New Bis-Pyrazole-Bis-Acetate Based Coordination Complexes: Influence of Counter-Anions and Metal Ions on the Supramolecular Structures
Abstract
:1. Introduction
- (1)
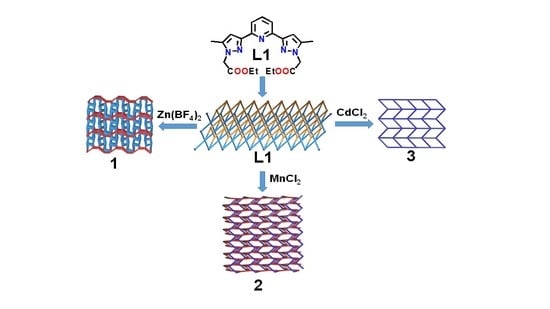
- The ligand L is an N-heterocyclic tridentate pyrazolyl pyridine compound capable of forming various coordination modes, and ligating topology with transition metal ions [21].
- (2)
- (3)
- (4)
- L is a new ligand, not yet reported.
2. Materials and Methods
Synthesis of Ligands and Coordination Complexes
3. Results
3.1. Synthesis, FT-IR and UV-Visible Spectroscopy
3.2. Crystal Structures
4. Discussions
4.1. Hirshfeld Surface Analyses
4.2. Influence of Counter-Anion and Metal Ion on the Conformation of the Ligand L and the Supramolecular Structures of the Coordination Complexes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adarsh, N.N.; Dastidar, P. Coordination polymers: What has been achieved in going from innocent 4,4′-bipyridine to bis-pyridyl ligands having a non-innocent backbone? Chem. Soc. Rev. 2012, 41, 3039–3060. [Google Scholar] [CrossRef] [PubMed]
- Smulders, M.M.J.; Riddell, I.A.; Browne, C.; Nitschke, J.R. Building on architectural principles for three-dimensional metallosupramolecular construction. Chem. Soc. Rev. 2013, 42, 1728–1754. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Duriska, M.B.; Neville, S.M.; Iremonger, S.S.; Boas, J.F.; Kepert, C.J.; Batten, S.R. Systematic Metal Variation and Solvent and Hydrogen-Gas Storage in Supramolecular Nanoballs. Angew. Chem. Int. Ed. 2009, 48, 8919–8922. [Google Scholar] [CrossRef]
- Chakrabarty, R.; Mukherjee, P.S.; Stang, P. Supramolecular Coordination: Self-Assembly of Finite Two- and Three-Dimensional Ensembles. Chem. Rev. 2011, 111, 6810–6918. [Google Scholar] [CrossRef] [Green Version]
- Domoto, Y.; Abe, M.; Kikuchi, T.; Fujita, M. Self-Assembly of Coordination Polyhedra with Highly Entangled Faces Induced by Metal–Acetylene Interactions. Angew. Chem. Int. Ed. 2020, 59, 3450–3454. [Google Scholar] [CrossRef]
- Janiak, C.; Vieth, J.K. MOFs, MILs and more: Concepts, properties and applications for porous coordination networks (PCNs). New J. Chem. 2010, 34, 2366–2388. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Champness, N.R.; Janiak, C. Recent advances in crystal engineering. CrystEngComm 2009, 12, 22–43. [Google Scholar] [CrossRef]
- Adarsh, N.N.; Dîrtu, M.M.; Guionneau, P.; Devlin, E.; Sanakis, Y.; Howard, J.A.K.; Chattopadhyay, B.; Garcia, Y. One-Dimensional Looped Chain and Two-Dimensional Square Grid Coordination Polymers: Encapsulation of Bis(1,2,4-Triazole)-trans-cyclohexane into the Voids. Eur. J. Inorg. Chem. 2019, 2019, 585–591. [Google Scholar] [CrossRef] [Green Version]
- Paul, M.; Adarsh, N.N.; Dastidar, P. CuII Coordination Polymers Capable of Gelation and Selective SO4–2 Separation. Cryst. Growth Des. 2012, 12, 4135–4143. [Google Scholar] [CrossRef]
