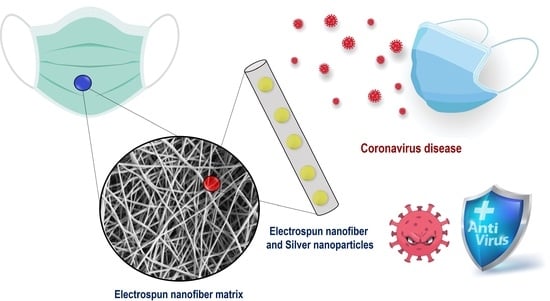
Silver Nanoparticles In Situ Synthesized and Incorporated in Uniaxial and Core–Shell Electrospun Nanofibers to Inhibit Coronavirus
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Polymeric Solutions for Electrospinning
2.2.2. Uniaxial Electrospinning
2.2.3. Coaxial Electrospinning
2.2.4. Nanofiber Characterization
2.2.5. Mechanical Analysis
2.2.6. Disintegration Assay
2.2.7. Virucidal Assay
3. Results and Discussion
4. Virucidal Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Available online: https://covid19.who.int/ (accessed on 26 November 2023).
- Yu, J.; Ouyang, W.; Chua, M.L.K.; Xie, C. SARS-CoV-2 Transmission in Patients With Cancer at a Tertiary Care Hospital in Wuhan, China. JAMA Oncol. 2020, 6, 1108–1110. [Google Scholar] [CrossRef]
- Sarangi, M.K.; Padhi, S.; Dheeman, S.; Karn, S.K.; Patel, L.D.; Yi, D.K.; Nanda, S.S. Diagnosis, Prevention, and Treatment of Coronavirus Disease: A Review. Expert Rev. Anti.-Infect. Ther. 2022, 20, 243–266. [Google Scholar] [CrossRef]
- Rasmi, Y.; Saloua, K.S.; Nemati, M.; Choi, J.R. Recent Progress in Nanotechnology for COVID-19 Prevention, Diagnostics and Treatment. Nanomaterials 2021, 11, 1788. [Google Scholar] [CrossRef]
- Baig, N.; Kammakakam, I.; Falath, W.; Kammakakam, I. Nanomaterials: A Review of Synthesis Methods, Properties, Recent Progress, and Challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Malik, S.; Muhammad, K. Nanotechnology: A Revolution in Modern Industry. Molecules 2023, 28, 661. [Google Scholar] [CrossRef] [PubMed]
- Sahu, T.; Ratre, Y.K.; Chauhan, S.; Bhaskar, L.V.K.S.; Nair, M.P.; Verma, H.K. Nanotechnology Based Drug Delivery System: Current Strategies and Emerging Therapeutic Potential for Medical Science. J. Drug Deliv. Sci. Technol. 2021, 63, 102487. [Google Scholar] [CrossRef]
- Zaheer, T.; Pal, K.; Zaheer, I. Topical Review on Nano-Vaccinology: Biochemical Promises and Key Challenges. Process Biochem. 2021, 100, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Yayehrad, A.T.; Siraj, E.A.; Wondie, G.B.; Alemie, A.A.; Derseh, M.T.; Ambaye, A.S. Could Nanotechnology Help to End the Fight against COVID-19? Review of Current Findings, Challenges and Future Perspectives. Int. J. Nanomed. 2021, 16, 5713–5743. [Google Scholar] [CrossRef] [PubMed]
- Phuna, Z.X.; Panda, B.P.; Hawala Shivashekaregowda, N.K.; Madhavan, P. Nanoprotection from SARS-CoV-2: Would Nanotechnology Help in Personal Protection Equipment (PPE) to Control the Transmission of COVID-19? Int. J. Environ. Health Res. 2022, 5, 670–699. [Google Scholar] [CrossRef] [PubMed]
- Husain, S.; Nandi, A.; Simnani, F.Z.; Saha, U.; Ghosh, A.; Sinha, A.; Sahay, A.; Samal, S.K.; Panda, P.K.; Verma, S.K. Emerging Trends in Advanced Translational Applications of Silver Nanoparticles: A Progressing Dawn of Nanotechnology. J. Funct. Biomater. 2023, 14, 47. [Google Scholar] [CrossRef]
