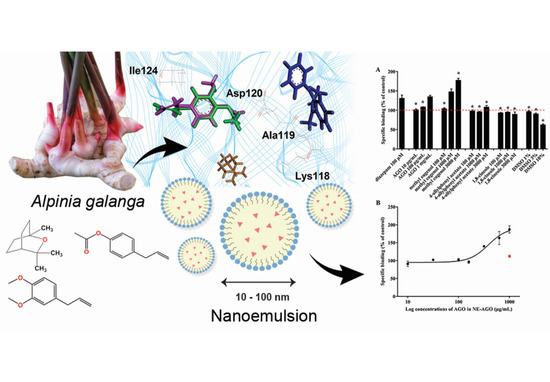
The Binding of Alpinia galanga Oil and Its Nanoemulsion to Mammal GABAA Receptors Using Rat Cortical Membranes and an In Silico Modeling Platform
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction and Chemical Analysis of AGO
2.3. Preparation and Characterization of NE-AGO
2.4. [3H]Muscimol Binding Assay
2.5. Computational Method
2.5.1. Preparation of Ligands for Molecular Docking
2.5.2. Molecular Docking and Dynamics Simulation
2.6. Cytotoxicity on Peripheral Blood Mononuclear Cells
2.7. Statistical Analysis
3. Results and Discussion
3.1. Extraction and Chemical Analysis of AGO
3.2. Preparation and Characterization of NE-AGO
3.3. [3H]Muscimol Binding
3.4. Molecular Docking and Dynamics Simulation
3.5. Cytotoxicity to Human Normal Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prakatthagomol, W.; Sirithunyalug, J.; Okonogi, S. Comparison of antibacterial activity against food-borne bacteria of Alpinia galanga, Curcuma longa, and Zingiber cassumunar. Chiang Mai Univ. J. Nat. Sci. 2012, 11, 177–186. [Google Scholar]
- Khumpirapang, N.; Klayraung, S.; Tima, S.; Okonogi, S. Development of microemulsion containing Alpinia galanga oil and its major compounds: Enhancement of antimicrobial activities. Pharmaceutics 2021, 13, 265. [Google Scholar] [CrossRef] [PubMed]
- Chudiwal, A.K.; Jain, D.P.; Somani, R.S. Alpinia galanga Willd—An overview on phyto-pharmacological properties. Indian J. Nat. Prod. Resour. 2010, 1, 143–149. [Google Scholar]
- Khumpirapang, N.; Pikulkaew, S.; Müllertz, A.; Rades, T.; Okonogi, S. Self-microemulsifying drug delivery system and nanoemulsion for enhancing aqueous miscibility of Alpinia galanga oil. PLoS ONE 2017, 12, e0188848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khumpirapang, N.; Pikulkaew, S.; Anuchapreeda, S.; Okonogi, S. Alpinia galanga oil-A new natural source of fish anaesthetic. Aquac. Res. 2018, 49, 1546–1556. [Google Scholar] [CrossRef]
- De Araújo, F.Y.R.; Silva, M.I.G.; Moura, B.A.; De Oliveira, G.V.; Leal, L.K.A.M.; Vasconcelos, S.M.M.; Viana, G.S.B.; De Moraes, M.O.; De Sousa, F.C.F.; Macêdo, D.S. Central nervous system effects of the essential oil of the leaves of Alpinia zerumbet in mice. J. Pharm. Pharmacol. 2009, 61, 1521–1527. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.A.; Rao, V.S.N. Antiinflammatory and antinociceptive effects of 1, 8-cineole a terpenoid oxide present in many plant essential oils. Phyther. Res. 2000, 14, 240–244. [Google Scholar] [CrossRef]
- Carlini, E.A.; Dallmeier, K.; Zelger, J.L. Methyleugenol as a surgical anesthetic in rodents. Experientia 1981, 37, 588–589. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.S.; Yeh, J.; Flood, P. Anesthesia matters: Patients anesthetized with propofol have less postoperative pain than those anesthetized with isoflurane. Anesth. Analg. 2008, 106, 264–269. [Google Scholar] [CrossRef]
- Garcia, P.S.; Kolesky, S.E.; Jenkins, A. General anesthetic actions on gabaa receptors. Curr. Neuropharmacol. 2010, 8, 2–9. [Google Scholar] [CrossRef]
- Hall, A.C.; Turcotte, C.M.; Betts, B.A.; Yeung, W.-Y.; Agyeman, A.S.; Burk, L.A. Modulation of human GABAA and glycine receptor currents by menthol and related monoterpenoids. Eur. J. Pharmacol. 2004, 506, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Olsen, R.W. GABAA receptor: Positive and negative allosteric modulators. Neuropharmacology 2018, 136, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Has, A.T.C.; Islam, M.R.; Baburin, I.; Hering, S.; Osman, H.; Mohamad, H.; Abdullah, J.M. The inhibitory activity of nutmeg essential oil on GABAAα1β2γ2s receptors. Biomed. Res. 2014, 25, 543–550. [Google Scholar]