- Neumann, T.; Gallo, G.; Dinnebier, R.E.; Näther, C. Synthesis, Crystal Structures, and Properties of Mn(NCS)2 Coordination Compounds with 4-Picoline as Coligand and Crystal Structure of Mn(NCS)2. Z. Anorg. Allg. Chem. 2020, 646, 88–94. [Google Scholar] [CrossRef] [Green Version]
- Caillé, F.; Bonnet, C.S.; Buron, F.; Villette, S.; Helm, L.; Petoud, S.; Suzenet, F.; Tóth, É. Isoquinoline-Based Lanthanide Complexes: Bright NIR Optical Probes and Efficient MRI Agents. Inorg. Chem. 2012, 51, 2522–2532. [Google Scholar] [CrossRef] [PubMed]
- Adarsh, N.N.; Novio, F.; Ruiz-Molina, D. Coordination polymers built from 1,4-bis(imidazol-1-ylmethyl)benzene: From crystalline to amorphous. Dalton Trans. 2016, 45, 11233–11255. [Google Scholar] [CrossRef] [PubMed]
- Viciano-Chumillas, M.; Tanase, S.; De Jongh, L.J.; Reedijk, J. Coordination Versatility of Pyrazole-Based Ligands towards High-Nuclearity Transition-Metal and Rare-Earth Clusters. Eur. J. Inorg. Chem. 2010, 2010, 3403–3418. [Google Scholar] [CrossRef]
- Batten, S.R.; Duriska, M.B.; Jensen, P.; Lu, J. Synthesis and Complexes of the New Scorpionate Ligand Tris[3-(4-benzonitrile)-pyrazol-1-yl]borate. Aust. J. Chem. 2007, 60, 72–74. [Google Scholar] [CrossRef]
- Ogryzek, M.; Chylewska, A.; Królicka, A.; Banasiuk, R.; Turecka, K.; Lesiak, D.; Nidzworski, D.; Makowski, M. Coordination chemistry of pyrazine derivatives analogues of PZA: Design, synthesis, characterization and biological activity. RSC Adv. 2016, 6, 52009–52025. [Google Scholar] [CrossRef] [Green Version]
- Radi, S.; Yahyi, A.; Ettouhami, A.; Jha, A.C.; Adarsh, N.N.; Robeyns, K.; Garcia, Y. Synthesis and crystal structures of mononuclear CuII/CoII coordination complexes from pyrazole-dicarboxylate acid derivatives. Polyhedron 2015, 85, 383–388. [Google Scholar] [CrossRef]
- Radi, S.; El-Massaoudi, M.; Benaissa, H.; Adarsh, N.N.; Ferbinteanu, M.; Devlin, E.; Sanakis, Y.; Garcia, Y. Crystal engineer-ing of a series of complexes and coordination polymers based on pyrazole-carboxylic acid ligands. New J. Chem. 2017, 41, 8232–8241. [Google Scholar] [CrossRef]
- El-Massaoudi, M.; Radi, S.; Mabkhot, Y.N.; Al-Showiman, S.; Ghabbour, H.A.; Ferbinteanu, M.; Adarsh, N.N.; Garcia, Y. Cu(II) and Mn(II) coordination complexes constructed by C linked bispyrazoles: Effect of anions and hydrogen bonding on the self assembly process. Inorganica Chim. Acta 2018, 482, 411–419. [Google Scholar] [CrossRef]
- Chkirate, K.; Fettach, S.; Karrouchi, K.; Sebbar, N.K.; Essassi, E.M.; Mague, J.T.; Radi, S.; Faouzi, M.E.A.; Adarsh, N.N.; Garcia, Y. Novel Co(II) and Cu(II) coordination complexes constructed from pyrazole-acetamide: Effect of hydrogen bonding on the self assembly process and antioxidant activity. J. Inorg. Biochem. 2019, 191, 21–28. [Google Scholar] [CrossRef]
