Ciprofloxacin-Loaded Polyvinylpyrrolidone Foils for the Topical Treatment of Wound Infections with Methicillin-Resistant Staphylococcus aureus (MRSA)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of PVP Foils Loaded with Ciprofloxacin (Cipro-Foils)
2.2. Ex Vivo Wound Infection Model and Treatment with Cipro-Foils
2.3. Fluorescence In Situ Hybridization (FISH)
2.4. Wound Extraction and Quantification of Bacteria
2.5. Protein Extraction and Measurement
2.6. ELISA and Multi-Analyte ELISA
2.7. Data Analysis and Statistics
3. Results
3.1. Characterization of Cipro-Foils
3.2. Visualization of Bacteria in the Wound Model (FISH)
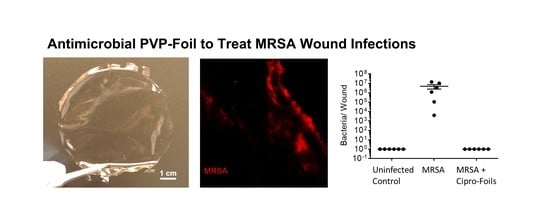
3.3. Antimicrobial Efficacy of Cipro-Foils
3.4. Skin Immune Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mahoney, A.R.; Safaee, M.M.; Wuest, W.M.; Furst, A.L. The silent pandemic: Emergent antibiotic resistances following the global response to SARS-CoV-2. IScience 2021, 24, 102304. [Google Scholar] [CrossRef]
- Sneka, P.; Mahalakshmi, K. Antimicrobial Resistance–A Silent Pandemic. Natl. J. Community Med. 2023, 14, 71–72. [Google Scholar] [CrossRef]
- Haque, M.; Sartelli, M.; McKimm, J.; Bakar, M.A. Health care-associated infections–an overview. Infect. Drug Resist. 2018, 11, 2321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimlichman, E.; Henderson, D.; Tamir, O.; Franz, C.; Song, P.; Yamin, C.K.; Keohane, C.; Denham, C.R.; Bates, D.W. Health care–associated infections: A meta-analysis of costs and financial impact on the US health care system. JAMA Intern. Med. 2013, 173, 2039–2046. [Google Scholar] [CrossRef]
- De Lissovoy, G.; Fraeman, K.; Hutchins, V.; Murphy, D.; Song, D.; Vaughn, B.B. Surgical site infection: Incidence and impact on hospital utilization and treatment costs. Am. J. Infect. Control 2009, 37, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Leaper, D.; Assadian, O.; Edmiston, C.E. Approach to chronic wound infections. Br. J. Dermatol. 2015, 173, 351–358. [Google Scholar] [CrossRef]
- Bjarnsholt, T.; Kirketerp-Møller, K.; Jensen, P.Ø.; Madsen, K.G.; Phipps, R.; Krogfelt, K.; Høiby, N.; Givskov, M. Why chronic wounds will not heal: A novel hypothesis. Wound Repair Regen. 2008, 16, 2–10. [Google Scholar] [CrossRef]
- Olsson, M.; Järbrink, K.; Divakar, U.; Bajpai, R.; Upton, Z.; Schmidtchen, A.; Car, J. The humanistic and economic burden of chronic wounds: A systematic review. Wound Repair Regen. 2019, 27, 114–125. [Google Scholar] [CrossRef] [Green Version]
- Martinengo, L.; Olsson, M.; Bajpai, R.; Soljak, M.; Upton, Z.; Schmidtchen, A.; Car, J.; Järbrink, K. Prevalence of chronic wounds in the general population: Systematic review and meta-analysis of observational studies. Ann. Epidemiol. 2019, 29, 8–15. [Google Scholar] [CrossRef]
- Yao, B.; Huang, S.; Gao, D.; Xie, J.; Liu, N.; Fu, X. Age-associated changes in regenerative capabilities of mesenchymal stem cell: Impact on chronic wounds repair. Int. Wound J. 2016, 13, 1252–1259. [Google Scholar] [CrossRef]
- Sen, C.K. Human wounds and its burden: An updated compendium of estimates. Adv. Wound Care 2019, 8, 39–48. [Google Scholar] [CrossRef] [Green Version]
- Kolluri, R.; Lugli, M.; Villalba, L.; Varcoe, R.; Maleti, O.; Gallardo, F.; Black, S.; Forgues, F.; Lichtenberg, M.; Hinahara, J. An estimate of the economic burden of venous leg ulcers associated with deep venous disease. Vasc. Med. 2022, 27, 63–72. [Google Scholar] [CrossRef]
