The Healthy Gluten-Free Diet: Practical Tips to Prevent Metabolic Disorders and Nutritional Deficiencies in Celiac Patients
Abstract
:1. Introduction
2. Impact of GFD on Nutritional Status in CD Patients
3. CD, GFD and Metabolic Disorders
3.1. CD, GFD and Lipid Profile
3.2. CD, GFD, Glucose Intolerance and Type 2 Diabetes (T2D)
4. CD, GFD, and Nutritional Deficiencies
4.1. At Diagnosis
4.2. In Patients Following GFD
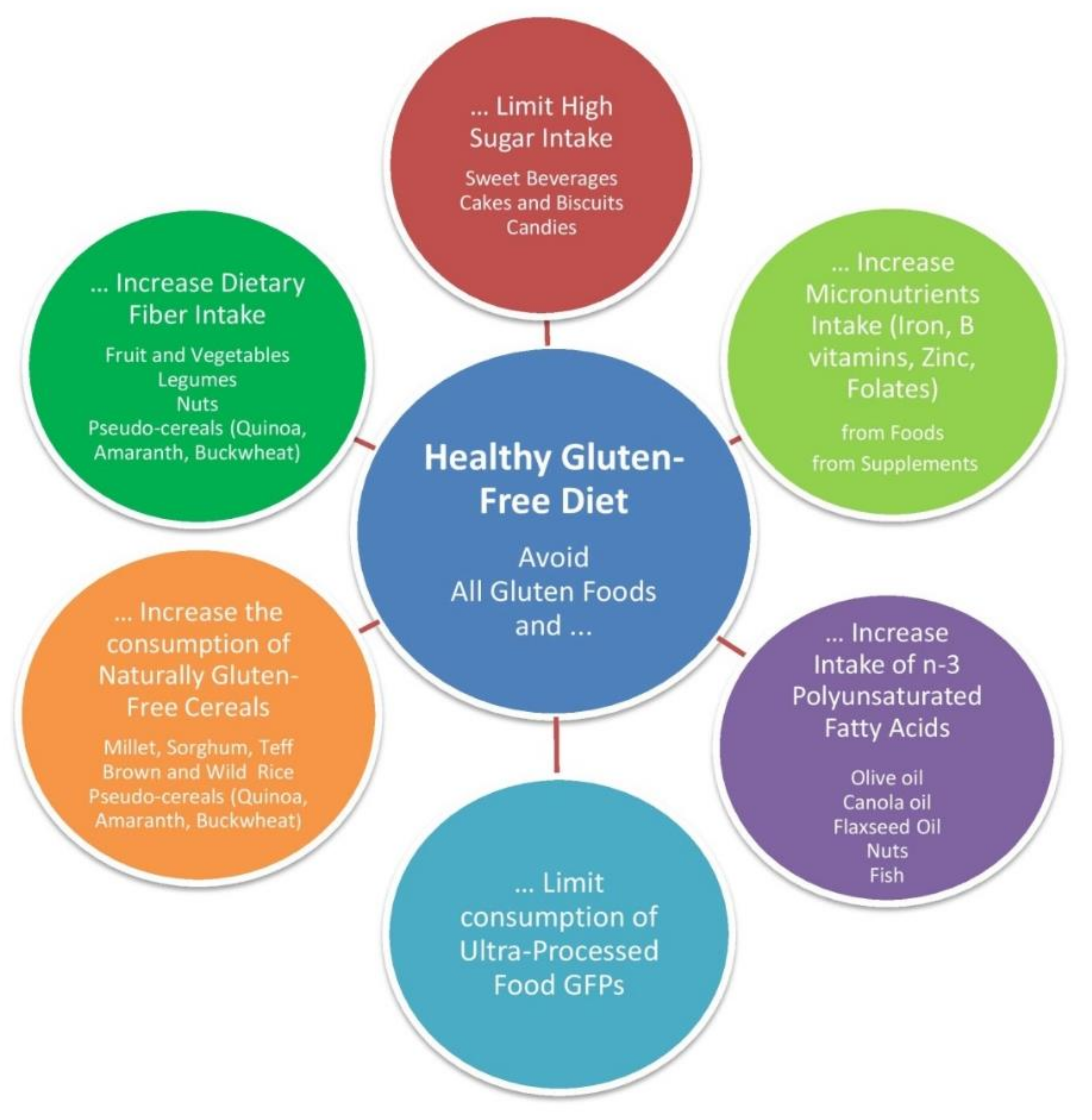
5. Nutritional Tips for an Adequate and Balanced Diet
5.1. Limit Sugar Consumption
5.2. Increase Dietary Fiber Intake
5.3. Increase Polyunsaturated Fatty Acid Intake
5.4. Limit Consumption of Ultra-Processed Food GFPs
5.5. Increase the Consumption of Naturally Gluten-Free Cereals, Including Pseudocereals
5.5.1. Rice
5.5.2. Maize
5.5.3. Sorghum, Millet and, Teff
| Pseudocereals | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Milled rice (2) | Brown rice (2) | Wild rice (2) | Maize * | Sorghum * | Millet * | Teff | Quinoa (1) | Amaranth (1) | Buckwheat | |
| Energy (kcal) | 360 | 362 | 357 | 361 | 359 | 382 | 367 | 368 | 371 | 343 |
| Protein (g) | 6.61 | 7.5 | 14.73 | 6.93 | 8.43 | 10.75 | 13.3 | 14.12 | 13.56 | 13.25 |
| Total lipid (fat) (g) | 0.58 | 2.68 | 1.08 | 3.86 | 3.34 | 4.25 | 2.38 | 6.07 | 7.02 | 3.4 |
| Fatty acids, total saturated (g) | 0.158 | 0.536 | 0.156 | 0.543 | 0.528 | 0.536 | 0.449 | 0.706 | 1.459 | 0.741 |
| Fatty acids, total monounsaturated (g) | 0.181 | 0.971 | 0.159 | 1.018 | 0.943 | 0.924 | 0.589 | 1.613 | 1.685 | 1.04 |
| Fatty acids, total polyunsaturated (g) | 0.155 | 0.959 | 0.676 | 1.759 | 1.403 | 2.618 | 1.071 | 3.292 | 2.778 | 1.039 |
| Fatty acids, total trans (g) | NR | NR | NR | NR | 0.004 | 0.002 | NR | NR | NR | NR |
| Carbohydrate (g) | 79.34 | 76.17 | 74.9 | 76.85 | 76.64 | 75.12 | 73.13 | 64.16 | 65.25 | 71.5 |
| Total dietary fiber (g) | NR | 3.4 | 6.2 | 7.3 | 6.6 | 3.5 | 8 | 7 | 6.7 | 10 |
| Starch (g) | NR | 80 | 69.3 | NR | 68 | 69.88 | 36.56 | 52.22 | 57.27 | 55 |
| Vitamin A (μg) | NR | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Vitamin B1 (thiamine, mg) | 0.07 | 0.413 | 0.115 | 0.246 | 6.6 | 0.413 | 0.39 | 0.36 | 0.116 | 0.101 |
| Vitamin B2 (riboflavin, mg) | 0.048 | 0.043 | 0.262 | 0.08 | 68 | 0.073 | 0.27 | 0.318 | 0.2 | 0.425 |
| Vitamin B3 (niacin, mg) | 1.6 | 4.308 | 6.733 | 1.9 | 4.496 | 6.02 | 3.363 | 1.52 | 0.923 | 7.02 |
| Vitamin B5 (pantothenic acid, mg) | 1.342 | 1.493 | 1.074 | 0.658 | 0.539 | 1.267 | 0.942 | 0.772 | 1.457 | 1.233 |
| Vitamin B6 (mg) | 0.145 | 0.509 | 0.391 | 0.37 | 0.325 | 0.372 | 0.482 | 0.487 | 0.591 | 0.21 |
| Folates (μg) | 9 | 0 | 95 | 25 | 25 | 42 | NR | 184 | 82 | 30 |
| Vitamin B12 (mg) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Choline (mg) | NR | NR | 35 | 21.6 | NR | NR | 13.1 | 70.2 | 69.8 | NR |
| Vitamin C (mg) | 0 | 0 | 0 | 0 | 0.8 | 0 | 0 | 0 | 4.2 | 0 |
| Vitamin D (μg) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Vitamin E (alpha-tocopherol, mg) | NR | NR | 0.82 | 0.42 | 0.5 | 0.11 | 0.08 | 2.44 | 1.19 | NR |
| Sodium (mg) | 1 | 4 | 7 | 5 | 3 | 4 | 12 | 5 | 4 | 1 |
| Calcium (mg) | 9 | 33 | 21 | 7 | 12 | 14 | 180 | 47 | 159 | 18 |
| Phosphorus (mg) | 108 | 264 | 433 | 272 | 278 | 285 | 429 | 457 | 557 | 347 |
| Iron (mg) | 0.8 | 1.8 | 1.96 | 2.38 | 3.14 | 3.94 | 7.63 | 4.57 | 7.61 | 2.2 |
| Magnesium (mg) | 35 | 143 | 177 | 93 | 123 | 119 | 184 | 197 | 248 | 231 |
| Potassium (mg) | 86 | 268 | 427 | 315 | 324 | 224 | 427 | 563 | 508 | 460 |
| Zinc (mg) | 1.16 | 2.02 | 5.96 | 1.73 | 1.63 | 2.63 | 3.63 | 3.1 | 2.87 | 2.4 |
| Copper (mg) | 0.11 | 0.277 | 0.524 | 0.23 | 0.253 | 0.535 | 0.81 | 0.59 | 0.525 | 1.1 |
| Selenium (μg) | NR | NR | 2.8 | 15.4 | 12.2 | 32.7 | 4.4 | 8.5 | 18.7 | 8.3 |
| Tryptophan (g) | 0.077 | 0.096 | 0.179 | 0.049 | 0.106 | 0.17 | 0.139 | 0.167 | 0.181 | 0.192 |
| Threonine (g) | 0.236 | 0.275 | 0.469 | 0.261 | 0.312 | 0.354 | 0.51 | 0.421 | 0.558 | 0.506 |
| Isoleucine (g) | 0.285 | 0.318 | 0.618 | 0.248 | 0.309 | 0.473 | 0.501 | 0.504 | 0.582 | 0.498 |
| Leucine (g) | 0.546 | 0.62 | 1.018 | 0.85 | 1.085 | 1.537 | 1.068 | 0.84 | 0.879 | 0.832 |
| Lysine (g) | 0.239 | 0.286 | 0.629 | 0.195 | 0.174 | 0.144 | 0.376 | 0.766 | 0.747 | 0.672 |
