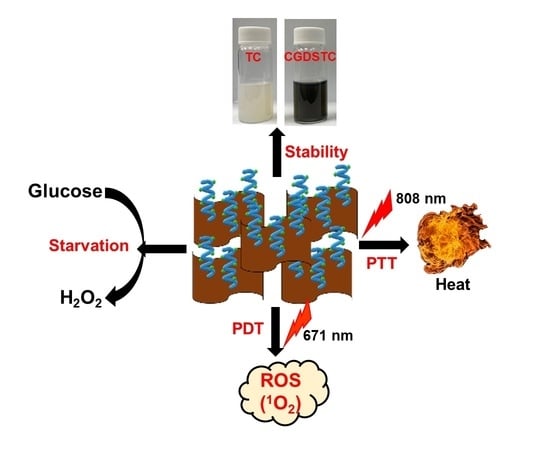
Engineering the Surface of Ti3C2 MXene Nanosheets for High Stability and Multimodal Anticancer Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Instrumentation and Characterization
2.3. Synthesis of Monolayer Ti3C2 MXene (TC)
2.4. Synthesis of DSTC NSs
2.5. Conjugation of GOx to DSTC NSs (GDSTC NSs)
2.6. Conjugation of Ce6 to GDSTC NSs (CGDSTC NSs)
2.7. Determination of Photothermal Effect of CGDSTC NSs
2.8. Determination of Photodynamic Properties of CGDSTC NSs
2.9. Detection of H2O2 Concentration
2.10. Detection of Solution pH
2.11. Detection of Dissolved O2
2.12. Determination of Gluconic Acid Concentration
2.13. Cytocompatibility of CGDSTC NSs
2.14. Cellular Internalization and Intracellular ROS Detection
2.15. In Vitro Therapeutic Efficacy of CGDSTC NSs
3. Results and Discussion
3.1. Synthesis and Characterization of CGDSTC NSs
3.2. Photothermal Properties of CGDSTC NSs
3.3. Photodynamic Properties of CGDSTC NSs
3.4. Enzymatic Activities of CGDSTC NSs
3.5. In Vitro Cytocompatibility of CGDSTC NSs
3.6. Cellular Internalization of CGDSTC NSs
3.7. Intracellular ROS Detection
3.8. Cooperative Effect of NSs on Cancer Cell Death
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rohaizad, N.; Mayorga-Martinez, C.C.; Fojtů, M.; Latiff, N.M.; Pumera, M. Two-Dimensional Materials in Biomedical, Biosensing and Sensing Applications. Chem. Soc. Rev. 2021, 50, 619–657. [Google Scholar] [CrossRef]
- Nguyen, E.P.; Silva, C.D.C.C.; Merkoçi, A. Recent advancement in biomedical applications on the surface of two-dimensional materials: From biosensing to tissue engineering. Nanoscale 2020, 12, 19043–19067. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, X.; Shen, S.; Liu, T.; Cheng, L.; Liu, Z. Two-Dimensional TiS2 Nanosheets for in Vivo Photoacoustic Imaging and Photothermal Cancer Therapy. Nanoscale 2015, 7, 6380–6387. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sui, L.; Huang, J.; Miao, L.; Nie, Y.; Wang, K.; Yang, Z.; Huang, Q.; Gong, X.; Nan, Y.; et al. MoS2-Based Nanocomposites for Cancer Diagnosis and Therapy. Bioact. Mater. 2021, 6, 4209–4242. [Google Scholar] [CrossRef]
- Manisekaran, R.; García-Contreras, R.; Chettiar, A.-D.R.; Serrano-Díaz, P.; Lopez-Ayuso, C.A.; Arenas-Arrocena, M.C.; Hernández-Padrón, G.; López-Marín, L.M.; Acosta-Torres, L.S. 2D Nanosheets—A New Class of Therapeutic Formulations against Cancer. Pharmaceutics 2021, 13, 1803. [Google Scholar] [CrossRef]
- Liu, H.; Mei, Y.; Zhao, Q.; Zhang, A.; Tang, L.; Gao, H.; Wang, W. Black Phosphorus, an Emerging Versatile Nanoplatform for Cancer Immunotherapy. Pharmaceutics 2021, 13, 1344. [Google Scholar] [CrossRef]
- Wen, J.; Yang, K.; Huang, J.; Sun, S. Recent advances in LDH-based nanosystems for cancer therapy. Mater. Des. 2020, 198, 109298. [Google Scholar] [CrossRef]
- Liu, C.; Qin, H.; Kang, L.; Chen, Z.; Wang, H.; Qiu, H.; Ren, J.; Qu, X. Graphitic Carbon Nitride Nanosheets as a Multifunctional Nanoplatform for Photochemical Internalization-Enhanced Photodynamic Therapy. J. Mater. Chem. B 2018, 6, 7908–7915. [Google Scholar] [CrossRef]
- Ciofani, M.E.; Sen, O.; Culha, M. Hexagonal Boron Nitride Nanoparticles for Prostate Cancer Treatment. ACS Appl. Nano Mater. 2020, 3, 2364–2372. [Google Scholar] [CrossRef]
- Saeb, M.R.; Rabiee, N.; Mozafari, M.; Verpoort, F.; Voskressensky, L.G.; Luque, R. Metal–Organic Frameworks (MOFs) for Cancer Therapy. Materials 2021, 14, 7277. [Google Scholar] [CrossRef] [PubMed]
