CuS-131I-PEG Nanotheranostics-Induced “Multiple Mild-Hyperthermia” Strategy to Overcome Radio-Resistance in Lung Cancer Brachytherapy
Abstract
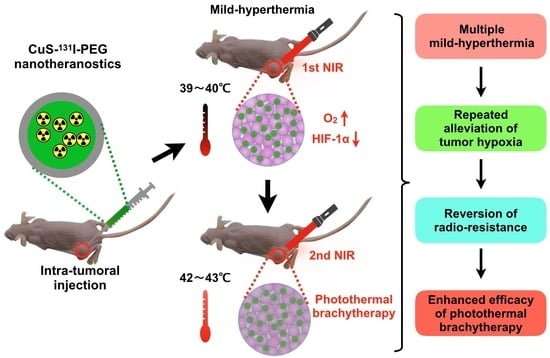
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PEGylated Nanotheranostics (CuS-131I-PEG)
2.3. Characterization of PEGylated Nanotheranostics (CuS-131I-PEG)
2.4. Cell Culture and Animal Model
2.5. In Vitro Serum Stability of PEGylated Nanotheranostics (CuS-131I-PEG)
2.6. In Vitro Cytotoxicity of PEGylated Nanotheranostics (CuS-131I-PEG)
2.7. In Vivo Photothermal Effect of PEGylated Nanotheranostics (CuS-131I-PEG)
2.8. In Vivo Photoacoustic Imaging
2.9. In Vivo SPECT/CT Imaging
2.10. Immunofluorescence Imaging
2.11. In Vivo Combined Photothermal Brachytherapy
2.12. Statistical Analysis
3. Results
3.1. Preparation and Characterization of CuS-131I-PEG nanotheranostics
3.2. Photothermal Properties of PEGylated Nanotheranostics
3.3. Cell Cytotoxicity of CuS-131I-PEG Nanotheranostics In Vitro
3.4. Intratumoral Distribution of CuS-131I-PEG Nanotheranostics by Photoacoustic Imaging
3.5. Intratumoral Accumulation of 131I in CuS-131I-PEG Nanotheranostics Monitored by SPECT/CT Imaging
3.6. In Vivo Anti-Tumor Efficiency of the Combined Photothermal Brachytherapy
3.7. Mechanism of Tumor Hypoxic Alleviation through Mild Hyperthermia
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al Torre, L.; Siegel, R.L.; Jemal, A. Lung Cancer Statistics. Adv. Exp. Med. Biol. 2016, 893, 1–19. [Google Scholar]
- Vinod, S.K.; Hau, E. Radiotherapy treatment for lung cancer: Current status and future directions. Respirology 2020, 25 (Suppl. 2), 61–71. [Google Scholar] [CrossRef]
- Khalifa, J.; Lerouge, D.; Le Péchoux, C.; Pourel, N.; Darréon, J.; Mornex, F.; Giraud, P. Radiotherapy for primary lung cancer. Cancer Radiother. 2022, 26, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Shirato, H.; Onimaru, R.; Ishikawa, M.; Kaneko, J.; Takeshima, T.; Mochizuki, K.; Shimizu, S.; Umegaki, K. Real-time 4-D radiotherapy for lung cancer. Cancer Sci. 2012, 103, 1–6. [Google Scholar] [CrossRef]
- Kubo, N.; Saitoh, J.I.; Shimada, H.; Shirai, K.; Kawamura, H.; Ohno, T.; Nakano, T. Dosimetric comparison of carbon ion and X-ray radiotherapy for Stage IIIA non-small cell lung cancer. J. Radiat. Res. 2016, 57, 548–554. [Google Scholar] [CrossRef] [Green Version]
- Chan, M.D.; Dupuy, D.E.; Mayo-Smith, W.W.; Ng, T.; Di Petrillo, T.A. Combined radiofrequency ablation and high-dose rate brachytherapy for early-stage non-small-cell lung cancer. Brachytherapy 2011, 10, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; Jiang, P.; Ji, Z.; Huo, X.; Sun, H.; Wang, J. Brachytherapy for lung cancer. Brachytherapy 2021, 20, 454–466. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, Y.; Dong, M.; Yang, J.; Weng, W.; Teng, L. Iodine-125 interstitial brachytherapy reduces tumor growth via Warburg effect inhibition in non-small cell lung cancer A549 xenografts. Oncol. Lett. 2018, 16, 5969–5977. [Google Scholar] [CrossRef] [Green Version]
- Peng, S.; Song, R.; Lin, Q.; Zhang, Y.; Yang, Y.; Luo, M.; Zhong, Z.; Xu, X.; Lu, L.; Yao, S.; et al. A Robust Oxygen Microbubble Radiosensitizer for Iodine-125 Brachytherapy. Adv. Sci. 2021, 8, 2002567. [Google Scholar] [CrossRef]
