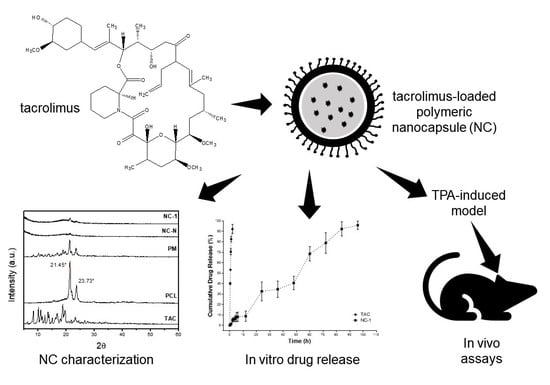
Characterization and In Vitro and In Vivo Evaluation of Tacrolimus-Loaded Poly(ε-Caprolactone) Nanocapsules for the Management of Atopic Dermatitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of Polymeric Nanocapsules Containing Tacrolimus (TAC)
2.3. Characterization of Polymeric NCs Containing TAC
2.3.1. Determination of Mean Diameter, Polydispersity Index (PDI), and Zeta Potential of NCs
2.3.2. Field Emission Scanning Electron Microscopy (FESEM) and Transmission Electron Microscopy (TEM)
2.3.3. X-ray Diffraction (XRD)
2.3.4. Fourier-Transform Infrared Spectroscopy (FTIR)
2.4. In Vitro Drug Dissolution Study
2.5. Evaluation of In Vivo Anti-Inflammatory Activity
2.5.1. Animals
2.5.2. Induction of Atopic Dermatitis in BALB/c Mice
2.5.3. Fibrinogen Dosage
2.5.4. Histological Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Preparation of NCs
3.2. Characterization of NCs Containing Tacrolimus
3.2.1. Determination of Mean Diameter, Polydispersity Index (PDI), and Zeta Potential of NCs
3.2.2. Field Emission Scanning Electron Microscopy (FESEM) and Transmission Electron Microscopy (TEM)
3.2.3. X-ray Diffraction (XRD)
3.2.4. Fourier-Transform Infrared Spectroscopy (FTIR)
3.3. In Vitro Drug Dissolution Study
3.4. Evaluation of In Vivo Anti-Inflammatory Activity
3.4.1. Fibrinogen Dosage
3.4.2. Histological Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Sroka-Tomaszewska, J.; Trzeciak, M. Molecular mechanisms of atopic dermatitis pathogenesis. Int. J. Mol. Sci. 2021, 22, 4130. [Google Scholar] [CrossRef]
- Wollenberg, A.; Thomsen, S.F.; Lacour, J.-P.; Jaumont, X.; Lazarewicz, S. Targeting immunoglobulin E in atopic dermatitis: A review of the existing evidence. World Allergy Organ. J. 2021, 14, 100519. [Google Scholar] [CrossRef]
- Girolomoni, G.; de Bruin-Weller, M.; Aoki, V.; Kabashima, K.; Deleuran, M.; Puig, L.; Bansal, A.; Rossi, A.B. Nomenclature and clinical phenotypes of atopic dermatitis. Ther. Adv. Chronic Dis. 2021, 12, 1–20. [Google Scholar] [CrossRef]
- Badloe, F.M.S.; De Vriese, S.; Coolens, K.; Schmidt-Weber, C.B.; Ring, J.; Gutermuth, J.; Krohn, I.K. IgE autoantibodies and autoreactive T cells and their role in children and adults with atopic dermatitis. Clin. Transl. Allergy 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Moreira, J.V.; Silva, S.C.M.; Cremasco, M.A. Evaluation of carbon: Nitrogen ratio in semi-defined culture medium to tacrolimus biosynthesis by Streptomyces tsukubaensis and the effect on bacterial growth. Biotechnol. Rep. 2020, 26, e00440. [Google Scholar] [CrossRef]
- Shoughy, S.S.; Aljassar, F.M.; Tabbara, K.F. Aqueous penetration of topical tacrolimus. Am. J. Ophthalmol. Case Rep. 2020, 17, 100582. [Google Scholar] [CrossRef]
- Shoughy, S.S. Topical tacrolimus in anterior segment inflammatory disorders. Eye Vis. 2017, 4, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Malecic, N.; Young, H. Tacrolimus for the management of psoriasis: Clinical utility and place in therapy. Psoriasis Targets Ther. 2016, 6, 153–163. [Google Scholar] [CrossRef]
- Nishiyama, M.; Izumi, S.; Okuhara, M. Discovery and development of FK506 (Tacrolimus), a potent immunosuppressant of microbial origin. In The Search for Anti-Inflammatory Drugs; Springer: Singapore, 1995; Volume 1, pp. 65–104. [Google Scholar]
- Pubchem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Tacrolimus (accessed on 10 October 2021).
