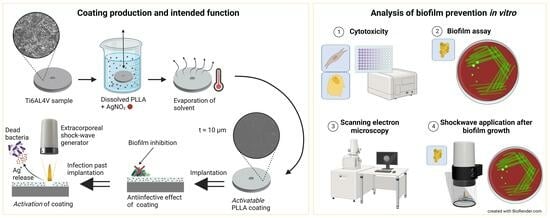
Shock Wave-Activated Silver-Loaded Biopolymer Implant Coating Eliminates Staphylococcus epidermidis on the Surface and in the Surrounding of Implants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Cytotoxicity
2.3. Biofilm Assay
2.4. SEM Analysis
2.5. Shock Wave Application after Biofilm Growth
2.6. Statistical Analysis
3. Results
3.1. Cytotoxicity
3.2. Biofilm Assay
3.3. Shock Wave Application after Biofilm Growth
4. Discussion
4.1. Cytotoxicity
4.2. Biofilm Assay
4.3. Shock Wave Application after Biofilm Growth
4.4. Limitations
5. Conclusions
6. Patents
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Franceschini, M.; Sandiford, N.A.; Cerbone, V.; Araujo, L.C.T.d.; Kendoff, D. Defensive antibacterial coating in revision total hip arthroplasty: New concept and early experience. Hip Int. 2020, 30, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Funovics, P.T.; Hipfl, C.; Hofstaetter, J.G.; Puchner, S.; Kotz, R.I.; Dominkus, M. Management of septic complications following modular endoprosthetic reconstruction of the proximal femur. Int. Orthop. (SICOT) 2011, 35, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Gosheger, G.; Gebert, C.; Ahrens, H.; Streitbuerger, A.; Winkelmann, W.; Hardes, J. Endoprosthetic reconstruction in 250 patients with sarcoma. Clin. Orthop. Relat. Res. 2006, 450, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Theil, C.; Röder, J.; Gosheger, G.; Deventer, N.; Dieckmann, R.; Schorn, D.; Hardes, J.; Andreou, D. What Is the Likelihood That Tumor Endoprostheses Will Experience a Second Complication after First Revision in Patients with Primary Malignant Bone Tumors and What Are Potential Risk Factors? Clin. Orthop. Relat. Res. 2019, 477, 2705–2714. [Google Scholar] [CrossRef] [PubMed]
- Leitner, L.; Posch, F.; Amerstorfer, F.; Sadoghi, P.; Leithner, A.; Glehr, M. The Dark Side of Arthroplasty: Competing Risk Analysis of Failed Hip and Knee Arthroplasty with Periprosthetic Joint Infection. J. Arthroplast. 2020, 35, 2601–2606.e1. [Google Scholar] [CrossRef]
- Zimmerli, W.; Sendi, P. Orthopaedic biofilm infections. APMIS 2017, 125, 353–364. [Google Scholar] [CrossRef]
- Boot, W.; Schmid, T.; D’Este, M.; Guillaume, O.; Foster, A.; Decosterd, L.; Richards, R.G.; Eglin, D.; Zeiter, S.; Moriarty, T.F. A Hyaluronic Acid Hydrogel Loaded with Gentamicin and Vancomycin Successfully Eradicates Chronic Methicillin-Resistant Staphylococcus aureus Orthopedic Infection in a Sheep Model. Antimicrob. Agents Chemother. 2021, 65, 10–128. [Google Scholar] [CrossRef]
- Foster, A.L.; Boot, W.; Stenger, V.; D’Este, M.; Jaiprakash, A.; Eglin, D.; Zeiter, S.; Richards, R.G.; Moriarty, T.F. Single-stage revision of MRSA orthopedic device-related infection in sheep with an antibiotic-loaded hydrogel. J. Orthop. Res. 2021, 39, 438–448. [Google Scholar] [CrossRef]
- Boo, G.-J.A.; Arens, D.; Metsemakers, W.-J.; Zeiter, S.; Richards, R.G.; Grijpma, D.W.; Eglin, D.; Moriarty, T.F. Injectable gentamicin-loaded thermo-responsive hyaluronic acid derivative prevents infection in a rabbit model. Acta Biomater. 2016, 43, 185–194. [Google Scholar] [CrossRef]
- Korah, L.V.; Anilkumar, G.; Thomas, S. 5—Hydrogels, DNA, and RNA polypeptides for the preparation of biomaterials. In Fundamental Biomaterials: Polymers; Thomas, S., Balakrishnan, P., Sreekala, M.S., Eds.; Woodhead Publishing: Duxford, UK, 2018; pp. 85–104. ISBN 978-0-08-102194-1. [Google Scholar]
- Lepperdinger, G.; Fehrer, C.; Reitinger, S. Chapter 4—Biodegradation of Hyaluronan. In Chemistry and Biology of Hyaluronan; Garg, H.G., Hales, C.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 71–82. ISBN 978-0-08-044382-9. [Google Scholar]