- Tang, W.; Yang, Z.; He, L.; Deng, L.; Fathi, P.; Zhu, S.; Li, L.; Shen, B.; Wang, Z.; Jacobson, O.; et al. A hybrid semiconducting organosilica-based O2 nanoeconomizer for on-demand synergistic photothermally boosted radiotherapy. Nat. Commun. 2021, 12, 523. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Chao, Y.; Zhou, X.; Liang, C.; Liu, J.; Zhang, R.; Cheng, L.; Yang, K.; Pan, W.; Zhu, M.; et al. Near-Infrared-Triggered in Situ Gelation System for Repeatedly Enhanced Photothermal Brachytherapy with a Single Dose. ACS Nano 2018, 12, 9412–9422. [Google Scholar] [CrossRef]
- Xiao, Y.; Peng, J.; Liu, Q.; Chen, L.; Shi, K.; Han, R.; Yang, Q.; Zhong, L.; Zha, R.; Qu, Y.; et al. Ultrasmall CuS@BSA nanoparticles with mild photothermal conversion synergistically induce MSCs-differentiated fibroblast and improve skin regeneration. Theranostics 2020, 10, 1500–1513. [Google Scholar] [CrossRef]
- Wan, X.; Liu, M.; Ma, M.; Chen, D.; Wu, N.; Li, L.; Li, Z.; Lin, G.; Wang, X.; Xu, G. The Ultrasmall Biocompatible CuS@BSA Nanoparticle and Its Photothermal Effects. Front. Pharmacol. 2019, 10, 141. [Google Scholar] [CrossRef]
- Bai, J.; Liu, Y.; Jiang, X. Multifunctional PEG-GO/CuS nanocomposites for near-infrared chemo-photothermal therapy. Biomaterials 2014, 35, 5805–5813. [Google Scholar] [CrossRef]
- Liang, L.; Peng, S.; Yuan, Z.; Wei, C.; He, Y.; Zheng, J.; Gu, Y.; Chen, H. Biocompatible tumor-targeting nanocomposites based on CuS for tumor imaging and photothermal therapy. RSC Adv. 2018, 8, 6013–6026. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Qian, Y.; Li, P.; Zhang, S.; Liu, J.; Sun, X.; Fulham, M.; Feng, D.; Huang, G.; Lu, W.; et al. 131I-Labeled Copper Sulfide-Loaded Microspheres to Treat Hepatic Tumors via Hepatic Artery Embolization. Theranostics 2018, 8, 785–799. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Yang, K.; Liang, C.; Zhong, X.; Ning, P.; Song, G.; Wang, D.; Ge, C.; Chen, C.; Chai, Z.; et al. Imaging-Guided Combined Photothermal and Radiotherapy to Treat Subcutaneous and Metastatic Tumors Using Iodine-131-Doped Copper Sulfide Nanoparticles. Adv. Funct. Mater. 2015, 25, 4689–4699. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99 Pt A, 28–51. [Google Scholar] [CrossRef] [Green Version]
- Lu, W.; Chen, D.; Yang, D.; Hong, H. Dual Functional Piscidin1: A Natural Chelating Ligand for 64Cu and an Anti-Cancer Peptide. J. Nucl. Med. 2017, 58, 4. [Google Scholar]
- Pallavicini, P.; Bernhard, C.; Chirico, G.; Dacarro, G.; Denat, F.; Donà, A.; Milanese, C.; Taglietti, A. Gold nanostars co-coated with the Cu(II) complex of a tetraazamacrocyclic ligand. Dalton Trans. 2015, 44, 5652–5661. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cui, Y.; Li, M.; Cui, K.; Li, R.; Xie, W.; Liu, L.; Xiao, Z. DNA-assembled visible nanodandelions with explosive hydrogen-bond breakage achieving uniform intra-tumor distribution (UITD)-guided photothermal therapy. Biomaterials 2022, 282, 121381. [Google Scholar] [CrossRef] [PubMed]