- Perumalla, A.V.S.; Hettiarachchy, N.S. Green tea and grape seed extracts—Potential applications in food safety and quality. Food Res. Int. 2011, 44, 827–839. [Google Scholar] [CrossRef]
- Khumpirapang, N.; von Gersdorff Jørgensen, L.; Müllertz, A.; Rades, T.; Okonogi, S. Formulation optimization, anesthetic activity, skin permeation, and transportation pathway of Alpinia galanga oil SNEDDS in zebrafish (Danio rerio). Eur. J. Pharm. Biopharm. 2021, 165, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Okonogi, S.; Phumat, P.; Khongkhunthian, S.; Chaijareenont, P.; Rades, T.; Müllertz, A. Development of self-nanoemulsifying drug delivery systems containing 4-allylpyrocatechol for treatment of oral infections caused by Candida albicans. Pharmaceutics 2021, 13, 167. [Google Scholar] [CrossRef] [PubMed]
- Stylios, G.K.; Giannoudis, P.V.; Wan, T. Applications of nanotechnologies in medical practice. Injury 2005, 36, S6–S13. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, K.; Conti, D.S.; da Rocha, S.R.; Zhang, Y. Application of an oregano oil nanoemulsion to the control of foodborne bacteria on fresh lettuce. Food Microbiol. 2015, 47, 69–73. [Google Scholar] [CrossRef]
- Anuchapreeda, S.; Fukumori, Y.; Okonogi, S.; Ichikawa, H. Preparation of lipid nanoemulsions incorporating curcumin for cancer therapy. J. Nanotechnol. 2012, 2012, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Kheawfu, K.; Pikulkaew, S.; Rades, T.; Müllertz, A.; Okonogi, S. Development and characterization of clove oil nanoemulsions and self-microemulsifying drug delivery systems. J. Drug Deliv. Sci. Technol. 2018, 46, 330–338. [Google Scholar] [CrossRef]
- Kheawfu, K.; Pikulkaew, S.; Hamamoto, H.; Sekimizu, K.; Okonogi, S. Influence of clove oil and eugenol on muscle contraction of silkworm (Bombyx mori). Drug Discov. Ther. 2017, 11, 64–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okonogi, S.; Chaiyana, W. Enhancement of anti-cholinesterase activity of Zingiber cassumunar essential oil using a microemulsion technique. Drug Discov. Ther. 2012, 6, 249–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ransom, R.W.; Stec, N.L. Cooperative modulation of [3H]MK-801 binding to the N-methyl-d-aspartate receptor-ion channel complex by l-glutamate, glycine, and polyamines. J. Neurochem. 1988, 51, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Karim, N.; Gavande, N.; Wellendorph, P.; Johnston, G.A.R.; Hanrahan, J.R.; Chebib, M. 3-Hydroxy-2′-methoxy-6-methylflavone: A potent anxiolytic with a unique selectivity profile at GABAA receptor subtypes. Biochem. Pharmacol. 2011, 82, 1971–1983. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Cheng, T.; Wang, Y.; Bryant, S.H. PubChem as a public resource for drug discovery. Drug Discov. Today 2010, 15, 1052–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, Y.; Wu, C.; Chowdhury, S.; Lee, M.C.; Xiong, G.; Zhang, W.; Yang, R.; Cieplak, P.; Luo, R.; Lee, T.; et al. A point-charge force field for molecular mechanics simulations of proteins based on condensed-phase quantum mechanical calculations. J. Comput. Chem. 2003, 24, 1999–2012. [Google Scholar] [CrossRef] [PubMed]