- Bélteky, P.; Rónavári, A.; Zakupszky, D.; Boka, E.; Igaz, N.; Szerencsés, B.; Pfeiffer, I.; Vágvölgyi, C.; Kiricsi, M.; Kónya, Z. Are Smaller Nanoparticles Always Better? Understanding the Biological Effect of Size-Dependent Silver Nanoparticle Aggregation under Biorelevant Conditions. Int. J. Nanomed. 2021, 16, 3021–3040. [Google Scholar] [CrossRef]
- Derouiche, S.; Chetehouna, S.; Djouadi, A.; Boulaares, I.; Guemari, I.Y. The Possible Mechanisms of Silver Nanoparticles against SARS-CoV-2. Front. Biomed. Technol. 2022, 9, 149–158. [Google Scholar] [CrossRef]
- Arjun, P.N.J.; Sankar, B.; Shankar, K.V.; Kulkarni, N.V.; Sivasankaran, S.; Shankar, B. Silver and Silver Nanoparticles for the Potential Treatment of COVID-19: A Review. Coatings 2022, 12, 1679. [Google Scholar] [CrossRef]
- Abulikemu, M.; Tabrizi, B.E.A.; Ghobadloo, S.M.; Mofarah, H.M.; Jabbour, G.E. Silver Nanoparticle-Decorated Personal Protective Equipment for Inhibiting Human Coronavirus Infectivity. ACS Appl. Nano Mater. 2022, 5, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Xie, J.; Liu, W.; Xia, Y. Electrospun Nanofibers: New Concepts, Materials, and Applications. Acc. Chem. Res. 2017, 50, 1976–1987. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; Klingner, A. A Review on Electrospun Polymeric Nanofibers: Production Parameters and Potential Applications. Polym. Test. 2020, 90, 106647. [Google Scholar] [CrossRef]
- Rahmati, M.; Mills, D.K.; Urbanska, A.M.; Saeb, M.R.; Venugopal, J.R.; Ramakrishna, S.; Mozafari, M. Electrospinning for Tissue Engineering Applications. Prog. Mater. Sci. 2021, 117, 100721. [Google Scholar] [CrossRef]
- Islam, M.S.; Ang, B.C.; Andriyana, A.; Afifi, A.M. A Review on Fabrication of Nanofibers via Electrospinning and Their Applications. SN Appl. Sci. 2019, 1, 1248. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Velgosova, O.; Vojtko, M.; Lisnichuk, M. Preparation, Structure, and Properties of PVA–AgNPs Nanocomposites. Polymers 2023, 15, 379. [Google Scholar] [CrossRef]
- ASTM Standard Designation D882-91; Standard Test Methods for Tensile Properties of Thin Plastic Sheeting. Annual Book of American Standard Testing Methods. American Society for Testing and Material: Philadelphia, PA, USA, 1996.
- Esenturk, I.; Gumrukcu, S.; Özdabak Sert, A.B.; Kök, F.N.; Döşler, S.; Gungor, S.; Erdal, M.S.; Sarac, A.S. Silk-Fibroin-Containing Nanofibers for Topical Sertaconazole Delivery: Preparation, Characterization, and Antifungal Activity. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 605–622. [Google Scholar] [CrossRef]
- ISO 21702:2019; Measurement of Antiviral Activity on Plastics and Other Non-Porous Surfaces. ISO: Geneva, Switzerland, 2019; p. 71365.
- BS EN 14476:2013+A2:2019; Chemical Disinfectants and Antiseptics. Quantitative Suspension Test for the Evaluation of Virucidal Activity in the Medical Area. Test Method and Requirements (Phase 2/Step 1). European Standard: Plzen, Czech Republic, 2019.