- Demidova-Rice, T.N.; Hamblin, M.R.; Herman, I.M. Acute and impaired wound healing: Pathophysiology and current methods for drug delivery, part 1: Normal and chronic wounds: Biology, causes, and approaches to care. Adv. Ski. Wound Care 2012, 25, 304. [Google Scholar] [CrossRef] [Green Version]
- Silver, S. Bacterial silver resistance: Molecular biology and uses and misuses of silver compounds. FEMS Microbiol. Rev. 2003, 27, 341–353. [Google Scholar] [CrossRef] [Green Version]
- Siddique, M.H.; Aslam, B.; Imran, M.; Ashraf, A.; Nadeem, H.; Hayat, S.; Khurshid, M.; Afzal, M.; Malik, I.R.; Shahzad, M. Research article effect of silver nanoparticles on biofilm formation and EPS production of multidrug-resistant Klebsiella pneumonia. Hindawi BioMed Res. Int 2020, 2020, 6398165. [Google Scholar] [CrossRef] [Green Version]
- Panáček, A.; Kvítek, L.; Smékalová, M.; Večeřová, R.; Kolář, M.; Röderová, M.; Dyčka, F.; Šebela, M.; Prucek, R.; Tomanec, O. Bacterial resistance to silver nanoparticles and how to overcome it. Nat. Nanotechnol. 2018, 13, 65–71. [Google Scholar] [CrossRef]
- Negut, I.; Grumezescu, V.; Grumezescu, A.M. Treatment strategies for infected wounds. Molecules 2018, 23, 2392. [Google Scholar] [CrossRef] [Green Version]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.; Eccleston, G.M. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef]
- Chandel, A.K.S.; Kumar, C.U.; Jewrajka, S.K. Effect of polyethylene glycol on properties and drug encapsulation–release performance of biodegradable/cytocompatible agarose–polyethylene glycol–polycaprolactone amphiphilic co-network gels. ACS Appl. Mater. Interfaces 2016, 8, 3182–3192. [Google Scholar] [CrossRef]
- Chandel, A.K.S.; Nutan, B.; Raval, I.H.; Jewrajka, S.K. Self-assembly of partially alkylated dextran-graft-poly [(2-dimethylamino) ethyl methacrylate] copolymer facilitating hydrophobic/hydrophilic drug delivery and improving conetwork hydrogel properties. Biomacromolecules 2018, 19, 1142–1153. [Google Scholar] [CrossRef]
- Thakur, S.; Anjum, M.M.; Jaiswal, S.; Kumar, A.; Deepak, P.; Anand, S.; Singh, S.; Rajinikanth, P.S. Novel Synergistic Approach: Tazarotene-Calcipotriol-Loaded-PVA/PVP-Nanofibers Incorporated in Hydrogel Film for Management and Treatment of Psoriasis. Mol. Pharm. 2023, 20, 997–1014. [Google Scholar] [CrossRef] [PubMed]
- Darvishan, S.; Pourmadadi, M.; Abdouss, M.; Mazinani, S.; Yazdian, F.; Rahdar, A.; Díez-Pascual, A.M. Gamma alumina coated-PAA/PVP hydrogel as promising quercetin nanocarrier: Physiochemical characterization and toxicity activity. J. Drug Deliv. Sci. Technol. 2023, 84, 104500. [Google Scholar] [CrossRef]
- Contardi, M.; Russo, D.; Suarato, G.; Heredia-Guerrero, J.A.; Ceseracciu, L.; Penna, I.; Margaroli, N.; Summa, M.; Spanò, R.; Tassistro, G. Polyvinylpyrrolidone/hyaluronic acid-based bilayer constructs for sequential delivery of cutaneous antiseptic and antibiotic. Chem. Eng. J. 2019, 358, 912–923. [Google Scholar] [CrossRef]
- Contardi, M.; Summa, M.; Picone, P.; Brancato, O.R.; Di Carlo, M.; Bertorelli, R.; Athanassiou, A. Evaluation of a multifunctional polyvinylpyrrolidone/hyaluronic acid-based bilayer film patch with anti-inflammatory properties as an enhancer of the wound healing process. Pharmaceutics 2022, 14, 483. [Google Scholar] [CrossRef] [PubMed]