- Yamamoto, K.; Klossek, A.; Fuchs, K.; Watts, B.; Raabe, J.; Flesch, R.; Rancan, F.; Pischon, H.; Radbruch, M.; Gruber, A.D.; et al. Soft X-ray microscopy for probing of topical tacrolimus delivery via micelles. Eur. J. Pharm. Biopharm. 2019, 139, 68–75. [Google Scholar] [CrossRef]
- Yu, K.; Wang, Y.; Wan, T.; Zhai, Y.; Cao, S.; Ruan, W.; Wu, C.; Xu, Y. Tacrolimus nanoparticles based on chitosan combined with nicotinamide: Enhancing percutaneous delivery and treatment efficacy for atopic dermatitis and reducing dose. Int. J. Nanomed. 2017, 13, 129–142. [Google Scholar] [CrossRef] [Green Version]
- Dheer, D.; Jyoti; Gupta, P.N.; Shankar, R. Tacrolimus: An updated review on delivering strategies for multifarious diseases. Eur. J. Pharm. Sci. 2018, 114, 217–227. [Google Scholar] [CrossRef]
- Savić, V.; Todosijević, M.; Ilić, T.; Lukic, M.; Mitsou, E.; Papadimitriou, V.; Avramiotis, S.; Marković, B.; Cekic, N.; Savić, S. Tacrolimus loaded biocompatible lecithin-based microemulsions with improved skin penetration: Structure characterization and in vitro/in vivo performances. Int. J. Pharm. 2017, 529, 491–505. [Google Scholar] [CrossRef]
- Ohtsuki, M.; Morimoto, H.; Nakagawa, H. Tacrolimus ointment for the treatment of adult and pediatric atopic dermatitis: Review on safety and benefits. J. Dermatol. 2018, 45, 936–942. [Google Scholar] [CrossRef]
- Nam, S.H.; Ji, X.Y.; Park, J.-S. Investigation of tacrolimus loaded nanostructured lipid carriers for topical drug delivery. Bull. Korean Chem. Soc. 2011, 32, 956–960. [Google Scholar] [CrossRef] [Green Version]
- Deng, S.; Gigliobianco, M.R.; Censi, R.; Di Martino, P. Polymeric nanocapsules as nanotechnological alternative for drug delivery system: Current status, challenges and opportunities. Nanomaterials 2020, 10, 847. [Google Scholar] [CrossRef]
- Fessi, H.; Puisieux, F.; Devissaguet, J.P.; Ammoury, N.; Benita, S. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int. J. Pharm. 1989, 55, R1–R4. [Google Scholar] [CrossRef]
- Dos Santos-Silva, A.M.; De Caland, L.B.; do Nascimento, E.G.; Oliveira, A.L.C.d.S.; de Araújo-Júnior, R.F.; Cornélio, A.M.; Fernandes-Pedrosa, M.F.; da Silva-Júnior, A.A. Self-assembled Benznidazole-loaded cationic nanoparticles containing Cholesterol/Sialic Acid: Physicochemical properties, in vitro drug release and in vitro anticancer efficacy. Int. J. Mol. Sci. 2019, 20, 2350. [Google Scholar] [CrossRef] [Green Version]
- Bao, Q.; Shen, J.; Jog, R.; Zhang, C.; Newman, B.; Wang, Y.; Choi, S.; Burgess, D.J. In vitro release testing method development for ophthalmic ointments. Int. J. Pharm. 2017, 526, 145–156. [Google Scholar] [CrossRef]
- Sheshala, R.; Anuar, N.K.; Abu Samah, N.H.; Wong, T.W. In vitro drug dissolution/permeation testing of nanocarriers for skin application: A comprehensive review. AAPS PharmSciTech 2019, 20, 164. [Google Scholar] [CrossRef]
- Camargo, G.d.A.; Lyra, A.M.; Barboza, F.M.; Fiorin, B.C.; Beltrame, F.L.; Nadal, J.M.; Novatski, A.; Farago, P.V. Validation of analytical methods for Tacrolimus determination in Poly(ε-caprolactone) nanocapsules and identification of drug degradation products. J. Nanosci. Nanotechnol. 2021, 21, 5920–5928. [Google Scholar] [CrossRef]
- Shi, C.; Liu, P.; He, Y.; Liu, Y.; Feng, X.; Fu, D. Drug-loaded poly(d,l-lactide-co-glycolide) microspheres as treatment for allergic contact dermatitis in mice model. J. Bioact. Compat. Polym. 2017, 32, 445–455. [Google Scholar] [CrossRef]
