A Comparative Study of Quercetin-Loaded Nanocochleates and Liposomes: Formulation, Characterization, Assessment of Degradation and In Vitro Anticancer Potential
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
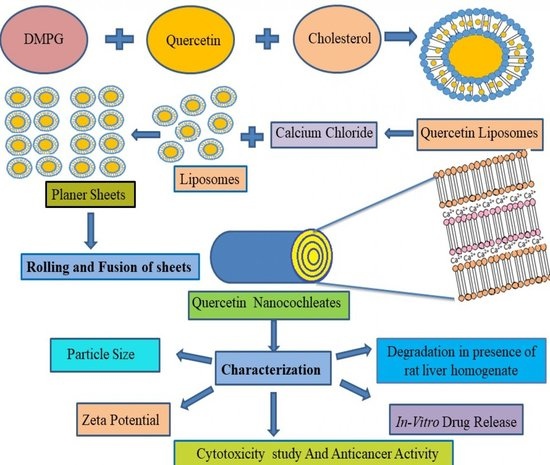
2.2. Preparation of Quercetin-Loaded Liposomes
- Step 1
- Step 2
2.3. Preparation of Quercetin-Loaded Nanocochleates
2.4. Particle Size Analysis
2.5. Zeta Potential Measurement
2.6. Determination of Encapsulation Efficiency (EE) of QL
2.7. Determination of Encapsulation Efficiency of QN
2.8. Surface Morphology
2.9. Differential Scanning Calorimetry (DSC)
2.10. In Vitro Release of Quercetin from QL and QN
2.11. Degradation Studies of Quercetin in the Presence of Rat Liver Homogenate (S9G)
2.12. Cytotoxicity Study
2.13. In Vitro Anticancer Activity
2.14. Stability Studies
3. Result and Discussion
3.1. Formulation and Evaluation of Quercetin-Loaded Liposomes
3.2. Preparation of Quercetin-Loaded Nanocochleates
3.3. Particle Size and Entrapment Efficiency Determination of Liposomes and Nanocochleates
3.4. Zeta Potential Measurements
3.5. Surface Morphology
3.6. DSC of Quercetin and QN
3.7. In Vitro Release Study
3.8. Degradation Studies of Quercetin in the Presence of Rat Liver Homogenate (S9G)
3.9. Cytotoxicity Study
3.10. In Vitro Anticancer Activity
3.11. Stability Studies of Developed Formulations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, P.F.; Travanut, A.; Conte, C.; Alexander, C. Reduction-responsive polymers for drug delivery in cancer therapy—Is there anything new to discover? WIREs Nanomed. Nanobiotechnol. 2021, 13, e1678. [Google Scholar] [CrossRef] [PubMed]
- Zare, M.; Norouzi Roshan, Z.; Assadpour, E.; Jafari, S.M. Improving the cancer prevention/treatment role of carotenoids through various nano-delivery systems. Crit. Rev. Food Sci. Nutr. 2021, 61, 522–534. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Zhou, T.; Jiang, Y.; Chang, S.K.; Yang, B. Prenylated flavonoids in foods and their applications on cancer prevention. Crit. Rev. Food Sci. Nutr. 2021, 62, 5067–5080. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, T.; Soares, G.A.B.e.; Chopra, H.; Rahman, M.M.; Hasan, Z.; Swain, S.S.; Cavalu, S. Applications of Phyto-Nanotechnology for the Treatment of Neurodegenerative Disorders. Materials 2022, 15, 804. [Google Scholar] [CrossRef]
- Ay, M.; Charli, A.; Jin, H.; Anantharam, V.; Kanthasamy, A.; Kanthasamy, A.G. Quercetin. In Nutraceuticals; Gupta, R.C., Lall, R., Srivastava, A., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 749–755. [Google Scholar] [CrossRef]
- Wang, Z.X.; Ma, J.; Li, X.Y.; Wu, Y.; Shi, H.; Chen, Y.; Lu, G.; Shen, H.M.; Lu, G.D.; Zhou, J. Quercetin induces p53-independent cancer cell death through lysosome activation by the transcription factor EB and Reactive Oxygen Species-dependent ferroptosis. Br. J. Pharmacol. 2021, 178, 1133–1148. [Google Scholar] [CrossRef] [PubMed]