- Jäger, M. Oberflächenmodifikationen von Implantaten. Teil 1: Werkstofftechnische und biologische Grundlagen. Orthopäde 2018, 47, 347–366. [Google Scholar] [CrossRef]
- Tande, A.J.; Patel, R. Prosthetic joint infection. Clin. Microbiol. Rev. 2014, 27, 302–345. [Google Scholar] [CrossRef] [PubMed]
- Walton, M.; Cotton, N.J. Long-term in vivo degradation of poly-L-lactide (PLLA) in bone. J. Biomater. Appl. 2007, 21, 395–411. [Google Scholar] [CrossRef]
- Bos, R.R.; Rozema, F.R.; Boering, G.; Nijenhuis, A.J.; Pennings, A.J.; Verwey, A.B.; Nieuwenhuis, P.; Jansen, H.W. Degradation of and tissue reaction to biodegradable poly(L-lactide) for use as internal fixation of fractures: A study in rats. Biomaterials 1991, 12, 32–36. [Google Scholar] [CrossRef]
- Capuana, E.; Lopresti, F.; Ceraulo, M.; La Carrubba, V. Poly-l-Lactic Acid (PLLA)-Based Biomaterials for Regenerative Medicine: A Review on Processing and Applications. Polymers 2022, 14, 1153. [Google Scholar] [CrossRef]
- Gristina, A.G. Biomaterial-centered infection: Microbial adhesion versus tissue integration. Science 1987, 237, 1588–1595. [Google Scholar] [CrossRef] [PubMed]
- Masters, E.A.; Trombetta, R.P.; de Mesy Bentley, K.L.; Boyce, B.F.; Gill, A.L.; Gill, S.R.; Nishitani, K.; Ishikawa, M.; Morita, Y.; Ito, H.; et al. Evolving concepts in bone infection: Redefining “biofilm”, “acute vs. chronic osteomyelitis”, “the immune proteome” and “local antibiotic therapy”. Bone Res. 2019, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Puetzler, J.; Hasselmann, J.; Nonhoff, M.; Fobker, M.; Niemann, S.; Theil, C.; Gosheger, G.; Schulze, M. On-Demand Release of Anti-Infective Silver from a Novel Implant Coating Using High-Energy Focused Shock Waves. Pharmaceutics 2023, 15, 2179. [Google Scholar] [CrossRef]
- Karacan, I.; Chou, J.; Ben-Nissan, B.; Macha, I.J.; Juritza, A.; Wang, A.H.; Müller, W.H.; Grossin, D.; Taraschi, V.; Oktar, F.N.; et al. Adhesion and Scratch Testing of Antibiotic Loaded Poly-Lactic Acid Biocomposite Thin Films on Metallic Implants. KEM 2018, 782, 195–200. [Google Scholar] [CrossRef]
- Schulze, M.; Fobker, M.; Puetzler, J.; Hillebrand, J.; Niemann, S.; Schulte, E.; Kurzynski, J.; Gosheger, G.; Hasselmann, J. Mechanical and microbiological testing concept for activatable anti-infective biopolymer implant coatings. Biomater. Adv. 2022, 138, 212917. [Google Scholar] [CrossRef]
- Puetzler, J.; Hasselmann, J.; Gosheger, G.; Niemann, S.; Fobker, M.; Hillebrand, J.; Schwarze, J.; Theil, C.; Schulze, M. On-demand activation of a novel anti-infective biopolymer implant coating with high-energy shockwaves. Orthop. Proc. 2022, 104-B, 87. [Google Scholar] [CrossRef]
- Schmidt-Braekling, T.; Streitbuerger, A.; Gosheger, G.; Boettner, F.; Nottrott, M.; Ahrens, H.; Dieckmann, R.; Guder, W.; Andreou, D.; Hauschild, G.; et al. Silver-coated megaprostheses: Review of the literature. Eur. J. Orthop. Surg. Traumatol. 2017, 27, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Wan, A.T.; Conyers, R.A.; Coombs, C.J.; Masterton, J.P. Determination of silver in blood, urine, and tissues of volunteers and burn patients. Clin. Chem. 1991, 37, 1683–1687. [Google Scholar] [CrossRef] [PubMed]
- Tweden, K.S.; Cameron, J.D.; Razzouk, A.J.; Holmberg, W.R.; Kelly, S.J. Biocompatibility of silver-modified polyester for antimicrobial protection of prosthetic valves. J. Heart Valve Dis. 1997, 6, 553–561. [Google Scholar] [PubMed]