| Methionine (g) | 0.155 | 0.169 | 0.438 | 0.145 | 0.145 | 0.319 | 0.428 | 0.309 | 0.226 | 0.172 |
| Cystine (g) | 0.135 | 0.091 | 0.174 | 0.125 | 0.165 | 0.178 | 0.236 | 0.203 | 0.191 | 0.229 |
| Phenylalanine (g) | 0.353 | 0.387 | 0.721 | 0.34 | 0.441 | 0.675 | 0.698 | 0.593 | 0.542 | 0.52 |
| Tyrosine (g) | 0.221 | 0.281 | 0.622 | 0.282 | 0.225 | 0.326 | 0.458 | 0.267 | 0.329 | 0.241 |
| Valine (g) | 0.403 | 0.44 | 0.858 | 0.351 | 0.387 | 0.584 | 0.686 | 0.594 | 0.679 | 0.678 |
| Arginine (g) | 0.551 | 0.569 | 1.136 | 0.345 | 0.33 | 0.37 | 0.517 | 1.091 | 1.06 | 0.982 |
| Histidine (g) | 0.155 | 0.19 | 0.384 | 0.211 | 0.167 | 0.257 | 0.301 | 0.407 | 0.389 | 0.309 |
| Alanine (g) | 0.383 | 0.437 | 0.825 | 0.518 | 0.758 | 1.282 | 0.747 | 0.588 | 0.799 | 0.748 |
| Aspartic acid (g) | 0.621 | 0.702 | 1.419 | 0.482 | 0.556 | 0.71 | 0.82 | 1.134 | 1.261 | 1.133 |
| Glutamic acid (g) | 1.288 | 1.528 | 2.565 | 1.3 | 1.741 | 2.599 | 3.349 | 1.865 | 2.259 | 2.046 |
| Glycine (g) | 0.301 | 0.369 | 0.672 | 0.284 | 0.313 | 0.271 | 0.477 | 0.694 | 1.636 | 1.031 |
| Proline (g) | 0.311 | 0.352 | 0.519 | 0.605 | 0.651 | 0.911 | 0.664 | 0.773 | 0.698 | 0.507 |
| Serine (g) | 0.347 | 0.388 | 0.778 | 0.329 | 0.411 | 0.782 | 0.622 | 0.567 | 1.148 | 0.685 |
5.5.4. Pseudocereals
5.6. Increase Micronutrients Intake from Food and Supplements if Necessary
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ludvigsson, J.F.; Leffler, D.A.; Bai, J.C.; Biagi, F.; Fasano, A.; Green, P.H.; Hadjivassiliou, M.; Kaukinen, K.; Kelly, C.P.; Leonard, J.N.; et al. The Oslo definitions for coeliac disease and related terms. Gut 2013, 14, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Newnham, E.D. Coeliac disease in the 21st century: Paradigm shifts in the modern age. J. Gastroenterol. Hepatol. 2017, 32, 82–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kupper, C. Dietary guidelines and implementation for celiac disease. Gastroenterology 2005, 128, S121–S127. [Google Scholar] [CrossRef] [PubMed]
- Aston, L.M. Glycaemic index and metabolic disease risk. Proc. Nutr. Soc. 2006, 65, 125–134. [Google Scholar] [CrossRef] [Green Version]
- Kabbani, T.A.; Goldberg, A.; Kelly, C.P.; Pallav, K.; Tariq, S.; Peer, A.; Hansen, J.; Dennis, M.; Leffler, D.A. Body mass index and the risk of obesity in coeliac disease treated with the gluten-free diet. Aliment. Pharmacol. Ther. 2012, 35, 723–729. [Google Scholar] [CrossRef]
- Dickey, W.; Kearney, N. Overweight in celiac disease: Prevalence, clinical characteristics, and effect of a gluten-free diet. Am. J. Gastroenterol. 2006, 101, 2356–2359. [Google Scholar] [CrossRef]
- Barone, M.; Della Valle, N.; Rosania, R.; Facciorusso, A.; Trotta, A.; Cantatore, F.P.; Falco, S.; Pignatiello, S.; Viggiani, M.T.; Amoruso, A.; et al. A comparison of the nutritional status between adult celiac patients on a long-term, strictly gluten-free diet and healthy subjects. Eur. J. Clin. Nutr. 2016, 70, 23–27. [Google Scholar] [CrossRef]
- Nikniaz, Z.; Farhangi, M.A.; Hosseinifard, H.; Nikniaz, L. Does a Gluten-free Diet Increase Body Mass Index and Lipid Profile in Celiac Patients? A Systematic Review and Meta-analysis. Mediterr. J. Nutr. Metab. 2019, 12, 341–352. [Google Scholar] [CrossRef]
- Tortora, R.; Capone, P.; De Stefano, G.; Imperatore, N.; Gerbino, N.; Donetto, S.; Monaco, V.; Caporaso, N.; Rispo, A. Metabolic syndrome in patients with coeliac disease on a gluten-free diet. Aliment. Pharmacol. Ther. 2015, 41, 352–359. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, A.; Singh, A.; Mehtab, W.; Gupta, V.; Chauhan, A.; Rajput, M.S.; Singh, N.; Ahuja, V.; Makharia, G.K. Patients with celiac disease are at high risk of developing metabolic syndrome and fatty liver. Intest. Res. 2021, 19, 106–114. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.; Brar, P.S.; Lee, A.R.; Green, P.H. Body mass index in celiac disease: Beneficial effect of a gluten-free diet. J. Clin. Gastroenterol. 2010, 44, 267–271. [Google Scholar] [CrossRef]
- Bardella, M.T.; Fredella, C.; Prampolini, L.; Molteni, N.; Giunta, A.M.; Bianchi, P.A. Body composition and dietary intakes in adult celiac disease patients consuming strict gluten-free diet. Am. J. Clin. Nutr. 2000, 72, 937–939. [Google Scholar] [CrossRef] [Green Version]