- Lebepe, T.C.; Oluwafemi, O.S. Photothermal Conversion Profiling of Large-Scaled Synthesized Gold Nanorods Using Binary Surfactant with Hydroquinone as a Reducing Agent. Nanomaterials 2022, 12, 1723. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, S.; Yang, S.; Liang, M.; Jiang, X.; Wu, W. Semiconductor Polymer with Strong NIR-II Absorption for Photoacoustic Imaging and Photothermal Therapy. ACS Appl. Bio. Mater. 2022, 5, 2224–2231. [Google Scholar] [CrossRef] [PubMed]
- Yim, W.; Borum, R.M.; Zhou, J.; Mantri, Y.; Wu, Z.; Zhou, J.; Jin, Z.; Creyer, M.; Jokerst, J.V. Ultrasmall gold nanorod-polydopamine hybrids for enhanced photoacoustic imaging and photothermal therapy in second near-infrared window. Nanotheranostics 2022, 6, 79–90. [Google Scholar] [CrossRef]
- Shu, X.; Chen, Y.; Yan, P.; Xiang, Y.; Shi, Q.Y.; Yin, T.; Wang, P.; Liu, L.H.; Shuai, X. Biomimetic nanoparticles for effective mild temperature photothermal therapy and multimodal imaging. J. Control. Release 2022, 347, 270–281. [Google Scholar] [CrossRef]
- Yu, N.; Zhao, L.; Cheng, D.; Ding, M.; Lyu, Y.; Zhao, J.; Li, J. Radioactive organic semiconducting polymer nanoparticles for multimodal cancer theranostics. J. Colloid Interface Sci. 2022, 619, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Gong, J.; Wang, P.; Zhu, J.; Yu, N.; Zhao, J.; Zhang, Q.; Li, J. 131I-Labeled gold nanoframeworks for radiotherapy-combined second near-infrared photothermal therapy of cancer. J. Mater. Chem. B 2021, 9, 9316–9323. [Google Scholar] [CrossRef]
- Han, T.H.; Park, M.K.; Nakamura, H.; Ban, H.S. Capsaicin inhibits HIF-1α accumulation through suppression of mitochondrial respiration in lung cancer cells. Biomed. Pharmacother. 2022, 146, 112500. [Google Scholar] [CrossRef]
- Luo, F.; Lu, F.T.; Cao, J.X.; Ma, W.J.; Xia, Z.F.; Zhan, J.H.; Zeng, K.M.; Huang, Y.; Zhao, H.Y.; Zhang, L. HIF-1α inhibition promotes the efficacy of immune checkpoint blockade in the treatment of non-small cell lung cancer. Cancer Lett. 2022, 531, 39–56. [Google Scholar] [CrossRef]
- Liu, P.; Huang, H.; Qi, X.; Bian, C.; Cheng, M.; Liu, L.; Xue, L.; Zhao, X.; Yi, T.; Quan, Y. Hypoxia-Induced LncRNA-MIR210HG Promotes Cancer Progression by Inhibiting HIF-1α Degradation in Ovarian Cancer. Front. Oncol. 2021, 11, 701488. [Google Scholar] [CrossRef]
- Ling, X.; Wan, J.; Peng, B.; Chen, J. Hsp70 Promotes SUMO of HIF-1α and Promotes Lung Cancer Invasion and Metastasis. J. Oncol. 2021, 2021, 7873085. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Qian, Y.; Xu, D.; Yin, Q.; Pan, H.J. Serum tumor markers, hypoxia-inducible factor-1α HIF-1α and vascular endothelial growth factor, in patients with non- small cell lung cancer before and after intervention. Asian Pac. J. Cancer Prev. 2013, 14, 3851–3854. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Li, J.; Wang, J.; Hu, Z. Long Non-Coding RNAs (lncRNAs) Tumor-Suppressive Role of lncRNA on Chromosome 8p12 (TSLNC8) Inhibits Tumor Metastasis and Promotes Apoptosis by Regulating Interleukin 6 (IL-6)/Signal Transducer and Activator of Transcription 3 (STAT3)/Hypoxia-Inducible Factor 1-alpha (HIF-1α) Signaling Pathway in Non-Small Cell Lung Cancer. Med. Sci. Monit. 2019, 25, 7624–7633. [Google Scholar] [PubMed]