- Schaudinn, C.; Dittmann, C.; Jurisch, J.; Laue, M.; Günday-Türeli, N.; Blume-Peytavi, U.; Vogt, A.; Rancan, F. Development, standardization and testing of a bacterial wound infection model based on ex vivo human skin. PLoS ONE 2017, 12, e0186946. [Google Scholar] [CrossRef] [Green Version]
- Serra, R.; Grande, R.; Butrico, L.; Rossi, A.; Settimio, U.F.; Caroleo, B.; Amato, B.; Gallelli, L.; De Franciscis, S. Chronic wound infections: The role of Pseudomonas aeruginosa and Staphylococcus aureus. Expert Rev. Anti-Infect. Ther. 2015, 13, 605–613. [Google Scholar] [CrossRef]
- Holloway, B.; Krishnapillai, V.; Morgan, A. Chromosomal genetics of Pseudomonas. Microbiol. Rev. 1979, 43, 73–102. [Google Scholar] [CrossRef]
- McDOUGAL, L.K.; Thornsberry, C. The role of beta-lactamase in staphylococcal resistance to penicillinase-resistant penicillins and cephalosporins. J. Clin. Microbiol. 1986, 23, 832–839. [Google Scholar] [CrossRef] [Green Version]
- Boles, B.R.; Thoendel, M.; Singh, P.K. Self-generated diversity produces “insurance effects” in biofilm communities. Proc. Natl. Acad. Sci. USA 2004, 101, 16630–16635. [Google Scholar] [CrossRef]
- Mah, T.-F.C.; O’Toole, G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef]
- Contardi, M.; Heredia-Guerrero, J.A.; Perotto, G.; Valentini, P.; Pompa, P.P.; Spanò, R.; Goldoni, L.; Bertorelli, R.; Athanassiou, A.; Bayer, I.S. Transparent ciprofloxacin-povidone antibiotic films and nanofiber mats as potential skin and wound care dressings. Eur. J. Pharm. Sci. 2017, 104, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S. Immune system and surgical site infection. J. Chemother. 2001, 13, 12–16. [Google Scholar] [CrossRef] [PubMed]
- De Jong, N.W.; Van Kessel, K.P.; Van Strijp, J.A. Immune evasion by Staphylococcus aureus. Microbiol. Spectr. 2019, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.Y. Molecular pathogenesis of Staphylococcus aureus infection. Pediatr. Res. 2009, 65, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Rancan, F.; Contardi, M.; Jurisch, J.; Blume-Peytavi, U.; Vogt, A.; Bayer, I.S.; Schaudinn, C. Evaluation of drug delivery and efficacy of ciprofloxacin-loaded povidone foils and nanofiber mats in a wound-infection model based on ex vivo human skin. Pharmaceutics 2019, 11, 527. [Google Scholar] [CrossRef] [Green Version]
- Potempa, J.; Pike, R.N. Corruption of innate immunity by bacterial proteases. J. Innate Immun. 2009, 1, 70–87. [Google Scholar] [CrossRef] [Green Version]
- Lindsay, S.; Oates, A.; Bourdillon, K. The detrimental impact of extracellular bacterial proteases on wound healing. Int. Wound J. 2017, 14, 1237–1247. [Google Scholar] [CrossRef]
- Vitellaro-Zuccarello, L.; Cappelletti, S.; Rossi, V.D.P.; Sari-Gorla, M. Stereological analysis of collagen and elastic fibers in the normal human dermis: Variability with age, sex, and body region. Anat. Rec. 1994, 238, 153–162. [Google Scholar] [CrossRef]
- Sugioka, K.; Kodama-Takahshi, A.; Sato, T.; Okada, K.; Murakami, J.; Park, A.-M.; Mishima, H.; Shimomura, Y.; Kusaka, S.; Nishida, T. Plasminogen-dependent collagenolytic properties of Staphylococcus aureus in collagen gel cultures of human corneal fibroblasts. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5098–5107. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.-W.; Stratton, C.W. Staphylococcus aureus: An old pathogen with new weapons. Clin. Lab. Med. 2010, 30, 179–208. [Google Scholar] [CrossRef]