- Więch, P.; Chmiel, Z.; Bazaliński, D.; Sałacińska, I.; Bartosiewicz, A.; Mazur, A.; Korczowski, B.; Binkowska-Bury, M.; Dąbrowski, M. The Relationship between Body Composition and a Gluten Free Diet in Children with Celiac Disease. Nutrients 2018, 10, 1817. [Google Scholar] [CrossRef] [Green Version]
- Lionetti, E.; Antonucci, N.; Marinelli, M.; Bartolomei, B.; Franceschini, E.; Gatti, S.; Catassi, G.N.; Verma, A.K.; Monachesi, C.; Catassi, C. Nutritional Status, Dietary Intake, and Adherence to the Mediterranean Diet of Children with Celiac Disease on a Gluten-Free Diet: A Case-Control Prospective Study. Nutrients 2020, 12, 143. [Google Scholar] [CrossRef] [Green Version]
- De Lorenzo, A.; Di Campli, C.; Andreoli, A.; Sasso, G.F.; Bonamico, M.; Gasbarrini, A. Assessment of body composition by bioelectrical impedance in adolescent patients with celiac disease. Am. J. Gastroenterol. 1999, 94, 2951–2955. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.; Cranney, A.; Zarkadas, M.; Graham, I.D.; Switzer, C.; Case, S.; Molloy, M.; Warren, R.E.; Burrows, V.; Butzner, J.D. Celiac Disease: Evaluation of the Diagnosis and Dietary Compliance in Canadian Children. Pediatrics 2005, 116, e754–e759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuccotti, G.; Fabiano, V.; Dilillo, D.; Picca, M.; Cravidi, C.; Brambilla, P. Intakes of nutrients in Italian children with celiac disease and the role of commercially available gluten-free products. J. Hum. Nutr. Diet 2013, 26, 436–444. [Google Scholar] [CrossRef]
- Anania, C.; Pacifico, L.; Olivero, F.; Perla, F.M.; Chiesa, C. Cardiometabolic risk factors in children with celiac disease on a gluten-free diet. World J. Clin. Pediatr. 2017, 6, 143–148. [Google Scholar] [CrossRef]
- Rosenthal, E.; Hoffman, R.; Aviram, M.; Benderly, A.; Erde, P.; Brook, J.G. Serum lipoprotein profile in children with celiac disease. J. Pediatr. Gastroenterol. Nutr. 1990, 11, 58–62. [Google Scholar] [CrossRef]
- Forchielli, M.L.; Diani, L.; Labriola, F.; Bolasco, G.; Rocca, A.; Salfi, N.C.; Leone, A.; Miserocchi, C.; Andreozzi, L.; Levi Della Vida, F.; et al. Gluten Deprivation: What Nutritional Changes Are Found During the First Year in Newly Diagnosed Coeliac Children? Nutrients 2019, 12, 60. [Google Scholar] [CrossRef] [Green Version]
- Valvano, M.; Longo, S.; Stefanelli, G.; Frieri, G.; Viscido, A.; Latella, G. Celiac Disease, Gluten-Free Diet, and Metabolic and Liver Disorders. Nutrients 2020, 12, 940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tortora, R.; Rispo, A.; Alisi, A.; Imperatore, N.; Crudele, A.; Ferretti, F.; Nobili, V.; Miele, L.; Gerbino, N.; Caporaso, N.; et al. PNPLA3 rs738409 Polymorphism Predicts Development and Severity of Hepatic Steatosis but Not Metabolic Syndrome in Celiac Disease. Nutrients 2018, 10, 1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciccone, A.; Gabrieli, D.; Cardinale, R.; Di Ruscio, M.; Vernia, F.; Stefanelli, G.; Necozione, S.; Melideo, D.; Viscido, A.; Frieri, G.; et al. Metabolic Alterations in Celiac Disease Occurring after Following a Gluten-Free Diet. Digestion 2019, 100, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Kabbani, T.A.; Kelly, C.P.; Betensky, R.A.; Hansen, J.; Pallav, K.; Villafuerte-Gálvez, J.A.; Vanga, R.; Mukherjee, R.; Novero, A.; Dennis, M.; et al. Patients with celiac disease have a lower prevalence of non-insulin-dependent diabetes mellitus and metabolic syndrome. Gastroenterology 2013, 144, 912–917. [Google Scholar] [CrossRef] [Green Version]
- Reilly, N.R.; Lebwohl, B.; Hultcrantz, R.; Green, P.H.R.; Ludvigsson, J.F. Increased risk of non-alcoholic fatty liver disease after diagnosis of celiac disease. J. Hepatol. 2015, 62, 1405–1411. [Google Scholar] [CrossRef] [Green Version]
- Caruso, R.; Pallone, F.; Stasi, E.; Romeo, S.; Monteleone, G. Appropriate nutrient supplementation in celiac disease. Ann. Med. 2013, 45, 522–531. [Google Scholar] [CrossRef]
- Dickey, W.; Ward, M.; Whittle, C.R.; Kelly, M.T.; Pentieva, K.; Horigan, G.; Patton, S.; McNulty, H. Homocysteine and related B-vitamin status in coeliac disease: Effects of gluten exclusion and histological recovery. Scand. J. Gastroenterol. 2008, 43, 682–688. [Google Scholar] [CrossRef]