- Zizkova, P.; Stefek, M.; Rackova, L.; Prnova, M.; Horakova, L. Novel quercetin derivatives: From redox properties to promising treatment of oxidative stress related diseases. Chem. Biol. Interact. 2017, 265, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Yang, Z.; Lu, N.; Peng, Y.-Y. Quercetin, but not rutin, attenuated hydrogen peroxide-induced cell damage via heme oxygenase-1 induction in endothelial cells. Arch. Biochem. Biophys. 2019, 676, 108157. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Zhang, Y.; Si, X.; Jin, Y.; Jiang, D.; Dai, Z.; Wu, Z. Quercetin alleviates oxidative damage by activating nuclear factor erythroid 2-related factor 2 signaling in porcine enterocytes. Nutrients 2021, 13, 375. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; Alsahli, M.A.; Almatroudi, A.; Verma, A.K.; Aloliqi, A.; Allemailem, K.S.; Khan, A.A.; Rahmani, A.H. Potential therapeutic targets of quercetin, a plant flavonol, and its role in the therapy of various types of cancer through the modulation of various cell signaling pathways. Molecules 2021, 26, 1315. [Google Scholar] [CrossRef] [PubMed]
- Dhanaraj, T.; Mohan, M.; Arunakaran, J. Quercetin attenuates metastatic ability of human metastatic ovarian cancer cells via modulating multiple signaling molecules involved in cell survival, proliferation, migration and adhesion. Arch. Biochem. Biophys. 2021, 701, 108795. [Google Scholar] [CrossRef] [PubMed]
- Maruszewska, A.; Tarasiuk, J. Quercetin triggers induction of apoptotic and lysosomal death of sensitive and multidrug resistant leukaemia HL60 cells. Nutr. Cancer 2021, 73, 484–501. [Google Scholar] [CrossRef]
- Lv, L.; Liu, C.; Chen, C.; Yu, X.; Chen, G.; Shi, Y.; Qin, F.; Ou, J.; Qiu, K.; Li, G. Quercetin and doxorubicin co-encapsulated biotin receptor-targeting nanoparticles for minimizing drug resistance in breast cancer. Oncotarget 2016, 7, 32184–32199. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.-S.; Hou, Y.-C.; Pai, M.-H.; Lin, M.-T.; Yeh, S.-L. Effects of quercetin combined with anticancer drugs on metastasis-associated factors of gastric cancer cells: in vitro and in vivo studies. J. Nutr. Biochem. 2018, 51, 105–113. [Google Scholar] [CrossRef]
- Scambia, G.; Ranelletti, F.O.; Panici, P.B.; Piantelli, M.; Bonanno, G.; de Vincenzo, R.; Ferrandina, G.; Pierelli, L.; Capelli, A.; Mancuso, S. Quercetin inhibits the growth of a multidrug-resistant estrogen-receptor-negative MCF-7 human breast-cancer cell line expressing type II estrogen-binding sites. Cancer Chemother. Pharmacol. 1991, 28, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Ding, H.; Tang, X.; Liang, M.; Li, S.; Zhang, J.; Cao, J. Quercetin induces pro-apoptotic autophagy via SIRT1/AMPK signaling pathway in human lung cancer cell lines A549 and H1299 in vitro. Thorac. Cancer 2021, 12, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, K.; King, J.W.; Howard, L.R.; Monrad, J.K. Solubility and solution thermodynamic properties of quercetin and quercetin dihydrate in subcritical water. J. Food Eng. 2010, 100, 208–218. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, inflammation and immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Savale, S. Formulation and evaluation of quercetin microemulsion for treatment of brain tumor via intranasal pathway. Asian J. Res. Bio. Pharm. Sci. 2017, 5, 91–95. [Google Scholar]