- Shalm, O.W.; Jain, N.C.; Carroll, W.J. Veterinary Hematology, 3rd ed.; Lea & Febiger: Philadelphia, PA, USA, 1975. [Google Scholar]
- Recio, M.C.; Giner, R.M.; Uriburu, L.; Máñez, S.; Cerdá, M.; De la Fuente, J.; Ríos, J.L. In vivo activity of pseudoguaianolide sesquiterpene lactones in acute and chronic inflammation. Life Sci. 2000, 66, 2509–2518. [Google Scholar] [CrossRef]
- Rudnik, L.A.C.; Farago, P.V.; Budel, J.M.; Lyra, A.; Barboza, F.M.; Klein, T.; Kanunfre, C.C.; Nadal, J.M.; Bandéca, M.C.; Raman, V.; et al. Co-loaded Curcumin and Methotrexate Nanocapsules Enhance Cytotoxicity against non-small-cell lung cancer cells. Molecules 2020, 25, 1913. [Google Scholar] [CrossRef]
- Camargo, G.d.A.; da Costa Filha, A.R.C.; Lyra, A.M.; Novatski, A.; Nadal, J.M.; de Lara, L.S.; Dias, D.T.; do Nascimento, E.A.; Silva, U.R.; Jacinto, C.; et al. Stability testing of tacrolimus-loaded poly(ԑ-caprolactone) nanoparticles by physicochemical assays and Raman spectroscopy. Vib. Spectrosc. 2020, 110, 103139. [Google Scholar] [CrossRef]
- Schaffazick, S.R.; Guterres, S.S.; Freitas, L.D.L.; Pohlmann, A.R. Caracterização e estabilidade físico-química de sistemas poliméricos nanoparticulados para administração de fármacos. Química Nova 2003, 26, 726–737. [Google Scholar] [CrossRef]
- Markwalter, C.E.; Pagels, R.F.; Wilson, B.K.; Ristroph, K.D.; Prud’Homme, R.K. Flash nanoprecipitation for the Encapsulation of Hydrophobic and Hydrophilic compounds in Polymeric Nanoparticles. J. Vis. Exp. 2019, 143, e58757. [Google Scholar] [CrossRef] [Green Version]
- Marcano, R.G.d.J.V.; Tominaga, T.T.; Khalil, N.M.; Pedroso, L.S.; Mainardes, R.M. Chitosan functionalized poly (ε-caprolactone) nanoparticles for amphotericin B delivery. Carbohydr. Polym. 2018, 202, 345–354. [Google Scholar] [CrossRef]
- Bhatia, S. Nanoparticles types, classification, characterization, fabrication methods and drug delivery applications. In Natural Polymer Drug Delivery Systems; Springer: Singapore, 2016; Volume 1, pp. 33–93. [Google Scholar]
- Kalita, S.; Devi, B.; Kandimalla, R.; Sharma, K.K.; Sharma, A.; Kalita, K.; Kataki, A.C.; Kotoky, J. Chloramphenicol encapsulated in poly-ε-caprolactone–pluronic composite: Nanoparticles for treatment of MRSA-infected burn wounds. Int. J. Nanomed. 2015, 10, 2971–2984. [Google Scholar] [CrossRef] [Green Version]
- Kong, N.; Deng, M.; Sun, X.-N.; Chen, Y.-D.; Sui, X.-B. Polydopamine-functionalized CA-(PCL-ran-PLA) Nanoparticles for target delivery of Docetaxel and Chemo-photothermal therapy of breast cancer. Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Parashar, P.; Rathor, M.; Dwivedi, M.; Saraf, S.A. Hyaluronic Acid decorated Naringenin Nanoparticles: Appraisal of chemopreventive and curative potential for lung cancer. Pharmaceutics 2018, 10, 33. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Zeng, Y.; Shi, S.; Xu, L.; Zhang, H.; Pathak, J.L.; Pan, Y. Design of polyaspartic acid peptide-poly (ethylene glycol)-poly (ε-caprolactone) nanoparticles as a carrier of hydrophobic drugs targeting cancer metastasized to bone. Int. J. Nanomed. 2017, 12, 3561–3575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaaban, M.I.; Shaker, M.A.; Mady, F.M. Imipenem/cilastatin encapsulated polymeric nanoparticles for destroying carbapenem-resistant bacterial isolates. J. Nanobiotechnol. 