Increasing the Batch Size of a QESD Crystallization by Using a MSMPR Crystallizer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mixed-Suspension, Mixed-Product Removal (MSMPR) Crystallizer
2.3. MSMPR QESD Crystallization of Metformin Hydrochloride
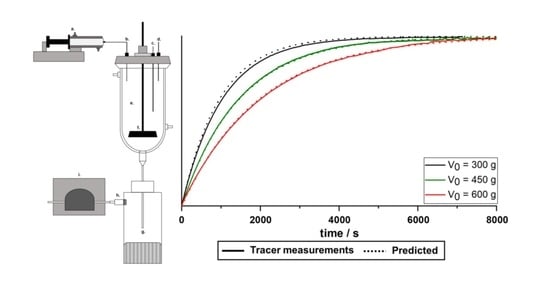
2.4. Determination of Residence Time Distribution (RTD) and Calculation of Solvent Fraction
2.5. Particle Size Distribution (PSD)
2.6. Microscopy
2.7. Flowability
2.8. Earth Mover’s Distance (EMD) for the Comparison of PSDs
2.9. Tableting
3. Results and Discussion
3.1. Residence Time Distribution (RTD) and Solvent Fraction (SF)
3.2. Reproducibility of MSMPR Batches
3.3. Considerations When Developing a MSMPR Crystallizer for QESD Crystallizations
3.3.1. Choosing a Suitable Transfer Pump for the Addition of the MF Solution
3.3.2. Continuously Removing Product from the Crystallizer
3.3.3. Influence of Stirrer Type and Material
3.3.4. Choice of Polymeric Stabilizer
3.4. Influence of MRT on Agglomerate Size
3.5. Increasing Batch Size
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poechlauer, P.; Manley, J.; Broxterman, R.; Gregertsen, B.; Ridemark, M. Continuous processing in the manufacture of active pharmaceutical ingredients and finished dosage forms: An industry perspective. Org. Process Res. Dev. 2012, 16, 1586–1590. [Google Scholar] [CrossRef]
- Lee, S.L.; O’Connor, T.F.; Yang, X.; Cruz, C.N.; Chatterjee, S.; Madurawe, R.D.; Moore, C.; Yu, L.X.; Woodcock, J. Modernizing pharmaceutical manufacturing: From batch to continuous production. J. Pharm. Innov. 2015, 10, 191–199. [Google Scholar] [CrossRef]
- Chen, J.; Sarma, B.; Evans, J.M.; Myerson, A.S. Pharmaceutical crystallization. Cryst. Growth Des. 2011, 11, 887–895. [Google Scholar] [CrossRef]
- Kawashima, Y.; Niwa, T.; Handa, T.; Takeuchi, H.; Iwamoto, T.; Itoh, K. Preparation of controlled-release microspheres of ibuprofen with acrylic polymers by a novel quasi-emulsion solvent diffusion method. J. Pharm. Sci. 1989, 78, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Morishima, K.; Kawashima, Y.; Kawashima, Y.; Takeuchi, H.; Niwa, T.; Hino, T. Micromeritic characteristics and agglomeration mechanisms in the spherical crystallization of bucillamine by the spherical agglomeration and the emulsion solvent diffusion methods. Powder Technol. 1993, 76, 57–64. [Google Scholar] [CrossRef]
- Chen, H.; Aburub, A.; Sun, C.C. Direct Compression Tablet Containing 99% Active Ingredient-A Tale of Spherical Crystallization. J. Pharm. Sci. 2019, 108, 1396–1400. [Google Scholar] [CrossRef]
- Hansen, J.; Kleinebudde, P. Enabling the direct compression of metformin hydrochloride through QESD crystallization. Int. J. Pharm. 2021, 605, 120796. [Google Scholar] [CrossRef]
- Hansen, J.; Kleinebudde, P. Improving flowability and reducing storage agglomeration of metformin hydrochloride through QESD crystallization. Eur. J. Pharm. Biopharm. 2021, 159, 170–176. [Google Scholar] [CrossRef]
- Arndt, O.-R.; Kleinebudde, P. Roll compaction and tableting of high loaded metformin formulations using efficient binders. AAPS PharmSciTech 2018, 19, 2068–2076. [Google Scholar] [CrossRef]
- Espitalier, F.; Biscans, B.; Laguerie, C. Particle design Part B: Batch quasi-emulsion process and mechanism of grain formation of ketoprofen. Chem. Eng. J. 1997, 68, 103–114. [Google Scholar] [CrossRef]