- Tran, T.H.; Guo, Y.; Song, D.; Bruno, R.S.; Lu, X. Quercetin-containing self-nanoemulsifying drug delivery system for improving oral bioavailability. J. Pharm. Sci. 2014, 103, 840–852. [Google Scholar] [CrossRef] [PubMed]
- Rasaee, S.; Ghanbarzadeh, S.; Mohammadi, M.; Hamishehkar, H. Nano phytosomes of quercetin: A promising formulation for fortification of food products with antioxidants. Pharm. Sci. 2014, 20, 96–101. [Google Scholar]
- Singh, A.; Dutta, P.; Kumar, H.; Kureel, A.K.; Rai, A.K. Synthesis of chitin-glucan-aldehyde-quercetin conjugate and evaluation of anticancer and antioxidant activities. Carbohydr. Polym. 2018, 193, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.H.; Yuan, L.; Meng, L.Y.; Qiu, J.L.; Wang, C.B. Quercetin-loaded mixed micelles exhibit enhanced cytotoxic efficacy in non-small cell lung cancer in vitro. Exp. Ther. Med. 2017, 14, 5503–5508. [Google Scholar] [CrossRef]
- Ravichandiran, V.; Masilamani, K.; Senthilnathan, B.; Maheshwaran, A.; Wui Wong, T.; Roy, P. Quercetin-decorated curcumin liposome design for cancer therapy: In-vitro and in-vivo studies. Curr. Drug Deliv. 2017, 14, 1053–1059. [Google Scholar] [CrossRef]
- Bothiraja, C.; Rajput, N.; Poudel, I.; Rajalakshmi, S.; Panda, B.; Pawar, A. Development of novel biofunctionalized chitosan decorated nanocochleates as a cancer targeted drug delivery platform. Artif. Cells Nanomed. Biotechnol. 2018, 46, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Pawar, A.Y.; Jadhav, K.R.; Sonkamble, N.B.; Kale, M.R. Nanocochleate: A novel drug delivery system. Asian J. Pharm. 2016, 10, S234–S242. [Google Scholar] [CrossRef]
- Asprea, M.; Tatini, F.; Piazzini, V.; Rossi, F.; Bergonzi, M.C.; Bilia, A.R. Stable, monodisperse, and highly cell-permeating nanocochleates from natural soy lecithin liposomes. Pharmaceutics 2019, 11, 34. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, T.; Joshi, N.; Kaviratna, A.; Ahamad, N.; Bhatia, E.; Banerjee, R. Aerosol delivery of paclitaxel-containing self-assembled nanocochleates for treating pulmonary metastasis: An approach supporting pulmonary mechanics. ACS Biomat. Sci. Eng. 2020, 7, 144–156. [Google Scholar] [CrossRef]
- Zarif, L.; Graybill, J.R.; Perlin, D.; Najvar, L.; Bocanegra, R.; Mannino, R.J. Antifungal activity of amphotericin B cochleates against Candida albicans infection in a mouse model. Antimicrob. Agents Chemother. 2000, 44, 1463–1469. [Google Scholar] [CrossRef]
- Tamargo, S.B.; Bui Thanh, T.; Pérez, M.; Otero, O.; Oliva, H.R.; Falero, G.; Pérez, J.L.; Cedré, M.B.; Okuskhanova, E.; Thiruvengadam, M.; et al. Nanocochleates containing N-Octylglicoside extracted Vibrio cholerae antigens elicited high vibriocidal antibodies titers after intragastric immunization in a mice model. Microb. Pathog. 2021, 156, 104902. [Google Scholar] [CrossRef]
- Nayek, S.; Venkatachalam, A.; Choudhury, S. Recent Nanocochleate Drug Delivery System for Cancer Treatment: A Review. Int. J. Curr. Pharm. Res. 2019, 11, 28–32. [Google Scholar] [CrossRef]
- Sonwane, S.A.; Chavan, M.J.; Hase, D.P.; Chumbhale, D.S.; Ambare, A.S.; Bodakhe, Y.T. Preparation, Characterization and in Vitro Anticancer Testing of Quercetin-Loaded Nanocochleates. 2017. Available online: https://research.pharmaguideline.com/2017/08/article-170812.html (accessed on 3 July 2022).