- Khonkarn, R.; Okonogi, S.; Ampasavate, C.; Anuchapreeda, S. Investigation of fruit peel extracts as sources for compounds with antioxidant and antiproliferative activities against human cell lines. Food Chem. Toxicol. 2010, 48, 2122–2129. [Google Scholar] [CrossRef]
- Okonogi, S.; Duangrat, C.; Anuchpreeda, S.; Tachakittirungrod, S.; Chowwanapoonpohn, S. Comparison of antioxidant capacities and cytotoxicities of certain fruit peels. Food Chem. 2007, 103, 839–846. [Google Scholar] [CrossRef]
- Ding, J.; Huang, C.; Peng, Z.; Xie, Y.; Deng, S.; Nie, Y.-Z.; Xu, T.-L.; Ge, W.-H.; Li, W.-G.; Li, F. Electrophysiological characterization of methyleugenol: A novel agonist of GABA(A) receptors. ACS Chem. Neurosci. 2014, 5, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Khumpirapang, N.; Chaichit, S.; Jiranusornkul, S.; Pikulkaew, S.; Müllertz, A.; Okonogi, S. In vivo anesthetic effect and mechanism of action of active compounds from Alpinia galanga oil on Cyprinus carpio (koi carp). Aquaculture 2018, 496, 176–184. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Li, Z.-H.; Wang, C.-F.; Wei, J.-Y.; Li, X.-L.; Wang, P.-J.; Zhou, Z.-F.; Du, S.-S.; Huang, D.-Y. Composition of the essential oil from Alpinia galanga rhizomes and its bioactivity on Lasioderma serricorne. Bull. Insectol. 2014, 67, 247–254. [Google Scholar]
- Faridah, Q.Z.; Abdelmageed, A.H.A.; AN, N.H.; Yaacob, M. Comparative study of essential oil composition of leaves and rhizomes of Alpinia conchigera Griff. at different post-harvest drying periods. J. Med. Plants Res. 2010, 4, 2700–2705. [Google Scholar]
- Song, R.; Shen, G.; Liu, Y.; Tang, F.; Chen, Q.; Sun, P. Preparation and characterization of an oil-in-water microemulsion of thiamethoxam and acetamiprid without organic solvent for unmanned aerial vehicle spraying. Colloids Surf. A Physicochem. Eng. Asp. 2020, 607, 125485. [Google Scholar] [CrossRef]
- Debnath, S.; Satayanarayana, K.V.; Kumar, G.V. Nanoemulsion—A method to improve the solubility of lipophilic drugs. Pharmanest 2011, 2, 72–83. [Google Scholar]
- Aktaş, Z. Effect of non-ionic reagent adsorption on zeta potential of fine coal particles. Turkish J. Chem. 2000, 24, 117–130. [Google Scholar]
- Marinova, K.G.; Alargova, R.G.; Denkov, N.D.; Velev, O.D.; Petsev, D.N.; Ivanov, A.I.B.; Borwankar, R.P. Charging of oil−water interfaces due to spontaneous adsorption of hydroxyl ions. Langmuir 1996, 12, 2045–2051. [Google Scholar] [CrossRef]
- Falk-Petersen, C.B.; Tsonkov, T.M.; Nielsen, M.S.; Harpsøe, K.; Bundgaard, C.; Frølund, B.; Kristiansen, U.; Gloriam, D.E.; Wellendorph, P. Discovery of a new class of orthosteric antagonists with nanomolar potency at extrasynaptic GABAA receptors. Sci. Rep. 2020, 10, 10078. [Google Scholar] [CrossRef]
- Tan, K.R.; Baur, R.; Charon, S.; Goeldner, M.; Sigel, E. Relative positioning of diazepam in the benzodiazepine-binding-pocket of GABAA receptors. J. Neurochem. 2009, 111, 1264–1273. [Google Scholar] [CrossRef]
- Singh, N.; Villoutreix, B.O. Demystifying the molecular basis of pyrazoloquinolinones recognition at the extracellular α1+/β3- interface of the GABAA receptor by molecular modeling. Front. Pharmacol. 2020, 11, 1457. [Google Scholar] [CrossRef]