- Chkirate, K.; Karrouchi, K.; Dege, N.; Sebbar, N.K.; Ejjoummany, A.; Radi, S.; Adarsh, N.N.; Talbaoui, A.; Ferbinteanu, M.; Essassi, E.M.; et al. Co(ii) and Zn(ii) pyrazolyl-benzimidazole complexes with remarkable antibacterial activity. New J. Chem. 2020, 44, 2210–2221. [Google Scholar] [CrossRef]
- Zhang, X.; Xing, N.; Bai, F.-Y.; Wan, L.; Shan, H.; Hou, Y.; Xing, Y.; Shi, Z. Multi-functional d10 metal–organic materials based on bis-pyrazole/pyridine ligands supported by a 2,6-di(3-pyrazolyl)pyridine with different spanning flexible dicarboxylate ligands: Synthesis, structure, photoluminescent and catalytic properties. CrystEngComm 2013, 15, 9135–9147. [Google Scholar] [CrossRef]
- Miao, L.-L.; Li, H.-X.; Yu, M.; Zhao, W.; Gong, W.-J.; Gao, J.; Ren, Z.-G.; Wang, H.-F.; Lang, J.-P. Preparation of a nitrate-coordinated copper(II) complex of 2-(pyrazol-3-yl)-6-(pyrazolate)pyridine as an efficient catalyst for methyl methacrylate polymerization. Dalton Trans. 2012, 41, 3424–3430. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.S.; Oh, B.J.; Ryu, J.Y.; Park, M.H.; Kim, M.; Lee, J.; Kim, Y. Synthesis, characterization, and cycloaddition reaction studies of zinc(II) acetate complexes containing 2,6-bis(pyrazol-1-yl)pyridine and 2,6-bis(3,5-dimethylpyrazol-1-yl)pyridine ligands. Polyhedron 2017, 125, 101–106. [Google Scholar] [CrossRef]
- Khmara, E.F.; Chizhov, D.L.; Sidorov, A.A.; Aleksandrov, G.G.; Slepukhin, P.A.; Kiskin, M.A.; Tokarev, K.L.; Filyakova, V.I.; Rusinov, G.L.; Smolyaninov, I.V.; et al. Synthesis, structure, electrochemical and magnetic properties of 2,6-bis(5-trifluoromethylpyrazol-3-yl)pyridine and its NiII complexes. Russ. Chem. Bull. 2012, 61, 313–325. [Google Scholar] [CrossRef]
- Jornet-Mollá, V.; Waerenborgh, J.C.; Romero, F.M. Synthesis, Structure, and Photomagnetic Properties of a Hydrogen-Bonded Lattice of [Fe(bpp)2]2+ Spin-Crossover Complexes and Nicotinate Anions. Crystals 2018, 8, 439. [Google Scholar] [CrossRef] [Green Version]
- Guan, Q.-L.; Liu, Z.; Wei, W.-J.; Xing, Y.-H.; Liu, J.; Zhang, R.; Hou, Y.-N.; Wang, X.; Bai, F.-Y. Synthesis, structure, spectroscopy of four novel supramolecular complexes and cytotoxicity study by application of multiple parallel perfused microbioreactors. New J. Chem. 2014, 38, 3258–3268. [Google Scholar] [CrossRef]
- Berdiell, I.C.; Kulmaczewski, R.; Halcrow, M.A. Iron(II) Complexes of 2,4-Dipyrazolyl-1,3,5-triazine Derivatives—The Influence of Ligand Geometry on Metal Ion Spin State. Inorg. Chem. 2017, 56, 8817–8828. [Google Scholar] [CrossRef]
- Ojwach, S.O.; Nyamato, G.S.; Omondi, B.; Darkwa, J.; Okoth, A.O. Multidentate bis(pyrazolylmethyl)pyridine ligands: Coordination chemistry and binding properties with zinc(II) and cadmium(II) cations. J. Coord. Chem. 2012, 65, 298–307. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Phase annealing in SHELX-90: Direct methods for larger structures. Acta Crystallogr. A 1990, 46, 467–473. [Google Scholar] [CrossRef]