- Turkevich, J. Colloidal Gold. Part I. Gold Bull. 1985, 18, 125–131. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A Simple Method of Estimating Fifty per Cent Endpoints. Am. J. Hyg. 1938, 27, 493–497. [Google Scholar]
- Paipitak, K.; Pornpra, T.; Mongkontalang, P.; Techitdheer, W.; Pecharapa, W. Characterization of PVA-Chitosan Nanofibers Prepared by Electrospinning. Procedia Eng. 2011, 8, 101–105. [Google Scholar] [CrossRef]
- Kim, G.H. Electrospun PCL Nanofibers with Anisotropic Mechanical Properties as a Biomedical Scaffold. Biomed. Mater. 2008, 3, 25010. [Google Scholar] [CrossRef]
- Yang, D.; Li, Y.; Nie, J. Preparation of Gelatin/PVA Nanofibers and Their Potential Application in Controlled Release of Drugs. Carbohydr. Polym. 2007, 69, 538–543. [Google Scholar] [CrossRef]
- Homayoni, H.; Abdolkarim, S.; Ravandi, H.; Valizadeh, M. Electrospinning of Chitosan Nanofibers: Processing Optimization. Carbohydr. Polym. 2009, 77, 656–661. [Google Scholar] [CrossRef]
- Ifuku, S. Chitin and Chitosan Nanofibers: Preparation and Chemical Modifications. Molecules 2014, 19, 18367–18380. [Google Scholar] [CrossRef]
- Lemma, S.M.; Bossard, F.; Rinaudo, M. Preparation of Pure and Stable Chitosan Nanofibers by Electrospinning in the Presence of Poly(Ethylene Oxide). Int. J. Mol. Sci. 2016, 17, 1790. [Google Scholar] [CrossRef] [PubMed]
- Qin, X. Coaxial Electrospinning of Nanofibers. In Electrospun Nanofibers; Elsevier: Amsterdam, The Netherlands, 2017; pp. 41–71. ISBN 9780081009116. [Google Scholar]
- Naganthran, A.; Verasoundarapandian, G.; Khalid, F.E.; Masarudin, M.J.; Zulkharnain, A.; Nawawi, N.M.; Karim, M.; Abdullah, C.A.C.; Ahmad, S.A. Synthesis, Characterization and Biomedical Application of Silver Nanoparticles. Materials 2022, 15, 427. [Google Scholar] [CrossRef]
- Gautam, A.; Singh, G.P.; Ram, S. A Simple Polyol Synthesis of Silver Metal Nanopowder of Uniform Particles. Synth. Met. 2007, 157, 5–10. [Google Scholar] [CrossRef]
- DeHoff, R.T. Thermodynamics in Materials Science; McGraw-Hill International Addition: Singapore, 1993. [Google Scholar]
- Wei, X.; Cai, J.; Lin, S.; Li, F.; Tian, F. Colloids and Surfaces B: Biointerfaces Controlled Release of Monodisperse Silver Nanoparticles via in Situ Cross-Linked Polyvinyl Alcohol as Benign and Antibacterial Electrospun Nanofibers. Colloids Surfaces B Biointerfaces 2021, 197, 111370. [Google Scholar] [CrossRef]
- Ahmed, M.; Hussein, M.; Guler, E.; Rayaman, E.; Emin, M.; Sahin, A.; Grinholc, M.; Sezgin, D.; Müge, Y.; Gunduz, O.; et al. Dual-Drug Delivery of Ag-Chitosan Nanoparticles and Phenytoin via Core-Shell PVA / PCL Electrospun Nanofibers. Carbohydr. Polym. 2021, 270, 118373. [Google Scholar] [CrossRef]
