmaceutics 13, no. 11: 1805. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics13111805