- La Brutel de Riviere, A.; Dossche, K.M.; Birnbaum, D.E.; Hacker, R. First clinical experience with a mechanical valve with silver coating. J. Heart Valve Dis. 2000, 9, 123–129; discussion 129–130. [Google Scholar]
- Gosheger, G.; Hardes, J.; Ahrens, H.; Streitburger, A.; Buerger, H.; Erren, M.; Gunsel, A.; Kemper, F.H.; Winkelmann, W.; Eiff, C. von. Silver-coated megaendoprostheses in a rabbit model—An analysis of the infection rate and toxicological side effects. Biomaterials 2004, 25, 5547–5556. [Google Scholar] [CrossRef] [PubMed]
- Hardes, J.; Ahrens, H.; Gebert, C.; Streitbuerger, A.; Buerger, H.; Erren, M.; Gunsel, A.; Wedemeyer, C.; Saxler, G.; Winkelmann, W.; et al. Lack of toxicological side-effects in silver-coated megaprostheses in humans. Biomaterials 2007, 28, 2869–2875. [Google Scholar] [CrossRef] [PubMed]
- Hardes, J.; von Eiff, C.; Streitbuerger, A.; Balke, M.; Budny, T.; Henrichs, M.P.; Hauschild, G.; Ahrens, H. Reduction of periprosthetic infection with silver-coated megaprostheses in patients with bone sarcoma. J. Surg. Oncol. 2010, 101, 389–395. [Google Scholar] [CrossRef]
- Hussmann, B.; Johann, I.; Kauther, M.D.; Landgraeber, S.; Jäger, M.; Lendemans, S. Measurement of the silver ion concentration in wound fluids after implantation of silver-coated megaprostheses: Correlation with the clinical outcome. Biomed Res. Int. 2013, 2013, 763096. [Google Scholar] [CrossRef]
- Fokter, S.K.; Gubeljak, N.; Punzón-Quijorna, E.; Pelicon, P.; Kelemen, M.; Vavpetič, P.; Predan, J.; Ferlič, L.; Novak, I. Total Knee Replacement with an Uncemented Porous Tantalum Tibia Component: A Failure Analysis. Materials 2022, 15, 2575. [Google Scholar] [CrossRef]
- Kravanja, K.A.; Finšgar, M. Analytical Techniques for the Characterization of Bioactive Coatings for Orthopaedic Implants. Biomedicines 2021, 9, 1936. [Google Scholar] [CrossRef]
- Sendi, P.; Zimmerli, W. Antimicrobial treatment concepts for orthopaedic device-related infection. Clin. Microbiol. Infect. 2012, 18, 1176–1184. [Google Scholar] [CrossRef]
- Zimmerli, W.; Sendi, P. Role of Rifampin against Staphylococcal Biofilm Infections In Vitro, in Animal Models, and in Orthopedic-Device-Related Infections. Antimicrob. Agents Chemother. 2019, 63, 10–128. [Google Scholar] [CrossRef] [PubMed]
- Schierholz, J.M.; Lucas, L.J.; Rump, A.; Pulverer, G. Efficacy of silver-coated medical devices. J. Hosp. Infect. 1998, 40, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Gordon, O.; Vig Slenters, T.; Brunetto, P.S.; Villaruz, A.E.; Sturdevant, D.E.; Otto, M.; Landmann, R.; Fromm, K.M. Silver coordination polymers for prevention of implant infection: Thiol interaction, impact on respiratory chain enzymes, and hydroxyl radical induction. Antimicrob. Agents Chemother. 2010, 54, 4208–4218. [Google Scholar] [CrossRef] [PubMed]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudré, C. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2019, 83, 37–54. [Google Scholar] [CrossRef] [PubMed]
- Mulley, G.; Jenkins, A.T.A.; Waterfield, N.R. Inactivation of the antibacterial and cytotoxic properties of silver ions by biologically relevant compounds. PLoS ONE 2014, 9, e94409. [Google Scholar] [CrossRef]
- Puetzler, J.; Milstrey, A.; Everding, J.; Raschke, M.; Arens, D.; Zeiter, S.; Geoff Richards, R.; Fintan Moriarty, T. Focused high-energy extracorporeal shockwaves as supplemental treatment in a rabbit model of fracture-related infection. J. Orthop. Res. 2020, 38, 1351–1358. [Google Scholar] [CrossRef]
- Milstrey, A.; Rosslenbroich, S.; Everding, J.; Raschke, M.J.; Richards, R.G.; Moriarty, T.F.; Puetzler, J. Antibiofilm efficacy of focused high-energy extracorporeal shockwaves and antibiotics in vitro. Bone Jt. Res. 2021, 10, 77–84. [Google Scholar] [CrossRef]