- Elsana, H.; Olusanya, T.O.B.; Carr-wilkinson, J.; Darby, S.; Faheem, A.; Elkordy, A.A. Evaluation of novel cationic gene based liposomes with cyclodextrin prepared by thin film hydration and microfluidic systems. Sci. Rep. 2019, 9, 15120. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.G.; Shah, D.A.; Dave, R.H. Formulation and evaluation of liposomes of fenofibrate prepared by thin film hydration technique. Int. J. Pharm. Sci. Res. 2018, 9, 3621–3637. [Google Scholar]
- Nadaf, S.J.; Killedar, S.G. Curcumin nanocochleates: Use of design of experiments, solid state characterization, in vitro apoptosis and cytotoxicity against breast cancer MCF-7 cells. J. Drug Deliv. Sci. Technol. 2018, 47, 337–350. [Google Scholar] [CrossRef]
- Oyungerel, G.; Batdemberel, G.; Munkhsaikhan, G. Study of particle size in natural and technological water suspensions using photon cross correlation spectroscopy with Nanophox. Phys. Chem. 2020, 10, 1–4. [Google Scholar] [CrossRef]
- Ghule, M.; Bhoyar, G. Formulation and Evaluation of modified liposome for transdermal drug. J. Dev. Drugs 2018, 7, 2–3. [Google Scholar]
- Landge, A.; Pawar, A.; Shaikh, K. Investigation of cochleates as carriers for topical drug delivery. Int. J. Pharm. Pharm. Sci. 2013, 5, 314–320. [Google Scholar]
- Çoban, Ö.; Değim, Z.; Yılmaz, Ş.; Altıntaş, L.; Arsoy, T.; Sözmen, M. Efficacy of targeted liposomes and nanocochleates containing imatinib plus dexketoprofen against fibrosarcoma. Drug Dev. Res. 2019, 80, 556–565. [Google Scholar] [CrossRef]
- Asprea, M.; Leto, I.; Bergonzi, M.C.; Bilia, A.R. Thyme essential oil loaded in nanocochleates: Encapsulation efficiency, in vitro release study and antioxidant activity. LWT-Food Sci. Tech. 2017, 77, 497–502. [Google Scholar] [CrossRef]
- Yücel, Ç.; Altintaş, Y.; Değim, Z.; Yılmaz, Ş.; Arsoy, T.; Altıntaş, L.; Çokçalışkan, C.; Sözmen, M. Novel approach to the treatment of diabetes: Embryonic stem cell and insulin-loaded liposomes and nanocochleates. J. Nanosci. Nanotechnol. 2019, 19, 3706–3719. [Google Scholar] [CrossRef]
- Delmarre, D.; Lu, R.; Tatton, N.; Krause-Elsmore, S.; Gould-Fogerite, S.; Mannino, R. Formulation of hydrophobic drugs into cochleate delivery vehicles: A simplified protocol & formulation kit. Drug Deliv. Technol. 2004, 4, 64–69. [Google Scholar]
- Newton, C.; Pangborn, W.; Nir, S.; Papahadjopoulos, D. Specificity of Ca2+ and Mg2+ binding to phosphatidylserine vesicles and resultant phase changes of bilayer membrane structure. Biochim. Biophys. Acta Biomembr. 1978, 506, 281–287. [Google Scholar] [CrossRef]
- Pedersen, U.R.; Leidy, C.; Westh, P.; Peters, G.H. The effect of calcium on the properties of charged phospholipid bilayers. Biochim. Biophys. Acta Biomembr. 2006, 1758, 573–582. [Google Scholar] [CrossRef]
- Poudel, I.; Ahiwale, R.; Pawar, A.; Mahadik, K.; Bothiraja, C. Development of novel biotinylated chitosan-decorated docetaxel-loaded nanocochleates for breast cancer targeting. Artif. Cells Nanomed. Biotechnol. 2018, 46, 229–240. [Google Scholar] [CrossRef]