- Powell, K.A.; Saleemi, A.N.; Rielly, C.D.; Nagy, Z.K. Periodic steady-state flow crystallization of a pharmaceutical drug using MSMPR operation. Chem. Eng. Process. Process Intensif. 2015, 97, 195–212. [Google Scholar] [CrossRef]
- Ferguson, S.; Ortner, F.; Quon, J.; Peeva, L.; Livingston, A.; Trout, B.L.; Myerson, A.S. Use of continuous MSMPR crystallization with integrated nanofiltration membrane recycle for enhanced yield and purity in API crystallization. Cryst. Growth Des. 2014, 14, 617–627. [Google Scholar] [CrossRef]
- Tahara, K.; O’Mahony, M.; Myerson, A.S. Continuous Spherical Crystallization of Albuterol Sulfate with Solvent Recycle System. Cryst. Growth Des. 2015, 15, 5149–5156. [Google Scholar] [CrossRef]
- Kinart, C.M.; Kinart, W.J.; Ćwiklińska, A. Density and viscosity at various temperatures for 2-methoxyethanol+ acetone mixtures. J. Chem. Eng. Data 2002, 47, 76–78. [Google Scholar] [CrossRef]
- Onyemelukwe, I.I.; Parsons, A.R.; Wheatcroft, H.P.; Robertson, A.; Nagy, Z.K.; Rielly, C.D. The role of residence time distribution in the continuous steady-state mixed suspension mixed product removal crystallization of glycine. Cryst. Growth Des. 2018, 19, 66–80. [Google Scholar] [CrossRef]
- Levenspiel, O. Chapter 11 Basics of Non-Ideal Flow. In Chemical Reaction Engineering, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 1998; p. 266. [Google Scholar]
- EDQM. 2.9.34. Bulk Density and Tapped Density of Powders. In European Pharmacopoeria, 10th ed.; Council of Europe: Strasbourg, France, 2018; pp. 384–387. [Google Scholar]
- EDQM. 2.9.36. Powder Flow. In European Pharmacopoeria, 10th ed.; Council of Europe: Strasbourg, France, 2018; pp. 387–390. [Google Scholar]
- Hu, M.; Jiang, X.; Absar, M.; Choi, S.; Kozak, D.; Shen, M.; Weng, Y.-T.; Zhao, L.; Lionberger, R. Equivalence testing of complex particle size distribution profiles based on earth mover’s distance. AAPS J. 2018, 20, 62. [Google Scholar] [CrossRef] [PubMed]
- Fell, J.; Newton, J. Determination of tablet strength by the diametral-compression test. J. Pharm. Sci. 1970, 59, 688–691. [Google Scholar] [CrossRef]
- Nocent, M.; Bertocchi, L.; Espitalier, F.; Baron, M.; Couarraze, G. Definition of a solvent system for spherical crystallization of salbutamol sulfate by quasi-emulsion solvent diffusion (QESD) method. J. Pharm. Sci. 2001, 90, 1620–1627. [Google Scholar] [CrossRef]
- Wang, T.; Lu, H.; Wang, J.; Xiao, Y.; Zhou, Y.; Bao, Y.; Hao, H. Recent progress of continuous crystallization. J. Ind. Eng. Chem. 2017, 54, 14–29. [Google Scholar] [CrossRef]
- Lawton, S.; Steele, G.; Shering, P.; Zhao, L.; Laird, I.; Ni, X.-W. Continuous crystallization of pharmaceuticals using a continuous oscillatory baffled crystallizer. Org. Process Res. Dev. 2009, 13, 1357–1363. [Google Scholar] [CrossRef]
- Wood, B.; Girard, K.P.; Polster, C.S.; Croker, D.M. Progress to date in the design and operation of continuous crystallization processes for pharmaceutical applications. Org. Process Res. Dev. 2019, 23, 122–144. [Google Scholar] [CrossRef]
- Peña, R.; Oliva, J.A.; Burcham, C.L.; Jarmer, D.J.; Nagy, Z.K. Process Intensification through Continuous Spherical Crystallization Using an Oscillatory Flow Baffled Crystallizer. Cryst. Growth Des. 2017, 17, 4776–4784. [Google Scholar] [CrossRef]