- Mu, X.; Liu, K.; Li, H.; Wang, F.-S.; Xu, R. Granulocyte-macrophage colony-stimulating factor: An immunotarget for sepsis and COVID-19. Cell. Mol. Immunol. 2021, 18, 2057–2058. [Google Scholar] [CrossRef]
- Macleod, T.; Berekmeri, A.; Bridgewood, C.; Stacey, M.; McGonagle, D.; Wittmann, M. The immunological impact of IL-1 family cytokines on the epidermal barrier. Front. Immunol. 2021, 12, 5563. [Google Scholar] [CrossRef]
- Miller, L.S.; Cho, J.S. Immunity against Staphylococcus aureus cutaneous infections. Nat. Rev. Immunol. 2011, 11, 505–518. [Google Scholar] [CrossRef] [Green Version]
- Zurek, O.W.; Pallister, K.B.; Voyich, J.M. Staphylococcus aureus inhibits neutrophil-derived IL-8 to promote cell death. J. Infect. Dis. 2015, 212, 934–938. [Google Scholar] [CrossRef] [Green Version]
- Tajima, A.; Iwase, T.; Shinji, H.; Seki, K.; Mizunoe, Y. Inhibition of endothelial interleukin-8 production and neutrophil transmigration by Staphylococcus aureus beta-hemolysin. Infect. Immun. 2009, 77, 327–334. [Google Scholar] [CrossRef] [Green Version]
- Murphy, J.; Ramezanpour, M.; Stach, N.; Dubin, G.; Psaltis, A.J.; Wormald, P.J.; Vreugde, S. Staphylococcus Aureus V8 protease disrupts the integrity of the airway epithelial barrier and impairs IL-6 production in vitro. Laryngoscope 2018, 128, E8–E15. [Google Scholar] [CrossRef]
- Rosselle, L.; Cantelmo, A.R.; Barras, A.; Skandrani, N.; Pastore, M.; Aydin, D.; Szunerits, S. An ‘on-demand’ photothermal antibiotic release cryogel patch: Evaluation of efficacy on an ex vivo model for skin wound infection. Biomater. Sci. 2020, 8, 5911–5919. [Google Scholar] [CrossRef]
- Haisma, E.M.; Rietveld, M.H.; de Breij, A.; van Dissel, J.T.; El Ghalbzouri, A.; Nibbering, P.H. Inflammatory and antimicrobial responses to methicillin-resistant Staphylococcus aureus in an in vitro wound infection model. PLoS ONE 2013, 8, e82800. [Google Scholar] [CrossRef] [Green Version]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rancan, F.; Jurisch, J.; Hadam, S.; Vogt, A.; Blume-Peytavi, U.; Bayer, I.S.; Contardi, M.; Schaudinn, C. Ciprofloxacin-Loaded Polyvinylpyrrolidone Foils for the Topical Treatment of Wound Infections with Methicillin-Resistant Staphylococcus aureus (MRSA). Pharmaceutics 2023, 15, 1876. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics15071876
Rancan F, Jurisch J, Hadam S, Vogt A, Blume-Peytavi U, Bayer IS, Contardi M, Schaudinn C. Ciprofloxacin-Loaded Polyvinylpyrrolidone Foils for the Topical Treatment of Wound Infections with Methicillin-Resistant Staphylococcus aureus (MRSA). Pharmaceutics. 2023; 15(7):1876. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics15071876
Chicago/Turabian StyleRancan, Fiorenza, Jana Jurisch, Sabrina Hadam, Annika Vogt, Ulrike Blume-Peytavi, Ilker S. Bayer, Marco Contardi, and Christoph Schaudinn. 2023. "Ciprofloxacin-Loaded Polyvinylpyrrolidone Foils for the Topical Treatment of Wound Infections with Methicillin-Resistant Staphylococcus aureus (MRSA)" Pharmaceutics 15, no. 7: 1876. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics15071876