- Sheldrick, G. A short history ofSHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Farrugia, L.J. WinGXsuite for small-molecule single-crystal crystallography. J. Appl. Crystallogr. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- Clark, R.C.; Reid, J.S. The analytical calculation of absorption in multifaceted crystals. Acta Crystallogr. A 1995, 51, 887–897. [Google Scholar] [CrossRef]
- Miyata, M.; Tohnai, N.; Hisaki, I.; Sasaki, T. Generation of Supramolecular Chirality around Twofold Rotational or Helical Axes in Crystalline Assemblies of Achiral Components. Symmetry 2015, 7, 1914–1928. [Google Scholar] [CrossRef] [Green Version]
- Dîrtu, M.M.; Adarsh, N.N.; Naik, A.D.; Robeyns, K.; Garcia, Y. Supramolecular homochiral helicity and zigzag hydrogen bonded chains in 1,2,4-triazole derived aminoester and aminoacid. New J. Chem. 2016, 40, 9025–9029. [Google Scholar] [CrossRef]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied Topological Analysis of Crystal Structures with the Program Package ToposPro. Cryst. Growth Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2008, 11, 19–32. [Google Scholar] [CrossRef]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. Hirshfeld sur-faces and two-dimensional fingerprint plots were generated by using CrystalExplorer17. In Crystal Explorer17; University of Western Australia: Crawley, Australia, 2017. [Google Scholar]
- Hirshfeld, H.L. Synthesis, Crystal structure, and Hirshfeld Surface Analysis of a New Mixed Ligand Copper (II) Com-plex. Theor. Chim. Acta. 1977, 44, 129–138. [Google Scholar] [CrossRef]
- McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. Towards quantitative analysis of intermolecular interactions with Hirshfeld surfaces. Chem. Commun. 2007, 3814–3816. [Google Scholar] [CrossRef]
- Adarsh, N.N.; Kumar, D.K.; Dastidar, P. Ligating topology and counter anion controlled formation of discrete metallo-macrocycle and 2D corrugated sheet in coordination compounds derived from a bis-pyridyl-bis-amide ligand and Cd (II)salts. Inorg. Chem. Commun. 2008, 11, 636–642. [Google Scholar] [CrossRef]
- Deng, H.-Y.; He, J.-R.; Pan, M.; Li, L.; Su, C.-Y. Synergistic metal and anion effects on the formation of coordination assemblies from a N,N′-bis(3-pyridylmethyl)naphthalene diimide ligand. CrystEngComm 2009, 11, 909–917. [Google Scholar] [CrossRef]
- Adarsh, N.N.; Kumar, D.K.; Suresh, E.; Dastidar, P. Coordination polymers derived from a bis-pyridyl-bis-amide ligand: Supramolecular structural diversities and anion binding properties. Inorganica Chim. Acta 2010, 363, 1367–1376. [Google Scholar] [CrossRef]
- Radi, S.; Toubi, Y.; Bacquet, M.; Degoutin, S.; Mabkhot, Y.N.; Garcia, Y. An inorganic–organic hybrid material made of a silica-immobilized Schiff base receptor and its preliminary use in heavy metal removal. RSC Adv. 2016, 6, 34212–34218. [Google Scholar] [CrossRef]