- Valente, F.X.; do Nascimento Campos, T.; de Sousa Moraes, L.F.; Hermsdorff, H.H.M.; de Morais Cardoso, L.; Pinheiro-Sant’Ana, H.M.; Gilberti, F.A.B.; Peluzio, M.D.C.G. B vitamins related to homocysteine metabolism in adults celiac disease patients: A cross-sectional study. Nutr. J. 2015, 14, 110. [Google Scholar] [CrossRef] [Green Version]
- Kemppainen, T.A.; Kosma, V.M.; Janatuinen, E.K.; Julkunen, R.J.; Pikkarainen, P.H.; Uusitupa, M.I. Nutritional status of newly diagnosed celiac disease patients before and after the institution of a celiac disease diet--association with the grade of mucosal villous atrophy. Am. J. Clin. Nutr. 1998, 67, 482–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haapalahti, M.; Kulmala, P.; Karttunen, T.J.; Paajanen, L.; Laurila, K.; Mäki, M.; Mykkänen, H.; Kokkonen, J. Nutritional status in adolescents and young adults with screen-detected celiac disease. J. Pediatr. Gastroenterol. Nutr. 2005, 40, 566–570. [Google Scholar] [CrossRef] [Green Version]
- Grace-Farfaglia, P. Bones of Contention: Bone Mineral Density Recovery in Celiac Disease—A Systematic Review. Nutrients 2015, 7, 3347–3369. [Google Scholar] [CrossRef] [Green Version]
- Fathi, F.; Ektefa, F.; Tafazzoli, M.; Rostami, K.; Rostami Nejad, M.; Fathi, M.; Rezaei-Tavirani, M.; Oskouie, A.A.; Zali, M.R. The concentration of serum zinc in celiac patients compared to healthy subjects in Tehran. Gastroenterol. Hepatol. Bed Bench. 2013, 6, 92–95. [Google Scholar]
- Erdem, T.; Ferat, Ç.; Nurdan, Y.A.; Halime, E.; Muhammed Selçuk, S.; Hamza, K.; Mukadder Ayşe, S. Vitamin and mineral deficiency in children newly diagnosed with celiac disease. Turk. J. Med. Sci. 2015, 45, 833–836. [Google Scholar]
- Black, M.M. Zinc deficiency and child development. Am. J. Clin. Nutr. 1998, 68, 464S–469S. [Google Scholar] [CrossRef] [Green Version]
- Jameson, S. Coeliac disease, insulin-like growth factor, bone mineral density, and zinc. Scand. J. Gastroenterol. 2000, 35, 894–896. [Google Scholar] [CrossRef]
- Wierdsma, N.J.; van Bokhorst-de van der Schueren, M.A.; Berkenpas, M.; Mulder, C.J.; van Bodegraven, A.A. Vitamin and mineral deficiencies are highly prevalent in newly diagnosed celiac disease patients. Nutrients 2013, 5, 3975–3992. [Google Scholar] [CrossRef]
- Alwitry, A. Vitamin A deficiency in coeliac disease. Br. J. Ophthalmol. 2000, 84, 1079–1080. [Google Scholar] [CrossRef] [Green Version]
- Welstead, L. The Gluten-Free Diet in the 3rd Millennium: Rules, Risks and Opportunities. Diseases 2015, 3, 136–149. [Google Scholar] [CrossRef]
- Theethira, T.G.; Dennis, M.; Leffler, D.A. Nutritional consequences of celiac disease and the gluten-free diet. Expert Rev. Gastroenterol. Hepatol. 2014, 8, 123–129. [Google Scholar] [CrossRef]
- Quero, J.S.; Jaime, B.E.; Martínez, A.R.; Martín, F.A.; Jiménez, R.G.; Murillo, M.R.; Martín, A.P. Nutritional assessment of gluten-free diet. Is gluten-free diet deficient in some nutrient? Anales de Pediatría 2015, 83, 33–39. [Google Scholar]
- Gelfond, D.; Fasano, A. Celiac disease in the pediatric population. Pediatr. Ann. 2006, 35, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Hill, I.D.; Bhatnagar, S.; Cameron, D.J.; De Rosa, S.; Maki, M.; Russell, G.J.; Troncone, R. Celiac disease: Working group report of the First World Congress of Pediatric Gastroenterology, Hepatology, and Nutrition. J. Pediatr. Gastroenterol. Nutr. 2002, 35, S78–S88. [Google Scholar] [CrossRef] [PubMed]
- Nestares, T.; Martín-Masot, R.; Labella, A.; Aparicio, V.A.; Flor-Alemany, M.; López-Frías, M.; Maldonado, J. Is a Gluten-Free Diet Enough to Maintain Correct Micronutrients Status in Young Patients with Celiac Disease? Nutrients 2020, 12, 844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Öhlund, K.; Olsson, C.; Hernell, O.; Öhlund, I. Dietary shortcomings in children on a gluten-free diet. J. Hum. Nutr. Diet. 2010, 23, 294–300. [Google Scholar] [CrossRef]
- Ballestero Fernández, C.; Varela-Moreiras, G.; Úbeda, N.; Alonso-Aperte, E. Nutritional Status in Spanish Children and Adolescents with Celiac Disease on a Gluten Free Diet Compared to Non-Celiac Disease Controls. Nutrients 2019, 11, 2329. [Google Scholar] [CrossRef] [Green Version]