- Mahapatra, A.; Garg, N.; Nayak, B.P.; Mishra, B.G.; Hota, G. Studies on the Synthesis of Electrospun PAN-Ag Composite Nanofibers for Antibacterial Application. J. Appl. Polym. Sci. 2012, 124, 1178–1185. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, Y.; Wang, Z.; Zou, X.; Zhao, Y.; Sun, L. Fabrication of Silver Nanoparticles Embedded into Polyvinyl Alcohol (Ag/PVA) Composite Nano Fi Brous Fi Lms through Electrospinning for Antibacterial and Surface-Enhanced Raman Scattering (SERS) Activities. Mater. Sci. Eng. C 2016, 69, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Ahmed, S. A Review on Chitosan and Its Nanocomposites in Drug Delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Xia, X. Types and Processing of Structured Functional Nanofibers: Core-Shell, Aligned, Porous and Gradient Nanofibers. In Functional Nanofibers and Their Applications; Woodhead Publishing Limited: Cambridge, UK, 2012; pp. 22–37. [Google Scholar]
- Beachley, V.; Wen, X. Effect of Electrospinning Parameters on the Nanofiber Diameter and Length. Mater. Sci. Eng. C Mater. Biol. Appl. 2009, 29, 663–668. [Google Scholar] [CrossRef]
- Lyalina, T.S.; Lunkov, A.P.; Varlamov, V.P. Obtaining of Metal Nanoparticles Using Reducing Agents and Chitosan. Appl. Biochem. Microbiol. 2022, 58, 97–104. [Google Scholar] [CrossRef]
- Le Ouay, B.; Stellacci, F. Antibacterial Activity of Silver Nanoparticles: A Surface Science Insight. Nano Today 2015, 10, 339–354. [Google Scholar] [CrossRef]
- Park, E.J.; Yi, J.; Kim, Y.; Choi, K.; Park, K. Silver Nanoparticles Induce Cytotoxicity by a Trojan-Horse Type Mechanism. Toxicol. In Vitro 2010, 24, 872–878. [Google Scholar] [CrossRef]
- Gomes, J.R.B.; Jorge, M.; Gomes, P. Interaction of Chitosan and Chitin with Ni, Cu and Zn Ions: A Computational Study. J. Chem. Thermodyn. 2014, 73, 121–129. [Google Scholar] [CrossRef]
- Janarthanan, G.; Kim, I.G.; Chung, E.J.; Noh, I. Comparative Studies on Thin Polycaprolactone-Tricalcium Phosphate Composite Scaffolds and Its Interaction with Mesenchymal Stem Cells. Biomater. Res. 2019, 23, 1. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, V.H.B.; da Silva, E.P.; Marques, V.d.S.; Rubira, A.F.; Silva, R.; Cava, C.E.; Lourenço, S.A.; Muniz, E.C. Electrospun Fibers of Poly (Vinyl Alcohol): Zinc Acetate (PVA:AcZn) and Further ZnO Production: Evaluation of PVA:AcZn Ratio and Annealing Temperature Effects on ZnO Structure. J. Nanoparticle Res. 2020, 22, 322. [Google Scholar] [CrossRef]
- De Souza Costa, E.; Pereira, M.M.; Mansur, H.S. Properties and Biocompatibility of Chitosan Films Modified by Blending with PVA and Chemically Crosslinked. J. Mater. Sci. Mater. Med. 2009, 20, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Ahad, N.; Saion, E.; Gharibshahi, E. Structural, Thermal, and Electrical Properties of Pva-Sodium Salicylate Solid Composite Polymer Electrolyte. J. Nanomater. 2012, 2012, 857569. [Google Scholar] [CrossRef]
- Balu, R.; Sampath Kumar, T.S.; Ramalingam, M.; Ramakrishna, S. Electrospun Polycaprolactone/Poly(1,4-Butylene Adipate-Co-Polycaprolactam) Blends: Potential Biodegradable Scaffold for Bone Tissue Regeneration. J. Biomater. Tissue Eng. 2011, 1, 30–39. [Google Scholar] [CrossRef]
- Okubo, N. Measuring Crystal Water in Hydrates by Thermogravimetry. Appl. Br. 1993, 63, 2–6. [Google Scholar]