- Radi, S.; Tighadouini, S.; Bacquet, M.; Degoutin, S.; Garcia, Y. New hybrid material based on a silica-immobilised conjugated β-ketoenol-bipyridine receptor and its excellent Cu(ii) adsorption capacity. Anal. Methods 2016, 8, 6923–6931. [Google Scholar] [CrossRef]
- Radi, S.; El-Massaoudi, M.; Bacquet, M.; Degoutin, S.; Adarsh, N.N.; Robeyns, K.; Garcia, Y. A novel environment-friendly hybrid material based on a modified silica gel with a bispyrazole derivative for the removal of ZnII, PbII, CdII and CuII traces from aqueous solutions. Inorg. Chem. Front. 2017, 4, 1821–1831. [Google Scholar] [CrossRef]
- El-Massaoudi, M.; Radi, S.; Bacquet, M.; Degoutin, S.; Garcia, Y. Highly efficient and selective adsorbent for potentially toxic metals removal from aquatic media. J. Environ. Chem. Eng. 2018, 6, 5980–5989. [Google Scholar] [CrossRef]
- Tighadouini, S.; Radi, S.; Elidrissi, A.; Haboubi, K.; Bacquet, M.; Degoutin, S.; Zaghrioui, M.; Garcia, Y. Removal of toxic heavy metals from river water samples using a porous silica surface modified with a new β-ketoenolic host. Beilstein J. Nanotechnol. 2019, 10, 262–273. [Google Scholar] [CrossRef] [Green Version]
- Tighadouini, S.; Radi, S.; Ferbinteanu, M.; Garcia, Y. Highly Selective Removal of Pb(II) by a Pyridylpyrazole-β-ketoenol Receptor Covalently Bonded onto the Silica Surface. ACS Omega 2019, 4, 3954–3964. [Google Scholar] [CrossRef] [Green Version]
- Tighadouini, S.; Radi, S.; El Massaoudi, M.; Lakbaibi, Z.; Ferbinteanu, M.; Garcia, Y. Efficient and Environmentally Friendly Adsorbent Based on β-Ketoenol-Pyrazole-Thiophene for Heavy-Metal Ion Removal from Aquatic Medium: A Combined Experimental and Theoretical Study. ACS Omega 2020, 5, 17324–17336. [Google Scholar] [CrossRef]
- Tighadouini, S.; Radi, S.; Elidrissi, A.; Zaghrioui, M.; Garcia, Y. Selective Confinement of CdII in Silica Particles Functionalized with β-Keto-Enol-Bisfuran Receptor: Isotherms, Kinetic and Thermodynamic Studies. Eur. J. Inorg. Chem. 2019, 2019, 3180–3186. [Google Scholar] [CrossRef]
- Tighadouini, S.; Radi, S.; Garcia, Y. Selective chemical adsorption of Cd(II) on silica covalently decorated with a β-ketoenol-thiophene-furan receptor. Mol. Syst. Des. Eng. 2020, 5, 1037–1047. [Google Scholar] [CrossRef]
- El-Massaoudi, M.; Radi, S.; Lamsayah, M.; Tighadouini, S.; Séraphin, K.K.; Kouassi, L.K.; Garcia, Y. Ultra-fast and highly efficient hybrid material removes Cu(II) from wastewater: Kinetic study and mechanism. J. Clean. Prod. 2021, 124757. [Google Scholar] [CrossRef]












| B | L | 1 | 2 | 3 | |
|---|---|---|---|---|---|
| Formula | C15H19N5O | C21H25N5O4 | C42H50B2F8N10O8Zn | C21H25Cl2MnN5O4 | C21H25CdCl2N5O4 |
| Mr | 285.35 | 411.46 | 1061.91 | 537.30 | 594.76 |
| T [K] | 293(2) | 297(2) | 150(2) | 150(2) | 150(2) |