- Pandey, N.; Dhiman, S.; Srivastava, T.; Majumder, S. Transition metal oxide nanoparticles are effective in inhibiting lung cancer cell survival in the hypoxic tumor microenvironment. Chem. Interactions 2016, 254, 221–230. [Google Scholar] [CrossRef]
- Pogorielov, M.; Smyrnova, K.; Kyrylenko, S.; Gogotsi, O.; Zahorodna, V.; Pogrebnjak, A. Mxenes—A New Class of Two-Dimensional Materials: Structure, Properties and Potential Applications. Nanomaterials 2021, 11, 3412. [Google Scholar] [CrossRef]
- Dai, C.; Chen, Y.; Jing, X.; Xiang, L.; Yang, D.; Lin, H.; Liu, Z.; Han, X.; Wu, R. Two-Dimensional Tantalum Carbide (MXenes) Composite Nanosheets for Multiple Imaging-Guided Photothermal Tumor Ablation. ACS Nano 2017, 11, 12696–12712. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Huang, J.; Lin, H.; Wang, Z.; Li, P.; Chen, Y. 2d Ultrathin Mxene-Based Drug-Delivery Nanoplatform for Synergistic Photothermal Ablation and Chemotherapy of Cancer. Adv. Healthc. Mater. 2018, 7, 1701394. [Google Scholar] [CrossRef]
- Lin, H.; Wang, X.; Yu, L.; Chen, Y.; Shi, J. Two-Dimensional Ultrathin MXene Ceramic Nanosheets for Photothermal Conversion. Nano Lett. 2016, 17, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zou, J.; Tang, Q.; Yang, X.; Zhang, Y.; Zhang, Q.; Huang, W.; Chen, P.; Shao, J.; Dong, X. Surface Modified Ti3C2 Mxene Nanosheets for Tumor Targeting Photothermal/Photodynamic/Chemo Synergistic Therapy. ACS Appl. Mater. Interfaces 2017, 9, 40077–40086. [Google Scholar] [CrossRef]
- Xing, C.; Chen, S.; Liang, X.; Liu, Q.; Qu, M.; Zou, Q.; Li, J.; Tan, H.; Liu, L.; Fan, D.; et al. Two-Dimensional MXene (Ti3C2)-Integrated Cellulose Hydrogels: Toward Smart Three-Dimensional Network Nanoplatforms Exhibiting Light-Induced Swelling and Bimodal Photothermal/Chemotherapy Anticancer Activity. ACS Appl. Mater. Interfaces 2018, 10, 27631–27643. [Google Scholar] [CrossRef]
- Huang, X.; Lu, Y.; Guo, M.; Du, S.; Han, N. Recent strategies for nano-based PTT combined with immunotherapy: From a biomaterial point of view. Theranostics 2021, 11, 7546–7569. [Google Scholar] [CrossRef]
- Han, L.; Lu, X.; Liu, K.; Wang, K.; Fang, L.; Weng, L.-T.; Zhang, H.; Tang, Y.; Ren, F.; Zhao, C.; et al. Mussel-Inspired Adhesive and Tough Hydrogel Based on Nanoclay Confined Dopamine Polymerization. ACS Nano 2017, 11, 2561–2574. [Google Scholar] [CrossRef]
- Varvara, M.; Bozzo, G.; Celano, G.; Disanto, C.; Pagliarone, C.N.; Celano, G.V. The Use of Ascorbic Acid as a Food Additive: Technical-Legal Issues. Ital. J. Food Saf. 2016, 5, 4313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lepock, J.R. Cellular effects of hyperthermia: Relevance to the minimum dose for thermal damage. Int. J. Hyperth. 2003, 19, 252–266. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Gao, X.; Huang, X.; Ge, H.; Xie, M.; Qian, J.; Song, J.; Li, Y.; Zhu, X.; Zhang, C. Polydopamine-Coated Nucleic Acid Nanogel for Sirna-Mediated Low-Temperature Photothermal Therapy. Biomaterials 2020, 245, 119976. [Google Scholar] [CrossRef]
- Long, Q.; Lin, T.-Y.; Huang, Y.; Li, X.; Ma, A.-H.; Zhang, H.; Carney, R.; Airhart, S.; Lam, K.S.; White, R.W.D.; et al. Image-guided photo-therapeutic nanoporphyrin synergized HSP90 inhibitor in patient-derived xenograft bladder cancer model. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 789–799. [Google Scholar] [CrossRef]
- Chen, B.-Q.; Kankala, R.K.; Zhang, Y.; Xiang, S.-T.; Tang, H.-X.; Wang, Q.; Yang, D.-Y.; Wang, S.-B.; Zhang, Y.S.; Liu, G.; et al. Gambogic acid augments black phosphorus quantum dots (BPQDs)-based synergistic chemo-photothermal therapy through downregulating heat shock protein expression. Chem. Eng. J. 2020, 390, 124312. [Google Scholar] [CrossRef]