- Gomaa, M.M.; Hugenschmidt, C.; Dickmann, M.; Abdel-Hady, E.E.; Mohamed, H.F.M.; Abdel-Hamed, M.O. Crosslinked PVA/SSA Proton Exchange Membranes: Correlation between Physiochemical Properties and Free Volume Determined by Positron Annihilation Spectroscopy. Phys. Chem. Chem. Phys. 2018, 20, 28287–28299. [Google Scholar] [CrossRef]
- Lozano-Sánchez, L.M.; Bagudanch, I.; Sustaita, A.O.; Iturbe-Ek, J.; Elizalde, L.E.; Garcia-Romeu, M.L.; Elías-Zúñiga, A. Single-Point Incremental Forming of Two Biocompatible Polymers: An Insight into Their Thermal and Structural Properties. Polymers 2018, 10, 391. [Google Scholar] [CrossRef]
- Koosha, M.; Mirzadeh, H. Electrospinning, Mechanical Properties, and Cell Behavior Study of Chitosan/PVA Nanofibers. J. Biomed. Mater. Res. 2015, 103, 3081–3093. [Google Scholar] [CrossRef]
- Sabarees, G.; Velmurugan, V.; Tamilarasi, G.P.; Alagarsamy, V. Recent Advances in Silver Nanoparticles Containing Nanofibers for Chronic Wound Management. Polymers 2022, 14, 3994. [Google Scholar] [CrossRef]
- Baker, S.R.; Banerjee, S.; Bonin, K.; Guthold, M. Determining the Mechanical Properties of Electrospun Poly-ε-Caprolactone (PCL) Nano Fi Bers Using AFM and a Novel Fi Ber Anchoring Technique. Mater. Sci. Eng. C 2016, 59, 203–212. [Google Scholar] [CrossRef]
- Yilmaz Atay, H. Antibacterial activity of chitosan-based systems. Funct. Chitosan 2019, 457–489. [Google Scholar] [CrossRef]
- Reichling, J. Antiviral and Virucidal Properties of Essential Oils and Isolated Compounds—A Scientific Approach. Planta Med. 2022, 88, 587–603. [Google Scholar] [CrossRef]
- Gurunathan, S.; Qasim, M.; Choi, Y.; Do, J.T.; Park, C.; Hong, K.; Kim, J.H.; Song, H. Antiviral Potential of Nanoparticles—Can Nanoparticles Fight against Coronaviruses? Nanomaterials 2020, 10, 1645. [Google Scholar] [CrossRef]
- Atay, H.Y. Antibacterial Activity of Chitosan-Based Systems; Springer: Berlin/Heidelberg, Germany, 2020; ISBN 9789811502637. [Google Scholar]
- Sarkar, J.; Das, S.; Aich, S.; Bhattacharyya, P.; Acharya, K. Antiviral Potential of Nanoparticles for the Treatment of Coronavirus Infections. J. Trace Elem. Med. Biol. 2022, 72, 126977. [Google Scholar] [CrossRef]
- Swathy, J.R.; Sankar, M.U.; Chaudhary, A.; Aigal, S.; Pradeep, T.; States, U.; Protection, E. Antimicrobial Silver: An Unprecedented Anion Effect. Sci. Rep. 2014, 7161, 7161. [Google Scholar] [CrossRef] [PubMed]
- Jeremiah, S.S.; Miyakawa, K.; Morita, T.; Yamaoka, Y. Potent Antiviral Effect of Silver Nanoparticles on SARS-CoV-2. Biochem. Biophys. Res. Commun. 2020, 533, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Behbudi, G. Effect of Silver Nanoparticles Disinfectant on COVID-19. Adv. Appl. Nano-Bio Technol. 2021, 2, 63–67. [Google Scholar]
- He, Q.; Lu, J.; Liu, N.; Lu, W.; Li, Y.; Shang, C.; Li, X.; Hu, L. Antiviral Properties of Silver Nanoparticles against SARS-CoV-2: Effects of Surface Coating and Particle Size. Nanomaterials 2022, 12, 990. [Google Scholar] [CrossRef]