- Oliva, J.A.; Wu, W.-L.; Greene, M.R.; Pal, K.; Nagy, Z.K. Continuous Spherical Crystallization of Lysozyme in an Oscillatory Baffled Crystallizer Using Emulsion Solvent Diffusion in Droplets. Cryst. Growth Des. 2020, 20, 934–947. [Google Scholar] [CrossRef]
- Hansen, J.; Kleinebudde, P. Towards a better understanding of the role of stabilizers in QESD crystallizations. Pharm. Res. 2022, 1–14. [Google Scholar] [CrossRef]
- Lamesic, D.; Planinsek, O.; Lavric, Z.; Ilic, I. Spherical agglomerates of lactose with enhanced mechanical properties. Int. J. Pharm. 2017, 516, 247–257. [Google Scholar] [CrossRef]
- Darmali, C.; Mansouri, S.; Yazdanpanah, N.; Nagy, Z.K.; Woo, M.W. Continuous lactose recovery from acid whey by mixed suspension mixed product removal (MSMPR) crystallizer in the presence of impurities. Chem. Eng. Process.-Process Intensif. 2021, 108752. [Google Scholar] [CrossRef]
- Hou, G.; Power, G.; Barrett, M.; Glennon, B.; Morris, G.; Zhao, Y. Development and characterization of a single stage mixed-suspension, mixed-product-removal crystallization process with a novel transfer unit. Cryst. Growth Des. 2014, 14, 1782–1793. [Google Scholar] [CrossRef]
- Peña, R.; Nagy, Z.K. Process Intensification through Continuous Spherical Crystallization Using a Two-Stage Mixed Suspension Mixed Product Removal (MSMPR) System. Cryst. Growth Des. 2015, 15, 4225–4236. [Google Scholar] [CrossRef]
- Tahara, K.; Kono, Y.; Myerson, A.S.; Takeuchi, H. Development of continuous spherical crystallization to prepare fenofibrate agglomerates with impurity complexation using mixed-suspension, mixed-product removal crystallizer. Cryst. Growth Des. 2018, 18, 6448–6454. [Google Scholar] [CrossRef]
- Liu, Y.C.; Domokos, A.; Coleman, S.; Firth, P.; Nagy, Z.K. Development of continuous filtration in a novel continuous filtration carousel integrated with continuous crystallization. Org. Process Res. Dev. 2019, 23, 2655–2665. [Google Scholar] [CrossRef]
- Houcine, I.; Plasari, E.; David, R. Effects of the stirred tank’s design on power consumption and mixing time in liquid phase. Chem. Eng. Technol. Ind. Chem.-Plant Equip.-Process Eng.-Biotechnol. 2000, 23, 605–613. [Google Scholar] [CrossRef]
- Chhabra, R.; Shankar, V. Chapter 7—Liquid Mixing. In Coulson and Richardson’s Chemical Engineering, 7th ed.; Chhabra, R., Shankar, V., Eds.; Butterworth-Heinemann: Oxford, UK, 2018; pp. 333–377. [Google Scholar]
- Krycer, I.; Pope, D.G.; Hersey, J.A. An evaluation of tablet binding agents part I. Solution binders. Powder Technol. 1983, 34, 39–51. [Google Scholar] [CrossRef]












| Batch Size/g | Hausner Ratio | Angle of Repose/° |
|---|---|---|
| 13 | 1.17 ± 0.01 | 30.6° ± 0.6 |
| 19 | 1.15 ± 0.02 | 31.3° ± 0.6 |
| 38 | 1.12 ± 0.01 | 27.3° ± 0.6 |
| 55 | 1.22 ± 0.05 | 31.7° ± 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hansen, J.; Kleinebudde, P. Increasing the Batch Size of a QESD Crystallization by Using a MSMPR Crystallizer. Pharmaceutics 2022, 14, 1227. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics14061227
Hansen J, Kleinebudde P. Increasing the Batch Size of a QESD Crystallization by Using a MSMPR Crystallizer. Pharmaceutics. 2022; 14(6):1227. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics14061227
Chicago/Turabian StyleHansen, Jerome, and Peter Kleinebudde. 2022. "Increasing the Batch Size of a QESD Crystallization by Using a MSMPR Crystallizer" Pharmaceutics 14, no. 6: 1227. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics14061227