- Mariani, P.; Viti, M.G.; Montuori, M.; La Vecchia, A.; Cipolletta, E.; Calvani, L.; Bonamico, M. The gluten-free diet: A nutritional risk factor for adolescents with celiac disease? J. Pediatr. Gastroenterol. Nutr. 1998, 27, 519–523. [Google Scholar] [CrossRef]
- Martin, J.; Geisel, T.; Maresch, C.; Krieger, K.; Stein, J. Inadequate nutrient intake in patients with celiac disease: Results from a German dietary survey. Digestion 2013, 87, 240–246. [Google Scholar] [CrossRef]
- Sue, A.; Dehlsen, K.; Ooi, C.Y. Paediatric Patients with Coeliac Disease on a Gluten-Free Diet: Nutritional Adequacy and Macro- and Micronutrient Imbalances. Curr. Gastroenterol. Rep. 2018, 20, 2. [Google Scholar] [CrossRef]
- Shepherd, S.J.; Gibson, P.R. Nutritional inadequacies of the gluten-free diet in both recently diagnosed and long-term patients with coeliac disease. J. Hum. Nutr. Diet 2013, 26, 349–358. [Google Scholar] [CrossRef]
- Penagini, F.; Dilillo, D.; Meneghin, F.; Mameli, C.; Fabiano, V.; Zuccotti, G.V. Gluten-free diet in children: An approach to a nutritionally adequate and balanced diet. Nutrients 2013, 5, 4553–4565. [Google Scholar] [CrossRef] [Green Version]
- Malik, V.S.; Hu, F.B. Sweeteners and Risk of Obesity and Type 2 Diabetes: The Role of Sugar-Sweetened Beverages. Curr. Diab. Rep. 2012, 12, 195–203. [Google Scholar] [CrossRef]
- De Queiroz, K.B.; Coimbra, R.S.; Ferreira, A.R.; Carneiro, C.M.; Paiva, N.C.; Costa, D.C.; Evangelista, E.A.; Guerra-Sá, R. Molecular mechanism driving retroperitoneal adipocyte hypertrophy and hyperplasia in response to a high-sugar diet. Mol. Nutr. Food Res. 2014, 58, 2331–2341. [Google Scholar] [CrossRef]
- Malik, V.S.; Schulze, M.B.; Hu, F.B. Intake of sugar-sweetened beverages and weight gain: A systematic review. Am. J. Clin. Nutr. 2006, 84, 274–288. [Google Scholar] [CrossRef]
- Kelishadi, R.; Mansourian, M.; Heidari-Beni, M. Association of fructose consumption and components of metabolic syndrome in human studies: A systematic review and meta-analysis. Nutrition 2014, 30, 503–510. [Google Scholar] [CrossRef]
- Stanhope, K.L. Sugar consumption, metabolic disease and obesity: The state of the controversy. Crit. Rev. Clin. Lab. Sci. 2016, 53, 52–67. [Google Scholar] [CrossRef]
- Babio, N.; Alcázar, M.; Castillejo, G.; Recasens, M.; Martínez-Cerezo, F.; Gutiérrez-Pensado, V.; Masip, G.; Vaqué, C.; Vila-Martí, A.; Torres-Moreno, M.; et al. Patients With Celiac Disease Reported Higher Consumption of Added Sugar and Total Fat Than Healthy Individuals. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 63–69. [Google Scholar] [CrossRef]
- Fry, L.; Madden, A.M.; Fallaize, R. An investigation into the nutritional composition and cost of gluten-free versus regular food products in the UK. J. Hum. Nutr. Diet 2018, 31, 108–120. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.H.Y.; Neal, B.; Trevena, H.; Crino, M.; Stuart-Smith, W.; Faulkner-Hogg, K.; Louie, J.C.Y.; Dunford, E. Are gluten-free foods healthier than non-gluten-free foods? An evaluation of supermarket products in Australia. Br. J. Nut. 2015, 114, 448–454. [Google Scholar] [CrossRef] [Green Version]
- DeVries, J.W. The definition of dietary fibre. Cereal Foods World 2001, 46, 112–129. [Google Scholar]
- Tucker, L.A.; Thomas, K.S. Increasing total fiber intake reduces risk of weight and fat gains in women. J. Nutr. 2009, 139, 576–581. [Google Scholar] [CrossRef] [Green Version]
- Chandalia, M.; Garg, A.; Lutjohann, D.; von Bergmann, K.; Grundy, S.M.; Brinkley, L.J. Beneficial effects of high dietary fiber intake in patients with type 2 diabetes mellitus. N. Engl. J. Med. 2000, 342, 1392–1398. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.A.; Kendall, C.W.C.; Vuksan, V.; Vidgen, E.; Parker, T.; Faulkner, D.; Mehling, C.C.; Garsetti, M.; Testolin, G.; Cunnane, S.C.; et al. Soluble fiber intake at a dose approved by the US Food and Drug Administration for a claim of health benefits: Serum lipid risk factors for cardiovascular disease assessed in a randomized controlled crossover trial. Am. J. Clin. Nutr. 2002, 75, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.; Rosner, B.; Willett, W.W.; Sacks, F.M. Cholesterol-lowering effects of dietary fiber: A meta-analysis. Am. J. Clin. Nutr. 1999, 69, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.P.; Chen, G.C.; Wang, X.P.; Qin, L.; Bai, Y. Dietary Fiber and Metabolic Syndrome: A Meta-Analysis and Review of Related Mechanisms. Nutrients 2017, 10, 24. [Google Scholar] [CrossRef] [Green Version]