- Gerdesmeyer, L.; von Eiff, C.; Horn, C.; Henne, M.; Roessner, M.; Diehl, P.; Gollwitzer, H. Antibacterial effects of extracorporeal shock waves. Ultrasound Med. Biol. 2005, 31, 115–119. [Google Scholar] [CrossRef]
- Qi, X.; Zhao, Y.; Zhang, J.; Han, D.; Chen, C.; Huang, Y.; Chen, X.; Zhang, X.; Wang, T.; Li, X. Increased Effects of Extracorporeal Shock Waves Combined with Gentamicin against Staphylococcus aureus Biofilms In Vitro and In Vivo. Ultrasound Med. Biol. 2016, 42, 2245–2252. [Google Scholar] [CrossRef]
- Gollwitzer, H.; Horn, C.; von Eiff, C.; Henne, M.; Gerdesmeyer, L. Antibakterieller Effekt hochenergetischer extrakorporaler Stosswellen: Ein in vitro Nachweis. Z. Orthop. Ihre Grenzgeb. 2004, 142, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Grecco, M.V.; Brech, G.C.; Greve, J.M.D. One-year treatment follow-up of plantar fasciitis: Radial shockwaves vs. conventional physiotherapy. Clinics (Sao Paulo) 2013, 68, 1089–1095. [Google Scholar] [CrossRef]
- Reilly, J.M.; Bluman, E.; Tenforde, A.S. Effect of Shockwave Treatment for Management of Upper and Lower Extremity Musculoskeletal Conditions: A Narrative Review. PM&R 2018, 10, 1385–1403. [Google Scholar] [CrossRef]
- Schmitz, C.; Császár, N.B.M.; Milz, S.; Schieker, M.; Maffulli, N.; Rompe, J.-D.; Furia, J.P. Efficacy and safety of extracorporeal shock wave therapy for orthopedic conditions: A systematic review on studies listed in the PEDro database. Br. Med. Bull. 2015, 116, 115–138. [Google Scholar] [CrossRef]
- Haupt, G.; Haupt, A.; Ekkernkamp, A.; Gerety, B.; Chvapil, M. Influence of shock waves on fracture healing. Urology 1992, 39, 529–532. [Google Scholar] [CrossRef]
- Frairia, R.; Berta, L. Biological effects of extracorporeal shock waves on fibroblasts. A review. Muscles Ligaments Tendons J. 2011, 1, 138–147. [Google Scholar]
- Koch, J.A.; Pust, T.M.; Cappellini, A.J.; Mandell, J.B.; Ma, D.; Shah, N.B.; Brothers, K.M.; Urish, K.L. Staphylococcus epidermidis Biofilms Have a High Tolerance to Antibiotics in Periprosthetic Joint Infection. Life 2020, 10, 253. [Google Scholar] [CrossRef]




| Coating | WST-1 NHF | WST-1 Saos-2 | Biofilm | Biofilm (with/without Shock Wave) |
|---|---|---|---|---|
| Ti6Al4V | - | - | 12 | 6/4 |
| Electroplated Silver | 1 | 1 | 1 | – |
| PLLA + 0% Ag | 6 | 6 | 6 | – |
| PLLA + 2% Ag | 6 | 6 | 3 | – |
| PLLA + 4% Ag | 6 | 6 | 6 | – |
| PLLA + 6% Ag | 6 | 6 | 5 | 6/6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schulze, M.; Nonhoff, M.; Hasselmann, J.; Fobker, M.; Niemann, S.; Theil, C.; Gosheger, G.; Puetzler, J. Shock Wave-Activated Silver-Loaded Biopolymer Implant Coating Eliminates Staphylococcus epidermidis on the Surface and in the Surrounding of Implants. Pharmaceutics 2023, 15, 2670. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics15122670
Schulze M, Nonhoff M, Hasselmann J, Fobker M, Niemann S, Theil C, Gosheger G, Puetzler J. Shock Wave-Activated Silver-Loaded Biopolymer Implant Coating Eliminates Staphylococcus epidermidis on the Surface and in the Surrounding of Implants. Pharmaceutics. 2023; 15(12):2670. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics15122670
Chicago/Turabian StyleSchulze, Martin, Melanie Nonhoff, Julian Hasselmann, Manfred Fobker, Silke Niemann, Christoph Theil, Georg Gosheger, and Jan Puetzler. 2023. "Shock Wave-Activated Silver-Loaded Biopolymer Implant Coating Eliminates Staphylococcus epidermidis on the Surface and in the Surrounding of Implants" Pharmaceutics 15, no. 12: 2670. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics15122670