2017, 15, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, M.F.; Ciciliatti, M.A.; Hechenleitner, A.A.W.; Peñalva, R.; Agüeros, M.; Irache, J.M.; de Oliveira, A.J.A.; Farooqi, A.A.; Pineda, E.A.G. Superparamagnetic maghemite loaded poly (ε-caprolactone) nanocapsules: Characterization and synthesis optimization. Matéria 2014, 19, 40–52. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Wang, B.; Wei, X.; Rao, W.; Ai, F.; Zhao, F.; Men, K.; Yang, B.; Liu, X.; Huang, M.; et al. Preparation, characterization and application of star-shaped PCL/PEG micelles for the delivery of doxorubicin in the treatment of colon cancer. Int. J. Nanomed. 2013, 8, 971–982. [Google Scholar] [CrossRef] [Green Version]
- Dantas, I.L.; Bastos, K.T.S.; Machado, M.; Galvão, J.G.; Lima, A.D.; Gonsalves, J.K.M.C.; Almeida, E.D.P.; Araújo, A.A.S.; de Meneses, C.T.; Sarmento, V.H.V.; et al. Influence of stearic acid and beeswax as solid lipid matrix of lipid nanoparticles containing tacrolimus. J. Therm. Anal. Calorim. 2018, 132, 1557–1566. [Google Scholar] [CrossRef]
- Ponnammal, P.; Kanaujia, P.; Yani, Y.; Ng, W.K.; Tan, R.B.H. Orally disintegrating tablets containing melt extruded amorphous solid dispersion of tacrolimus for dissolution enhancement. Pharmaceutics 2018, 10, 35. [Google Scholar] [CrossRef] [Green Version]
- Obaidat, R.M.; Tashtoush, B.M.; Awad, A.A.; Al Bustami, R.T. Using supercritical fluid technology (SFT) in preparation of Tacrolimus solid dispersions. AAPS PharmSciTech 2016, 18, 481–493. [Google Scholar] [CrossRef]
- Binkhathlan, Z.; Badran, M.M.; Alomrani, A.; Aljuffali, I.A.; Alghonaim, M.; Al-Muhsen, S.; Halwani, R.; Alshamsan, A. Reutilization of tacrolimus extracted from expired prograf® capsules: Physical, chemical, and pharmacological assessment. AAPS PharmSciTech 2015, 17, 978–987. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Han, X.; Wang, J.; Wang, Y. Preparation, characterization and in vivo evaluation of amorphous tacrolimus nanosuspensions produced using CO2-assisted in situ nanoamorphization method. Int. J. Pharm. 2016, 505, 35–41. [Google Scholar] [CrossRef]
- Gomes, M.L.S.; Nascimento, N.d.S.; Borsato, D.M.; Pretes, A.P.; Nadal, J.M.; Novatski, A.; Gomes, R.Z.; Fernandes, D.; Farago, P.V.; Zanin, S.M.W. Long-lasting anti-platelet activity of cilostazol from poly(ε-caprolactone)-poly(ethylene glycol) blend nanocapsules. Mater. Sci. Eng. C 2019, 94, 694–702. [Google Scholar] [CrossRef]
- Carletto, B.; Berton, J.; Ferreira, T.N.; Dalmolin, L.F.; Paludo, K.S.; Mainardes, R.M.; Farago, P.V.; Favero, G.M. Resveratrol-loaded nanocapsules inhibit murine melanoma tumor growth. Colloids Surf. B Biointerfaces 2016, 144, 65–72. [Google Scholar] [CrossRef]
- Yamashita, K.; Nakate, T.; Okimoto, K.; Ohike, A.; Tokunaga, Y.; Ibuki, R.; Higaki, K.; Kimura, T. Establishment of new preparation method for solid dispersion formulation of tacrolimus. Int. J. Pharm. 2003, 267, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Serrano, D.R.; Gallagher, K.H.; Healy, A.M. Emerging nanonisation technologies: Tailoring crystalline versus amorphous nanomaterials. Curr. Top. Med. Chem. 2015, 15, 2327–2340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abriata, J.P.; Turatti, R.C.; Luiz, M.T.; Raspantini, G.L.; Tofani, L.B.; do Amaral, R.L.F.; Swiech, K.; Marcato, P.D.; Marchetti, J.M. Development, characterization and biological in vitro assays of paclitaxel-loaded PCL polymeric nanoparticles. Mater. Sci. Eng. C 2019, 96, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, M.; Mazon, L.R.; de Meneses, A.C.; Silva, L.L.; de Araujo, P.H.H.; Fiori, M.A.; de Oliveira, D. Encapsulation of geranyl cinnamate in polycaprolactone nanoparticles. Mater. Sci. Eng. C 2019, 97, 198–207. [Google Scholar] [CrossRef]