- Miere, F.; Fritea, L.; Cavalu, S.; Vicas, S.I. Formulation, characterization, and advantages of using liposomes in multiple therapies. Pharmacophore 2020, 11, 1–12. [Google Scholar]
- Liu, M.; Zhong, X.; Yang, Z. Chitosan functionalized nanocochleates for enhanced oral absorption of cyclosporine A. Sci. Rep. 2017, 7, 41322. [Google Scholar] [CrossRef]
- Hoskins, D.; Turban, R.C.; Colbourn, C.J. Experimental designs in software engineering: D-optimal designs and covering arrays. In Proceedings of the 2004 ACM Workshop on Interdisciplinary Software Engineering Research, Newport Beach, CA, USA, 5 November 2004; pp. 55–66. [Google Scholar]
- Maherani, B.; Wattraint, O. Liposomal structure: A comparative study on light scattering and chromatography techniques. J. Dispers. Sci. Technol. 2017, 38, 1633–1639. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; HasanzadehDavarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Tabandeh, H.; Mortazavi, S.A. An investigation into some effective factors on encapsulation efficiency of alpha-tocopherol in MLVs and the release profile from the corresponding liposomal gel. Iran. J. Pharm. Res. 2013, 12, 21–30. [Google Scholar]
- Selvamani, V. Stability Studies on Nanomaterials Used in Drugs. In Characterization and Biology of Nanomaterials for Drug Delivery; Mohapatra, S.S., Ranjan, S., Dasgupta, N., Mishra, R.K., Thomas, S., Eds.; Elsevier: New York, NY, USA, 2019; pp. 425–444. [Google Scholar] [CrossRef]
- Pawar, A.P.; Vinugala, D.; Bothiraja, C. Nanocochleates derived from nanoliposomes for paclitaxel oral use: Preparation, characterization, in vitro anticancer testing, bioavailability and biodistribution study in rats. Biomed. Pharmacother. 2014, 113, 3502. [Google Scholar] [CrossRef]
- Ferreira, L.M.B.; Kiill, C.P.; Pedreiro, L.N.; Santos, A.M.; Gremião, M.P.D. Supramolecular design of hydrophobic and hydrophilic polymeric nanoparticles. In Design and Development of New Nanocarriers; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 181–221. [Google Scholar] [CrossRef]
- Alam, M.M.; Abdullah, K.; Singh, B.R.; Naqvi, A.H.; Naseem, I. Ameliorative effect of quercetin nanorods on diabetic mice: Mechanistic and therapeutic strategies. RSC Adv. 2016, 6, 55092–55103. [Google Scholar] [CrossRef]
- Wu, N.; Zhang, Y.; Ren, J.; Zeng, A.; Liu, J. Preparation of quercetin–nicotinamide cocrystals and their evaluation under in vivo and in vitro conditions. RSC Adv. 2020, 10, 21852–21859. [Google Scholar] [CrossRef] [PubMed]