- You, C.; Li, Y.-J.; Dong, Y.; Ning, L.; Zhang, Y.; Yao, L.; Wang, F. Low-Temperature Trigger Nitric Oxide Nanogenerators for Enhanced Mild Photothermal Therapy. ACS Biomater. Sci. Eng. 2020, 6, 1535–1542. [Google Scholar] [CrossRef]
- Garrido, C.; Schmitt, E.; Candé, C.; Vahsen, N.; Parcellier, A.; Kroemer, G. HSP27 and HSP70: Potentially Oncogenic Apoptosis Inhibitors. Cell Cycle 2003, 2, 579–584. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Ding, L.; Chen, Y.; Shi, J. Augmenting Tumor—Starvation Therapy by Cancer Cell Autophagy Inhibition. Adv. Sci. 2020, 7, 1902847. [Google Scholar] [CrossRef] [Green Version]
- Ciou, T.-Y.; Korupalli, C.; Chou, T.-H.; Hsiao, C.-H.; Getachew, G.; Bela, S.; Chang, J.-Y. Biomimetic Nanoreactor for Cancer Eradication via Win–Win Cooperation between Starvation/Photo/Chemodynamic Therapies. ACS Appl. Bio Mater. 2021, 4, 5650–5660. [Google Scholar] [CrossRef]
- Hu, J.J.; Liu, M.D.; Gao, F.; Chen, Y.; Peng, S.Y.; Li, Z.H.; Cheng, H.; Zhang, X.Z. Photo-Controlled Liquid Metal Nanoparticle-Enzyme for Starvation/Photothermal Therapy of Tumor by Win-Win Cooperation. Biomaterials 2019, 217, 119303. [Google Scholar] [CrossRef]
- Shen, Z.; Ma, Q.; Zhou, X.; Zhang, G.; Hao, G.; Sun, Y.; Cao, J. Strategies to Improve Photodynamic Therapy Efficacy by Relieving the Tumor Hypoxia Environment. NPG Asia Mater. 2021, 13, 39. [Google Scholar] [CrossRef]
- Liu, X.; Li, B.; Fu, F.; Xu, K.; Zou, R.; Wang, Q.; Zhang, B.; Chen, Z.; Hu, J. Facile synthesis of biocompatible cysteine-coated CuS nanoparticles with high photothermal conversion efficiency for cancer therapy. Dalton Trans. 2014, 43, 11709–11715. [Google Scholar] [CrossRef] [PubMed]
- Soleymaniha, M.; Shahbazi, M.-A.; Rafieerad, A.R.; Maleki, A.; Amiri, A. Promoting Role of MXene Nanosheets in Biomedical Sciences: Therapeutic and Biosensing Innovations. Adv. Health Mater. 2018, 8, 1801137. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-R.; Kim, Y.-J. Hydrophilic Chlorin e6-Poly(amidoamine) Dendrimer Nanoconjugates for Enhanced Photodynamic Therapy. Nanomaterials 2018, 8, 445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [Green Version]
- Getachew, G.; Korupalli, C.; Rasal, A.S.; Chang, J.-Y. ROS generation/scavenging modulation of carbon dots as phototherapeutic candidates and peroxidase mimetics to integrate with polydopamine nanoparticles/GOx towards cooperative cancer therapy. Compos. Part B Eng. 2021, 226, 109364. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korupalli, C.; You, K.-L.; Getachew, G.; Rasal, A.S.; Dirersa, W.B.; Zakki Fahmi, M.; Chang, J.-Y. Engineering the Surface of Ti3C2 MXene Nanosheets for High Stability and Multimodal Anticancer Therapy. Pharmaceutics 2022, 14, 304. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics14020304
Korupalli C, You K-L, Getachew G, Rasal AS, Dirersa WB, Zakki Fahmi M, Chang J-Y. Engineering the Surface of Ti3C2 MXene Nanosheets for High Stability and Multimodal Anticancer Therapy. Pharmaceutics. 2022; 14(2):304. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics14020304
Chicago/Turabian StyleKorupalli, Chiranjeevi, Kai-Long You, Girum Getachew, Akash S. Rasal, Worku Batu Dirersa, Mochamad Zakki Fahmi, and Jia-Yaw Chang. 2022. "Engineering the Surface of Ti3C2 MXene Nanosheets for High Stability and Multimodal Anticancer Therapy" Pharmaceutics 14, no. 2: 304. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics14020304