- Thompson, T.; Dennis, M.; Higgins, L.A.; Lee, A.R.; Sharrett, M.K. Gluten-free diet survey: Are Americans with coeliac disease consuming recommended amounts of fibre, iron, calcium and grain foods? J. Hum. Nutr. Diet 2005, 18, 163–169. [Google Scholar] [CrossRef]
- Saturni, L.; Ferretti, G.; Bacchetti, T. The gluten-free diet: Safety and nutritional quality. Nutrients 2010, 2, 16–34. [Google Scholar] [CrossRef] [Green Version]
- Phillips, C.M.; Kesse-Guyot, E.; McManus, R.; Hercberg, S.; Lairon, D.; Planells, R.; Roche, H.M. High dietary saturated fat intake accentuates obesity risk associated with the fat mass and obesity–associated gene in adults. J. Nutr. 2012, 142, 824–831. [Google Scholar] [CrossRef]
- Raatz, S.; Conrad, Z.; Johnson, L.; Picklo, M.; Jahns, L. Relationship of the Reported Intakes of Fat and Fatty Acids to Body Weight in US Adults. Nutrients 2017, 9, 438. [Google Scholar] [CrossRef] [Green Version]
- Moleres, A.; Ochoa, M.C.; Rendo-Urteaga, T.; Martínez-González, M.A.; Azcona San Julián, M.C.; Martínez, J.A.; Marti, A. Dietary fatty acid distribution modifies obesity risk linked to the rs9939609 polymorphism of the fat mass and obesity-associated gene in a Spanish case–control study of children. Br. J. Nutr. 2012, 107, 533–538. [Google Scholar] [CrossRef] [Green Version]
- Diaf, M.; Khaled, M.B.; Sellam, F. Correlation between dietary fat intake and atherogenic indices in normal, overweight and obese adults with or without type 2 diabetes. Rom. J. Diabetes Nutr. Metab. Dis. 2015, 22, 347–360. [Google Scholar] [CrossRef] [Green Version]
- Lund, A.S.Q.; Hasselbalch, A.L.; Gamborg, M.; Skogstrand, K.; Hougaard, D.M.; Heitmann, B.L. N-3 polyunsaturated fatty acids, body fat and inflammation. Obes. Facts 2013, 6, 369–379. [Google Scholar] [CrossRef]
- Cardel, M.; Lemas, D.J.; Jackson, K.H.; Friedman, J.E.; Fernández, J.R. Higher intake of PUFAs is associated with lower total and visceral adiposity and higher lean mass in a racially diverse sample of children. J. Nutr. 2015, 145, 2146–2152. [Google Scholar] [CrossRef]
- Suara, S.B.; Siassi, F.; Saaka, M.; Foroshani, A.R.; Asadi, S.; Sotoudeh, G. Dietary fat quantity and quality in relation to general and abdominal obesity in women: A cross-sectional study from Ghana. Lipids Health Dis. 2020, 19, 67. [Google Scholar] [CrossRef] [Green Version]
- Nimptsch, K.; Berg-Beckhoff, G.; Linseisen, J. Effect of dietary fatty acid intake on prospective weight change in the Heidelberg cohort of the European prospective investigation into Cancer Micallef M, Munro I, Phang M, Garg, M. Plasma n-3 Polyunsaturated Fatty Acids are negatively associated with obesity. Br. J. Nutr. 2009, 102, 1370–1374. [Google Scholar]
- Klein-Platat, C.; Drai, J.; Oujaa, M.; Schlienger, J.L.; Simon, C. Plasma fatty acid composition is associated with the metabolic syndrome and low-grade inflammation in overweight adolescents. Am. J. Clin. Nutr. 2005, 82, 1178–1184. [Google Scholar] [CrossRef] [Green Version]
- Bjermo, H.; Iggman, D.; Kullberg, J.; Dahlman, I.; Johansson, L.; Persson, L.; Berglund, J.; Pulkki, K.; Basu, S.; Uusitupa, M.; et al. Effects of n-6 PUFAs compared with SFAs on liver fat, lipoproteins, and inflammation in abdominal obesity: A randomized controlled trial. Am. J. Clin. Nutr. 2012, 95, 1003–1012. [Google Scholar] [CrossRef] [Green Version]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–579. [Google Scholar] [CrossRef]
- Maggio, A.; Orecchio, S. Fatty Acid Composition of Gluten-Free Food (Bakery Products) for Celiac People. Foods 2018, 7, 95. [Google Scholar] [CrossRef] [Green Version]
- Caponio, F.; Summo, C.; Clodoveo, M.; Pasqualone, A. Evaluation of the nutritional quality of the lipid fraction of gluten-free biscuits. Euro Food Res. Technol. 2008, 227, 135–139. [Google Scholar] [CrossRef]
- Hadj Ahmed, S.; Kharroubi, W.; Kaoubaa, N.; Zarrouk, A.; Batbout, F.; Gamra, H.; Najjar, M.F.; Lizard, G.; Hininger-Favier, I.; Hammami, M. Correlation of trans fatty acids with the severity of coronary artery disease lesions. Lipids Health Dis. 2018, 17, 52. [Google Scholar] [CrossRef] [Green Version]