- Moghimipour, E.; Handali, S. Utilization of thin film method for preparation of celecoxib loaded liposomes. Adv. Pharm. Bull. 2012, 2, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Mali, A.; Joshi, P.; Bothiraja, C.; Pawar, A. Fabrication and application of dimyristoyl phosphatidylcholine biomaterial-based nanocochleates dry powder inhaler for controlled release resveratrol delivery. Future J. Pharm. Sci. 2021, 7, 47. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Prajapati, A. Quercetin in anti-diabetic research and strategies for improved quercetin bioavailability using polymer-based carriers—A review. RSC Adv. 2015, 5, 97547–97562. [Google Scholar] [CrossRef]
- Bhosale, R.R.; Ghodake, P.P.; Mane, A.; Ghadge, A. Nanocochleates: A novel carrier for drug transfer. J. Sci. Ind. Res. 2013, 2, 964–969. [Google Scholar]
- Fischer, D.; Li, Y.; Ahlemeyer, B.; Krieglstein, J.; Kissel, T. In vitro cytotoxicity testing of polycations: Influence of polymer structure on cell viability and hemolysis. Biomaterials 2003, 24, 1121–1131. [Google Scholar] [CrossRef]
- Rekha, S.; Anila, E.I. In vitro cytotoxicity studies of surface modified CaS nanoparticles on L929 cell lines using MTT assay. Mater. Lett. 2019, 236, 637–639. [Google Scholar] [CrossRef]
- Mutlu, N.B.; Değim, Z.; Yılmaz, Ş.; Eşsiz, D.; Nacar, A. New perspective for the treatment of Alzheimer diseases: Liposomal rivastigmine formulations. Drug Dev. Ind. Pharm. 2011, 37, 775–789. [Google Scholar] [CrossRef]
- Sakaguchi, H.; Ashikaga, T.; Miyazawa, M.; Kosaka, N.; Ito, Y.; Yoneyama, K.; Sono, S.; Itagaki, H.; Toyoda, H.; Suzuki, H. The relationship between CD86/CD54 expression and THP-1 cell viability in an in vitro skin sensitization test–human cell line activation test (h-CLAT). Cell Biol. Toxicol. 2009, 25, 109–126. [Google Scholar] [CrossRef]
- Gibellini, L.; Pinti, M.; Nasi, M.; Montagna, J.P.; De Biasi, S.; Roat, E.; Bertoncelli, L.; Cooper, E.L.; Cossarizza, A. Quercetin and cancer chemoprevention. Evid. Based Complementary Altern. Med. 2011, 2011, 591356. [Google Scholar] [CrossRef] [PubMed]
- Zarif, L.; Perlin, D. Amphotericin B nanocochleates: From formulation to oral efficacy. Drug Deliv. Technol. 2002, 2, 34–37. [Google Scholar]
- Cavalu, S.; Bisboaca, S.; Mates, I.M.; Pasca, P.M.; Laslo, V.; Costea, T.; Fritea, L.; Vicas, S. Novel Formulation Based on Chitosan-Arabic Gum Nanoparticles Entrapping Propolis Extract Production, physico-chemical and structural characterization. Rev. Chim. 2018, 69, 3756–3760. [Google Scholar] [CrossRef]

















| Formulation | DMPG (mg) | Cholesterol (mg) | Quercetin (mg) |
|---|---|---|---|
| QL1 | 30 | 10 | 5 |
| QL2 | 30 | 10 | 10 |
| QL3 | 30 | 10 | 15 |
| QL4 | 50 | 10 | 5 |
| QL5 | 50 | 10 | 10 |
| QL6 | 50 | 10 | 15 |
| QL7 | 70 | 10 | 5 |
| QL8 | 70 | 10 | 10 |
| QL9 | 70 | 10 | 15 |
| Formulation | DMPG (mg) | Cholesterol (mg) | Quercetin (mg) |
|---|---|---|---|
| QN1 | 30 | 10 | 5 |
| QN2 | 50 | 10 | 5 |
| QN3 | 70 | 10 | 5 |
| Experimental Response for Entrapment Efficiency (%) | ||||||
|---|---|---|---|---|---|---|
| Source | Sum of Squares | Df | Mean Square | F Value | p-value | |
| Model | 1293.09 | 8 | 161.64 | 6.366 × 107 | Prob > F | Significant |