- Ferraboschi, P.; Colombo, D.; De Mieri, M.; Grisenti, P. Evaluation, synthesis and characterization of tacrolimus impurities. J. Antibiot. 2012, 65, 349–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pretsch, E.; Buhlmann, P.; Badertscher, M. Structure Determination of Organic Compounds; Springer: Singapore, 2009; p. 431. [Google Scholar]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Spectrometric Identification of Organic Compounds, 7th ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2005. [Google Scholar]
- Paarakh, M.P.; Jose, P.A.; Setty, C.; Christoper, G.V.P. Release kinetics—Concepts and applications. Int. J. Pharm. Res. Technol. 2019, 8, 12–20. [Google Scholar] [CrossRef]
- Singhvi, G.; Singh, M. Review: In vitro drug release characterization models. Int. J. Pharm. Sci. Res. 2011, 2, 77–84. [Google Scholar]
- Da Costa, P.J.C. Avaliação in vitro da lioequivalência de formulações farmacêuticas. Rev. Bras. Ciências Farm. 2002, 38, 141–153. [Google Scholar] [CrossRef] [Green Version]
- Villanova, J.C.O.; Oréfice, R.L.; Cunha, A.S. Aplicações farmacêuticas de polímeros. Polímeros 2010, 20, 51–64. [Google Scholar] [CrossRef] [Green Version]
- Dash, T.K.; Konkimalla, V.B. Poly-ε-caprolactone based formulations for drug delivery and tissue engineering: A review. J. Control. Release 2012, 158, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Lenzi, E.K.; Novatski, A.; Farago, P.V.; Almeida, M.A.; Zawadzki, S.F.; Neto, R.M. Diffusion processes and drug release: Capsaicinoids—Loaded Poly (ε-caprolactone) microparticles. PLoS ONE 2016, 11, e0157662. [Google Scholar] [CrossRef] [Green Version]
- Carletto, B.; Koga, A.Y.; Novatski, A.; Mainardes, R.M.; Lipinski, L.C.; Farago, P.V. Ursolic acid-loaded lipid-core nanocapsules reduce damage caused by estrogen deficiency in wound healing. Colloids Surf. B Biointerfaces 2021, 203, 111720. [Google Scholar] [CrossRef]
- Roshal, M. Thrombin time and fibrinogen determination. In Transfusion Medicine and Hemostasis; Elsevier: Amsterdam, The Netherlands, 2013; Volume 1, pp. 793–798. [Google Scholar]
- Luyendyk, J.P.; Schoenecker, J.G.; Flick, M.J. The multifaceted role of fibrinogen in tissue injury and inflammation. Blood 2019, 133, 511–520. [Google Scholar] [CrossRef]
- De Almeida, V.V.; Silva-Herdade, A.; Calado, A.; Rosário, H.S.; Saldanha, C. Fibrinogen modulates leukocyte recruitment in vivo during the acute inflammatory response. Clin. Hemorheol. Microcirc. 2015, 59, 97–106. [Google Scholar] [CrossRef]
- Gabriel, D.; Mugnier, T.; Courthion, H.; Kranidioti, K.; Karagianni, N.; Denis, M.C.; Lapteva, M.; Kalia, Y.; Möller, M.; Gurny, R. Improved topical delivery of tacrolimus: A novel composite hydrogel formulation for the treatment of psoriasis. J. Control. Release 2016, 242, 16–24. [Google Scholar] [CrossRef]