- Segura, M.E.; Rosell, C.M. Chemical composition and starch digestibility of different gluten-free breads. Plant. Foods Hum. Nutr. 2011, 3, 224–230. [Google Scholar] [CrossRef] [Green Version]
- Miranda, J.; Lasa, A.; Bustamante, M.A.; Churruca, I.; Simon, E. Nutritional differences between a gluten-free diet and a diet containing equivalent products with gluten. Plant. Foods Hum. Nutr. 2014, 69, 182–187, Erratum in: 2014, 69, 290. [Google Scholar] [CrossRef]
- Melini, V.; Melini, F. Gluten-Free Diet: Gaps and Needs for a Healthier Diet. Nutrients 2019, 11, 170. [Google Scholar] [CrossRef] [Green Version]
- Nestares, T.; Martín-Masot, R.; Flor-Alemany, M.; Bonavita, A.; Maldonado, J.; Aparicio, V.A. Influence of Ultra-Processed Foods Consumption on Redox Status and Inflammatory Signaling in Young Celiac Patients. Nutrients 2021, 13, 156. [Google Scholar] [CrossRef]
- Berti, C.; Riso, P.; Monti, L.D.; Porrini, M. In vitro starch digestibility and in vivo glucose response of gluten-free foods and their gluten counterparts. Eur. J. Nutr. 2004, 43, 198–204. [Google Scholar] [CrossRef]
- Cicero, A.F.; Gaddi, A. Rice bran oil and gamma-oryzanol in the treatment of hyperlipoproteinaemias and oTher. conditions. PhytoTher. Res. 2001, 15, 277–289. [Google Scholar] [CrossRef]
- Wu, F.; Yang, N.; Toure, A.; Jin, Z.; Xu, X. Germinated brown rice and its role in human health. Crit. Rev. Food Sci. 2013, 53, 451–463. [Google Scholar] [CrossRef]
- Mattei, J.; Malik, V.; Wedick, N.M.; Hu, F.B.; Spiegelman, D.; Willett, W.C.; Campos, H. Global Nutrition Epidemiologic Transition Initiative. Reducing the global burden of type 2 diabetes by improving the quality of staple foods: The global nutrition and epidemiologic transition initiative. Glob. Health 2015, 11, 23. [Google Scholar] [CrossRef] [Green Version]
- Shao, Y.F.; Bao, J.S. Polyphenols in whole rice grain: Genetic diversity and health benefits. Food Chem. 2015, 180, 86–97. [Google Scholar] [CrossRef]
- Shobana, S.; Lakshmipriya, N.; Bai, M.R.; Rajagopal, G.; Vaidya, R.; Malleshi, N.; Mohan, V. Even minimal polishing of an Indian parboiled brown rice variety leads to increased glycemic responses. Asia Pac. J. Clin. Nutr. 2017, 26, 829–836. [Google Scholar]
- Krittanawong, C.; Tunhasiriwet, A.; Zhang, H.; Prokop, L.J.; Chirapongsathorn, S.; Sun, T.; Wang, Z. Is white rice consumption a risk for metabolic and cardiovascular outcomes? A systematic review and meta-analysis. Heart Asia 2017, 9, e010909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Surendiran, G.; Alsaif, M.; Kapourchali, F.R.; Moghadasian, M.H. Nutritional constituents and health benefits of wild rice (Zizania spp.). Nutr. Rev. 2014, 72, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, U.; Robin, F. Slowly digestible starch-its structure and health implications: A review. Trends Food Sci. Technol. 2007, 18, 346–355. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, P.; Agellon, L.B.; Zhai, C.K. Wild rice (Zizania latifolia (Griseb) Turcz) improves the serum lipid profile and antioxidant status of rats fed with a high fat/cholesterol diet. Br. J. Nutr. 2009, 102, 1723–1727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, S.; Zhang, H.; Qin, L.; Zhai, C. Effects of dietary carbohydrate replaced with wild rice (Zizania latifolia (Griseb) Turcz) on insulin resistance in rats fed with highfat/cholesterol diet. Nutrients 2013, 5, 552–564. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Y.; Liu, Q.; Beta, T. Antioxidant properties of commercial wild rice and analysis of soluble and insoluble phenolic acids. Food Chem. 2010, 121, 140–147. [Google Scholar] [CrossRef]
- Prasanna, B.M.; Vasal, S.K.; Kassahun, B.; Singh, N.N. Quality protein maize. Curr. Sci. 2001, 81, 1308–1318. [Google Scholar]
- Nuss, E.T.; Tanumihardjo, S.A. Maize: A paramount staple crop in the context of global nutrition. Compr. Rev. Food Sci. Food Saf. 2010, 9, 417–436. [Google Scholar] [CrossRef]
- Ai, Y.; Jane, J.L. Macronutrients in Corn and Human Nutrition. Compr. Rev. Food Sci. Food Saf. 2016, 15, 581–598. [Google Scholar] [CrossRef] [Green Version]