- Jeyakumar, M.; Webb, P.; Baxter, J.D.; Scanlan, T.S.; Katzenellenbogen, J.A. Quantification of ligand-regulated nuclear receptor corepressor and coactivator binding, key interactions determining ligand potency and efficacy for the thyroid hormone receptor. Biochemistry 2008, 47, 7465–7476. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.-P.; Tsai, W.-J.; Lin, Y.-L.; Liao, J.-F.; Chen, C.-F.; Kuo, Y.-C. The extracts from Nelumbo Nucifera suppress cell cycle progression, cytokine genes expression, and cell proliferation in human peripheral blood mononuclear cells. Life Sci. 2004, 75, 699–716. [Google Scholar] [CrossRef]
- Anazetti, M.C.; Melo, P.S.; Durán, N.; Haun, M. Comparative cytotoxicity of dimethylamide-crotonin in the promyelocytic leukemia cell line (HL60) and human peripheral blood mononuclear cells. Toxicology 2003, 188, 261–274. [Google Scholar] [CrossRef]
- Ferreira-Da-Silva, F.W.; da Silva-Alves, K.S.; Alves-Fernandes, T.A.; Coelho-De-Souza, A.N.; Leal-Cardoso, J.H. Effects of 1,8-cineole on Na+ currents of dissociated superior cervical ganglia neurons. Neurosci. Lett. 2015, 595, 45–49. [Google Scholar] [CrossRef] [Green Version]
- Day, T.K.; Skarda, R.T. The pharmacology of local anesthetics. Veter-Clin. N. Am. Equine Pract. 1991, 7, 489–500. [Google Scholar] [CrossRef]







| No. | Components | Retention Time (min) | Amount (%) |
|---|---|---|---|
| 1 | β-Pinene | 5.08 | 0.72 ± 0.07 |
| 2 | 1,8-Cineole | 6.62 | 41.94 ± 0.13 |
| 3 | α-Terpineol | 11.49 | 2.64 ± 0.06 |
| 4 | Terpinen-4-ol | 11.89 | 3.07 ± 0.04 |
| 5 | Chavicol | 15.85 | 1.33 ± 0.08 |
| 6 | 4-Allylphenyl acetate | 19.02 | 35.70 ± 0.14 |
| 7 | Geranyl acetate | 20.40 | 0.55 ± 0.01 |
| 8 | Methyl eugenol | 21.39 | 3.23 ± 0.02 |
| 9 | α-Farnesene | 22.32 | 0.58 ± 0.05 |
| 10 | β-Bisaboloene | 25.29 | 0.78 ± 0.01 |
| 11 | β-Sesquiphellandrene | 25.87 | 0.53 ± 0.01 |
| 12 | Eugenyl acetate | 26.22 | 1.19 ± 0.02 |
| 13 | 9-Octadecenoic acid | 44.86 | 2.34 ± 0.12 |
| 14 | 9-Octadecenamide | 46.53 | 3.39 ± 0.07 |
| Total | 97.99 ± 0.34 | ||
| Ligand | MM-GBSA Binding Energy (kcal/mol) |
|---|---|
| Methyl eugenol | −22.16 |
| 1,8-Cineole | −16.63 |
| 4-Allylphenyl acetate | −23.72 |
| Diazepam | −15.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khumpirapang, N.; Suknuntha, K.; Wongrattanakamon, P.; Jiranusornkul, S.; Anuchapreeda, S.; Wellendorph, P.; Müllertz, A.; Rades, T.; Okonogi, S. The Binding of Alpinia galanga Oil and Its Nanoemulsion to Mammal GABAA Receptors Using Rat Cortical Membranes and an In Silico Modeling Platform. Pharmaceutics 2022, 14, 650. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics14030650
Khumpirapang N, Suknuntha K, Wongrattanakamon P, Jiranusornkul S, Anuchapreeda S, Wellendorph P, Müllertz A, Rades T, Okonogi S. The Binding of Alpinia galanga Oil and Its Nanoemulsion to Mammal GABAA Receptors Using Rat Cortical Membranes and an In Silico Modeling Platform. Pharmaceutics. 2022; 14(3):650. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics14030650
Chicago/Turabian StyleKhumpirapang, Nattakanwadee, Krit Suknuntha, Pathomwat Wongrattanakamon, Supat Jiranusornkul, Songyot Anuchapreeda, Petrine Wellendorph, Anette Müllertz, Thomas Rades, and Siriporn Okonogi. 2022. "The Binding of Alpinia galanga Oil and Its Nanoemulsion to Mammal GABAA Receptors Using Rat Cortical Membranes and an In Silico Modeling Platform" Pharmaceutics 14, no. 3: 650. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics14030650