- Schneider, S.R.; Mayer, L.C.; Lichtblau, M.; Berlier, C.; Schwarz, E.I.; Saxer, S.; Furian, M.; Bloch, K.E.; Ulrich, S. Effect of Normobaric Hypoxia on Exercise Performance in Pulmonary Hypertension: Randomized Trial. Chest 2021, 159, 757–771. [Google Scholar] [CrossRef]
- Tang, W.; Yang, Z.; Wang, S.; Wang, Z.; Song, J.; Yu, G.; Fan, W.; Dai, Y.; Wang, J.; Shan, L.; et al. Organic semiconducting photoacoustic nanodroplets for laseractivatable ultrasound imaging and combinational cancer therapy. ACS Nano 2018, 12, 2610–2622. [Google Scholar] [CrossRef]
- Song, G.; Liang, C.; Yi, X.; Zhao, Q.; Cheng, L.; Yang, K.; Liu, Z. Perfluorocarbon-Loaded Hollow Bi2Se3 Nanoparticles for Timely Supply of Oxygen under Near-Infrared Light to Enhance the Radiotherapy of Cancer. Adv. Mater. 2016, 28, 2716–2723. [Google Scholar] [CrossRef]
- Hu, D.R.; Zhong, L.; Wang, M.Y.; Li, H.H.; Qu, Y.; Liu, Q.Y.; Han, R.; Yuan, L.P.; She, K.; Peng, J.R.; et al. Perfluorocarbon-loaded and redox-activatable photosensitizing agent with oxygen supply for enhancement of fluorescence/photoacoustic imaging guided tumor photodynamic therapy. Adv. Funct. Mater. 2019, 29, 1806199. [Google Scholar] [CrossRef]
- Liu, C.P.; Wu, T.H.; Liu, C.Y.; Chen, K.C.; Chen, Y.X.; Chen, G.S.; Lin, S.Y. Self-supplying O2 through the catalase-like activity of gold nanoclusters for photodynamic therapy against hypoxic cancer cells. Small 2017, 13, 1700278. [Google Scholar] [CrossRef]
- Prasad, P.; Gordijo, C.R.; Abbasi, A.Z.; Maeda, A.; Ip, A.; Rauth, A.M.; DaCosta, R.S.; Wu, X.Y. Multifunctional albumin-MnO2 nanoparticles modulate solid tumor microenvironment by attenuating hypoxia, acidosis, vascular endothelial growth factor and enhance radiation response. ACS Nano 2014, 8, 3202–3212. [Google Scholar] [CrossRef]
- Atkinson, R.L.; Zhang, M.; Diagaradjane, P.; Peddibhotla, S.; Contreras, A.; Hilsenbeck, S.G.; Woodward, W.A.; Krishnan, S.; Chang, J.C.; Rosen, J.M. Thermal Enhancement with Optically Activated Gold Nanoshells Sensitizes Breast Cancer Stem Cells to Radiation Therapy. Sci. Transl. Med. 2010, 2, 55ra79. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Y.; Yan, H.; Wang, H.; Zhang, Y.; Li, M.; Cui, K.; Xiao, Z.; Liu, L.; Xie, W. CuS-131I-PEG Nanotheranostics-Induced “Multiple Mild-Hyperthermia” Strategy to Overcome Radio-Resistance in Lung Cancer Brachytherapy. Pharmaceutics 2022, 14, 2669. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics14122669
Cui Y, Yan H, Wang H, Zhang Y, Li M, Cui K, Xiao Z, Liu L, Xie W. CuS-131I-PEG Nanotheranostics-Induced “Multiple Mild-Hyperthermia” Strategy to Overcome Radio-Resistance in Lung Cancer Brachytherapy. Pharmaceutics. 2022; 14(12):2669. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics14122669
Chicago/Turabian StyleCui, Yanna, Hui Yan, Haoze Wang, Yongming Zhang, Meng Li, Kai Cui, Zeyu Xiao, Liu Liu, and Wenhui Xie. 2022. "CuS-131I-PEG Nanotheranostics-Induced “Multiple Mild-Hyperthermia” Strategy to Overcome Radio-Resistance in Lung Cancer Brachytherapy" Pharmaceutics 14, no. 12: 2669. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics14122669