- Boscardin, P.M.D. Avaliação Antiinflamatória e Citotóxica do Óleo Essencial de Eucalyptus Benthamii Maiden et Cambage. Ph.D. Thesis, Universidade Federal do Paraná, Curitiba, Brazil, 2012. [Google Scholar]
- Li, G.; Fan, Y.; Fan, C.; Li, X.; Wang, X.; Li, M.; Liu, Y. Tacrolimus-loaded ethosomes: Physicochemical characterization and in vivo evaluation. Eur. J. Pharm. Biopharm. 2012, 82, 49–57. [Google Scholar] [CrossRef]
- Kandikattu, H.K.; Mishra, A. Immunomodulatory effects of tacrolimus (FK506) for the treatment of allergic diseases. Int. J. Cell Boil. Physiol. 2018, 1, 5–13. [Google Scholar] [CrossRef]
- Jain, S.; Addan, R.; Kushwah, V.; Harde, H.; Mahajan, R.R. Comparative assessment of efficacy and safety potential of multifarious lipid based Tacrolimus loaded nanoformulations. Int. J. Pharm. 2019, 562, 96–104. [Google Scholar] [CrossRef] [PubMed]










| Group | Treatment |
|---|---|
| G1 (n = 10) | Animals that did not receive any treatment (basal group) |
| G2 (n = 10) | Animals treated with TPA (inflammatory control group—ICG) |
| G3 (n = 10) | Animals treated with TPA and NC-N (0.44 mL/animal 1,3) (NC-N Group) |
| G4 (n = 10) | Animals treated with TPA and TAC at a concentration of 0.1% (w/v) (0.4 mL/animal 1) (TAC Group) |
| G5 (n = 10) | Animals treated with TPA and TAC-loaded PCL nanocapsules at a drug concentration of 0.1% (w/v) (0.44 mL/animal 1,2,3) (NC-1 Group) |
| Formulation | Mean Particle Size (nm) | PDI | Zeta Potential (mV) | |||
|---|---|---|---|---|---|---|
| Mean | SD * | Mean | SD * | Mean | SD * | |
| NC-1 | 226.64 | ±32.28 | 0.23 | ±0.04 | −38.11 | ±3.12 |
| NC-N | 250.53 | ±43.11 | 0.24 | ±0.04 | −42.60 | ±5.58 |
| Model | Equation | Linear Equation | r2 Adjusted |
|---|---|---|---|
| Zero-order | Q = Q0 + K0t | y = 1.0506x + 1.511 | 0.9947 |
| First-order | dC/dT = −Kt | y = −0.0122x + 2.0517 | 0.9143 |
| Higuchi | Q = KH t1/2 | y = 0.0945x + 1.2057 | 0.9532 |
| Korsmeyer–Peppas | Mt/M∞ = Ktn | y = 0.8816x + 0.2487 | 0.9846 |
| Hixson–Crowell | Q01/3 − Q11/3 = KHC t | y = −0.0294x + 4.7112 | 0.9675 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camargo, G.d.A.; Ferreira, L.; Schebelski, D.J.; Lyra, A.M.; Barboza, F.M.; Carletto, B.; Koga, A.Y.; Semianko, B.C.; Dias, D.T.; Lipinski, L.C.; et al. Characterization and In Vitro and In Vivo Evaluation of Tacrolimus-Loaded Poly(ε-Caprolactone) Nanocapsules for the Management of Atopic Dermatitis. Pharmaceutics 2021, 13, 2013. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics13122013
Camargo GdA, Ferreira L, Schebelski DJ, Lyra AM, Barboza FM, Carletto B, Koga AY, Semianko BC, Dias DT, Lipinski LC, et al. Characterization and In Vitro and In Vivo Evaluation of Tacrolimus-Loaded Poly(ε-Caprolactone) Nanocapsules for the Management of Atopic Dermatitis. Pharmaceutics. 2021; 13(12):2013. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics13122013
Chicago/Turabian StyleCamargo, Guilherme dos Anjos, Leandro Ferreira, Diego José Schebelski, Amanda Martinez Lyra, Fernanda Malaquias Barboza, Bruna Carletto, Adriana Yuriko Koga, Betina Christi Semianko, Daniele Toniolo Dias, Leandro Cavalcante Lipinski, and et al. 2021. "Characterization and In Vitro and In Vivo Evaluation of Tacrolimus-Loaded Poly(ε-Caprolactone) Nanocapsules for the Management of Atopic Dermatitis" Pharmaceutics 13, no. 12: 2013. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics13122013