- Karagoz, S.; Burak Kiremitler, N.; Sarp, G.; Pekdemir, S.; Salem, S.; Goksu, A.G.; Serdar Onses, M.; Sozdutmaz, I.; Sahmetlioglu, E.; Ozkara, E.S.; et al. Antibacterial, Antiviral, and Self-Cleaning Mats with Sensing Capabilities Based on Electrospun Nanofibers Decorated with ZnO Nanorods and Ag Nanoparticles for Protective Clothing Applications. ACS Appl. Mater. Interfaces 2021, 13, 5678–5690. [Google Scholar] [CrossRef] [PubMed]








| Solution | Material | %(w/V) | Solvent | Conditions |
|---|---|---|---|---|
| 1 | PCL | 10.0 | DMF/DCM (1:1) | 1 h at 25 °C |
| 2 | CHT | 10.0 | H2O and 2% CH3COOH | 3 h at 60 °C |
| 3 | PVA | 12.0 | H2O | 3 h at 80 °C * |
| 4 | PVA-AgNO3 | 12.0; 0.25 | H2O | 3 h at 80 °C * |
| Sample | Young’s Modulus (MPa) | Tensile Strength (10−3 MPa) | Elongation at Break (%) |
|---|---|---|---|
| PVA | 0.164 ± 0.008 | 10.8 ± 1.0 | 21.9 ± 6.9 |
| PVA AgNPs | 0.084 ± 0.001 | 9.3 ± 0.10 | 113.6 ± 15.6 |
| PVA/CHT | 0.020 ± 0.004 | 1.25 ± 0.10 | 6.2 ± 2.0 |
| PVA/CHT/AgNPs | 0.030 ± 0.003 | 9.50 ± 0.05 | 15.5 ± 2.4 |
| PCL[PVA/CHT] | 0.013 ± 0.005 | 1.45 ± 0.05 | 12.1 ± 0.3 |
| PCL[PVA/CHT/AgNPs] | 0.049 ± 0.002 | 3.70 ± 0.70 | 16.1 ± 1.5 |
| Time (h) | PVA | PVA/CHT | PCL[PVA/CHT] | AgNPs | PVA/AgNPs | PVA/CHT/AgNps | PCL[PVA/CHT/AgNPs] |
|---|---|---|---|---|---|---|---|
| Log ± SD | Log ± SD | Log ± SD | Log ± SD | Log ± SD | Log ± SD | Log ± SD | |
| 0.5 | 0.8 ± 0.5 | 1.5 ± 0.5 | 1.50 ± 0.5 | 5.3 ± 0.5 | 5.5 ± 0.5 | 5.3 ± 0.4 | 5.0 ± 0.7 |
| 1 | 1.0 ± 0.0 | 1.8 ± 0.4 | 1.50 ± 0.5 | 5.3 ± 0.5 | 5.5 ± 0.5 | 5.3 ± 0.4 | 5.3 ± 0.4 |
| 8 | 1.0 ± 0.4 | 2.0 ± 0.7 | 1.75 ± 0.4 | 5.8 ± 0.5 | 5.3 ± 0.4 | 5.5 ± 0.5 | 5.5 ± 0.5 |
| 24 | 0.8 ± 0.4 | 2.0 ± 0.0 | 2.00 ± 0.0 | 5.3 ± 0.5 | 5.5 ± 0.5 | 5.5 ± 0.5 | 5.5 ± 0.5 |
| 48 | 1.0 ± 0.0 | 2.0 ± 0.0 | 2.00 ± 0.0 | 6.5 ± 0.6 | 5.5 ± 0.5 | 5.5 ± 0.5 | 5.5 ± 0.5 |
| 64 | 1.0 ± 0.0 | 2.0 ± 0.0 | 2.00 ± 0.0 | 6.8 ± 0.5 | 5.5 ± 0.5 | 5.5 ± 0.5 | 5.5 ± 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Freitas, C.F.; Souza, P.R.; Jacinto, G.S.; Braga, T.L.; Ricken, Y.S.; Souza, G.K.; Caetano, W.; Radovanovic, E.; Arns, C.W.; Rai, M.; et al. Silver Nanoparticles In Situ Synthesized and Incorporated in Uniaxial and Core–Shell Electrospun Nanofibers to Inhibit Coronavirus. Pharmaceutics 2024, 16, 268. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics16020268
de Freitas CF, Souza PR, Jacinto GS, Braga TL, Ricken YS, Souza GK, Caetano W, Radovanovic E, Arns CW, Rai M, et al. Silver Nanoparticles In Situ Synthesized and Incorporated in Uniaxial and Core–Shell Electrospun Nanofibers to Inhibit Coronavirus. Pharmaceutics. 2024; 16(2):268. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics16020268
Chicago/Turabian Stylede Freitas, Camila F., Paulo R. Souza, Gislaine S. Jacinto, Thais L. Braga, Yara S. Ricken, Gredson K. Souza, Wilker Caetano, Eduardo Radovanovic, Clarice W. Arns, Mahendra Rai, and et al. 2024. "Silver Nanoparticles In Situ Synthesized and Incorporated in Uniaxial and Core–Shell Electrospun Nanofibers to Inhibit Coronavirus" Pharmaceutics 16, no. 2: 268. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics16020268