| γ [Å] | 0.71073 | 0.71073 | 0.71073 | 0.71073 | 0.71073 |
| Crystal system | Orthorhombic | Triclinic | Monoclinic | Monoclinic | Monoclinic |
| Space group | P212121 | P-1 | P21/c | P21/c | P2/n |
| a [Å] | 9.036(1) | 6.687(1) | 22.848(6) | 21.183(5) | 11.785(3) |
| b [Å] | 12.456(1) | 11.333(4) | 29.051(7) | 18.572(5) | 9.296(2) |
| c [Å] | 13.962(1) | 15.576(3) | 14.640(4) | 12.688(3) | 11.852(3) |
| α [°] | 90 | 71.24(2) | 90 | 90 | 90 |
| β [°] | 90 | 79.22(1) | 91.163(4) | 100.812(3) | 110.813(2) |
| γ [°] | 90 | 74.17(1) | 90 | 90 | 90 |
| V [Å3] | 1571.6(3) | 1069.0(5) | 9715(4) | 4903(2) | 1213.7(5) |
| Z | 4 | 2 | 4 | 8 | 2 |
| ρc [g cm−3] | 1.206 | 1.278 | 1.452 | 1.456 | 1.627 |
| μ [mm−1] | 0.080 | 0.091 | 0.597 | 0.793 | 1.157 |
| F(000) | 608 | 436 | 4384 | 2216 | 600 |
| θ range | 3.26–24.94 | 2.90–25.24 | 2.18–24.96 | 2.24–26.03 | 2.86–26.44 |
| Independent reflns | 2838 | 3817 | 20,039 | 10,094 | 2513 |
| Abs. correction | Multi-scan | Multi-scan | Empirical | Empirical | Empirical |
| Refinement method | Full-matrix least-squares on F2 | ||||
| GoF on F2 | 1.094 | 1.097 | 1.020 | 1.063 | 1.079 |
| Final R indices [I > 2 σ(I)] | R1 = 0.0510 | R1 = 0.087 | R1 = 0.0518 | R1 = 0.0715 | R1 = 0.0264 |
| wR2 = 0.1211 | wR2 = 0.1770 | wR2 = 0.1303 | wR2 = 0.1539 | wR2 = 0.0709 | |
| R indices (all data) | R1 = 0.0607 | R1 = 0.1283 | R1 = 0.0779 | R1 = 0.1008 | R1 = 0.0295 |
| wR2 = 0.1276 | wR2 = 0.1992 | wR2 = 0.1448 | wR2 = 0.1752 | wR2 = 0.0730 | |
| B (%) | L (%) | 1 (%) | 2 (%) | 3 (%) | |
|---|---|---|---|---|---|
| O···H | 2.5 | 13.9 | 13.8 | 14.5 | 15.8 |
| N···H | 22.2 | 9.6 | 1.8 | 4.9 | 5.8 |
| C···H | 25.9 | 14.9 | 8.9 | 10 | 10.9 |
| H···H | 49.3 | 57.3 | 51.9 | 46.6 | 43.9 |
| F···H | – | – | 21.1 | – | – |
| Cl···H | – | – | – | 18.2 | 17.8 |
| C···C | 0.1 | 0.9 | – | 2.7 | 3.1 |
| Cl···O | – | – | – | 0.5 | 0.6 |
| C···O | – | 0.9 | 2.0 | – | – |
| N···C | – | 1.8 | – | – | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oulmidi, A.; Radi, S.; Miras, H.N.; Adarsh, N.N.; Garcia, Y. New Bis-Pyrazole-Bis-Acetate Based Coordination Complexes: Influence of Counter-Anions and Metal Ions on the Supramolecular Structures. Sustainability 2021, 13, 288. https://0-doi-org.brum.beds.ac.uk/10.3390/su13010288
Oulmidi A, Radi S, Miras HN, Adarsh NN, Garcia Y. New Bis-Pyrazole-Bis-Acetate Based Coordination Complexes: Influence of Counter-Anions and Metal Ions on the Supramolecular Structures. Sustainability. 2021; 13(1):288. https://0-doi-org.brum.beds.ac.uk/10.3390/su13010288
Chicago/Turabian StyleOulmidi, Afaf, Smaail Radi, Haralampos N. Miras, Nayarassery N. Adarsh, and Yann Garcia. 2021. "New Bis-Pyrazole-Bis-Acetate Based Coordination Complexes: Influence of Counter-Anions and Metal Ions on the Supramolecular Structures" Sustainability 13, no. 1: 288. https://0-doi-org.brum.beds.ac.uk/10.3390/su13010288