- Afify, A.E.-M.M.R.; El-Beltagi, H.S.; El-Salam, S.M.A.; Omran, A.A. Biochemical changes in phenols, flavonoids, tannins, vitamin E, β–carotene and antioxidant activity during soaking of three white sorghum varieties. Asian Pac. J. Trop Biomed. 2012, 2, 203–209. [Google Scholar] [CrossRef] [Green Version]
- Vázquez-Araújo, L.; Chambers Iv, E.; Cherdchu, P. Consumer Input for Developing Human Food Products Made with Sorghum Grain. J. Food Sci. 2012, 77, 384–389. [Google Scholar] [CrossRef]
- Awika, J.M.; Rooney, L.W. Sorghum phytochemicals and their potential impact on human health. Phytochemistry 2004, 65, 1199–1221. [Google Scholar] [CrossRef]
- Anunciação, P.C.; Cardoso, L.d.M.; Gomes, J.V.P.; Della Lucia, C.M.; Carvalho, C.W.P.; Galdeano, M.C.; Queiroz, V.A.V.; Alfenas, R.C.G.; Martino, H.S.D.; Pinheiro-Sant’Ana, H.M. Comparing sorghum and wheat whole grain breakfast cereals: Sensorial acceptance and bioactive compound content. Food Chem. 2017, 221, 984–989. [Google Scholar] [CrossRef]
- Bora, P.; Ragaee, S.; Marcone, M. Characterisation of several types of millets as functional food ingredients. Int. J. Food Sci. Nutr. 2019, 70, 714–724. [Google Scholar] [CrossRef]
- Martínez-Villaluenga, C.; Peñas, E.; Hernández-Ledesma, B. Pseudocereal grains: Nutritional value, health benefits and current applications for the development of gluten-free foods. Food Chem. Toxicol. 2020, 137, 111178. [Google Scholar] [CrossRef]
- Repo-Carrasco-Valencia, R.; Hellström, J.K.; Pihlava, J.M.; Mattila, P.H. Flavonoids and oTher. phenolic compounds in Andean indigenous grains: Quinoa (Chenopodium quinoa), kañiwa (Chenopodium pallidicaule) and kiwicha (Amaranthus caudatus). Food Chem. 2010, 120, 128–133. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, S.; Jiang, Y.; Wei, Y.; Zhou, X. Regulatory function of buckwheat-resistant starch supplementation on lipid profile and gut microbiota in mice fed with a high-fat diet. J. Food Sci. 2019, 84, 2674–2681. [Google Scholar] [CrossRef]
- Motta, C.; Castanheira, I.; Gonzales, G.B.; Delgado, I.; Torres, D.; Santos, M.; Matos, A.S. Impact of cooking methods and malting on amino acids content in amaranth, buckwheat and quinoa. J. Food Compos. Anal. 2019, 76, 58–65. [Google Scholar] [CrossRef]
- Rodrigo, L. Celiac disease. World J. Gastroenterol. 2006, 12, 6585–6593. [Google Scholar] [CrossRef]
- Rizzoli, R. Dairy products, yogurts, and bone health. Am. J. Clin. Nutr. 2014, 99 (Suppl. 5), 1256S–1262S. [Google Scholar] [CrossRef] [Green Version]
- Geissler, C.; Singh, M. Iron, Meat and Health. Nutrients 2011, 3, 283–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubio-Tapia, A.; Hill, I.D.; Kelly, C.P.; Calderwood, A.H.; Murray, J.A. ACG Clinical Guidelines: Diagnosis and Management of Celiac Disease. Am. J. Gastroenterol. 2013, 108, 656–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rondanelli, M.; Faliva, M.A.; Gasparri, C.; Peroni, G.; Naso, M.; Picciotto, G.; Riva, A.; Nichetti, M.; Infantino, V.; Alalwan, T.A.; et al. Micronutrients Dietary Supplementation Advices for Celiac Patients on Long-Term Gluten-Free Diet with Good Compliance: A Review. Medicina 2019, 55, 337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rinninella, E.; Cintoni, M.; Raoul, P.; Triarico, S.; Dionisi, T.; Gasbarrini, G.B.; Gasbarrini, A.; Mele, M.C. The Healthy Gluten-Free Diet: Practical Tips to Prevent Metabolic Disorders and Nutritional Deficiencies in Celiac Patients. Gastroenterol. Insights 2021, 12, 166-182. https://0-doi-org.brum.beds.ac.uk/10.3390/gastroent12020015
Rinninella E, Cintoni M, Raoul P, Triarico S, Dionisi T, Gasbarrini GB, Gasbarrini A, Mele MC. The Healthy Gluten-Free Diet: Practical Tips to Prevent Metabolic Disorders and Nutritional Deficiencies in Celiac Patients. Gastroenterology Insights. 2021; 12(2):166-182. https://0-doi-org.brum.beds.ac.uk/10.3390/gastroent12020015
Chicago/Turabian StyleRinninella, Emanuele, Marco Cintoni, Pauline Raoul, Silvia Triarico, Tommaso Dionisi, Giovanni Battista Gasbarrini, Antonio Gasbarrini, and Maria Cristina Mele. 2021. "The Healthy Gluten-Free Diet: Practical Tips to Prevent Metabolic Disorders and Nutritional Deficiencies in Celiac Patients" Gastroenterology Insights 12, no. 2: 166-182. https://0-doi-org.brum.beds.ac.uk/10.3390/gastroent12020015