| A-A | 400.06 | 2 | 200.03 | 6.366 × 107 | <0.0001 | |
| B-B | 907.62 | 2 | 453.81 | 6.366 × 107 | <0.0001 | |
| AB | 180.38 | 4 | 45.09 | <0.0001 | ||
| Pure Error | 0.000 | 5 | 0.000 | <0.0001 | ||
| Cor Total | 13 | |||||
| Experimental Response for Particle Size (nm) | ||||||
| Source | Sum of Squares | Df | Mean Square | F Value | p-value | |
| Model | 85,538.86 | 8 | 10,692.36 | 6.366 × 107 | <0.0001 | Significant |
| A-A | 50,304.12 | 2 | 25,152.06 | 6.366 × 107 | <0.0001 | |
| B-B | 17,901.63 | 2 | 8950.82 | 6.366 × 107 | <0.0001 | |
| AB | 24,594.72 | 4 | 6148.68 | 6.366 × 107 | <0.0001 | |
| Pure Error | 0.000 | 5 | 0.000 | |||
| Cor Total | 85,538.86 | 13 | 10,692.36 | |||
| Formulation | EE % | Vesicles Size (nm) | Zeta Potential (mV) | Appearance |
|---|---|---|---|---|
| QL1 | 70.5 ± 5.23 | 213 ± 3 | −45.32 | Spherical |
| QL2 | 55 ± 3.23 | 345 ± 2 | −47.34 | Spherical |
| QL3 | 35 ± 4.35 | 233 ± 4 | −48.65 | Spherical |
| QL4 | 72.4 ± 5.34 | 150 ± 2 | −53.65 | Spherical |
| QL5 | 66 ± 2.34 | 132 ± 5 | −55.34 | Spherical |
| QL6 | 59 ± 1.35 | 145 ± 3 | −56.25 | Spherical |
| QL7 | 74.2 ± 2.34 | 111.06 ± 2 | −40.33 | Spherical |
| QL8 | 65 ± 1.56 | 238 ± 3 | −46.24 | Spherical |
| QL9 | 58 ± 2.54 | 324 ± 6 | −51.22 | Spherical |
| Formulation | % EE | Particle Size (nm) | Zeta Potential (mV) |
|---|---|---|---|
| QN1 | 78.2 ± 4.23 | 670 ± 3 | −39.54 |
| QN2 | 85 ± 3.25 | 544 ± 2 | −26.32 |
| QN3 | 88.62 ± 4.20 | 502 ± 4 | −18.52 |
| Formulation | % EE | Particle Size (nm) | Zeta Potential (mV) | Appearance | ||||
|---|---|---|---|---|---|---|---|---|
| 0 Day | 90 Day | 0 Day | 90 Day | 0 Day | 90 Day | 0 Day | 90 Day | |
| QL7 | 74.2 ± 2.34 | 66 ± 2.21 | 111.06 ± 2 | 454.42 ± 2.8 | −40.33 | −53.68 | Spherical | Spherical |
| QN3 | 88.62 ± 4.20 | 87.23 ± 3.24 | 502 ± 4 | 460.67 ± 3.33 | −18 | −39.60 | Rod-shaped | Rod-shaped |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munot, N.; Kandekar, U.; Giram, P.S.; Khot, K.; Patil, A.; Cavalu, S. A Comparative Study of Quercetin-Loaded Nanocochleates and Liposomes: Formulation, Characterization, Assessment of Degradation and In Vitro Anticancer Potential. Pharmaceutics 2022, 14, 1601. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics14081601
Munot N, Kandekar U, Giram PS, Khot K, Patil A, Cavalu S. A Comparative Study of Quercetin-Loaded Nanocochleates and Liposomes: Formulation, Characterization, Assessment of Degradation and In Vitro Anticancer Potential. Pharmaceutics. 2022; 14(8):1601. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics14081601
Chicago/Turabian StyleMunot, Neha, Ujjwala Kandekar, Prabhanjan S. Giram, Kavita Khot, Abhinandan Patil, and Simona Cavalu. 2022. "A Comparative Study of Quercetin-Loaded Nanocochleates and Liposomes: Formulation, Characterization, Assessment of Degradation and In Vitro Anticancer Potential" Pharmaceutics 14, no. 8: 1601. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics14081601