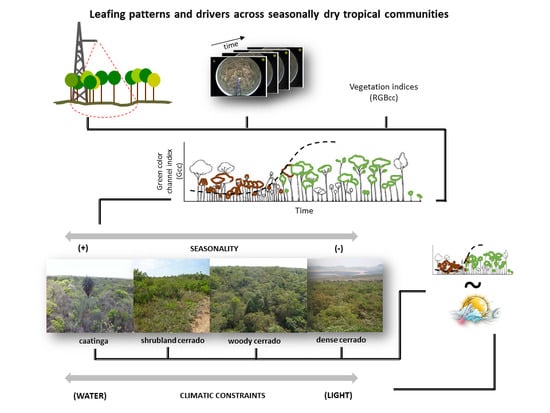
Leafing Patterns and Drivers across Seasonally Dry Tropical Communities
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Sites
2.1.1. Caatinga
2.1.2. Cerrado
- Cerrado shrubland—the cerrado shrubland (campo sujo) is characterized by a dominant grassy herbaceous layer with scattered shrubs and small trees [24,42]. This site is part of the Itirapina Ecological Station, with an area of 2300 ha and an altitude of 700 m. Previous vegetation surveys found that 79% of the species from the herbaceous-shrubland layer belong to Asteraceae, Fabaceae, Poaceae, and Cyperaceae, and 21% of the small tree species were mainly from Fabaceae, Myrtaceae, and Melastomataceae [43]. As reported by [44], local historic climatic data show a total mean annual precipitation of 1524 mm and mean temperature of 20.7 °C. During the three years we monitored the area, the mean annual precipitation was 1272 mm and a mean annual temperature 23.5 °C (Figure 2e,f). Historic climatic information was made available by the Centro de Recursos Hídricos e Estudos Ambientais (CRHEA-EESC/USP), located about 15 km from the study sites.
- Woody cerrado—this site is located in a private area of 260 hectares and 700 m a.s.l. adjacent to the cerrado shrubland site. This woody cerrado is a remnant of the original Cerrado that once covered the entire region, and therefore, faces the same climatic regime as the cerrado shrubland site. The vegetation is a cerrado sensu stricto, which is characterized by a dominant woody layer (trees and shrubs ranging from 3 to 12 m in height) with discontinuous crown cover and a scattered herbaceous layer [44]. According to local meteorological data (2011–2015), mean annual total precipitation was 1478 mm and mean annual temperature 22.9 °C (Figure 2h,i). The most common plant families are Myrtaceae, Fabaceae, and Malpighiaceae [44], and the vegetation has been classified as semi-deciduous based on long-term leaf exchange strategies [4].
- Dense cerrado—this site is a dense woody cerrado that belongs to the Reserva Ecológica Pé-de-Gigante (PEG), located within the Parque Estadual do Vassununga, in Santa Rita do Passa Quatro, SP. The PEG reserve comprises a continuous area of 1060 ha and 649 m a.s.l. covered by a heterogeneous landscape of Cerrado vegetation types, from open grasslands to dense woody cerrado. As reported by Batalha et al. [24], local climatic data (1986–1995) presented a total mean annual rainfall of 1499 mm and mean annual temperature of 21.5 °C. Our local climatic data (2013 to 2015) showed a total mean annual precipitation of 1150 mm and a mean annual temperature of 22.5 °C (Figure 2k,l). The study site is a transition from woody cerrado to cerradão [42], which we classified as dense cerrado; it is characterized by a less discontinuous top canopy, nearly no herbaceous layer, and a high density of shrubs and trees [45]. Dominant woody layer reaches 10 to 15 m height is composed mainly of Ptedoron pubecens, Copaifera langsdofii, and Anadenanthera peregrina var. falcata (all Fabaceae) [46].
2.2. Near-Surface Phenology Monitoring
2.3. Defining the Growing Season
2.4. Environmental Cues of Leafing Phenology
2.5. Data Analysis
3. Results
3.1. Detecting Growing Seasons Across Sites
3.2. Model Predictions
4. Discussion
4.1. Communities Leafing Patterns
4.2. Deciduous Leaf Exchange Strategies and Life Forms
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reich, P.B. Phenology of tropical forests: Patterns, causes, and consequences. Can. J. Bot. 1995, 73, 164–174. [Google Scholar] [CrossRef]
- Rötzer, T.; Grote, R.; Pretzsch, H. The timing of bud burst and its effect on tree growth. Int. J. Biometeorol. 2004, 48, 109–118. [Google Scholar] [CrossRef]
- Richardson, A.D.; Keenan, T.F.; Migliavacca, M.; Ryu, Y.; Sonnentag, O.; Toomey, M. Climate change, phenology, and phenological control of vegetation feedbacks to the climate system. Agric. For. Meteorol. 2013, 169, 156–173. [Google Scholar] [CrossRef]
- Camargo, M.G.G.; Carvalho, G.H.; Alberton, B.C.; Reys, P.; Morellato, L.P.C. Leafing patterns and leaf exchange strategies of a cerrado woody community. Biotropica 2018, 50, 442–454. [Google Scholar] [CrossRef]
- Van Schaik, C.P.; Terborgh, J.W.; Wright, S.J. The Phenology of Tropical Forests—Adaptive Significance and Consequences for Primary Consumers. Annu. Rev. Ecol. Syst. 1993, 24, 353–377. [Google Scholar] [CrossRef]
- Wright, S.J.; van Schaik, C.P. Light and the Phenology of Tropical Trees. Am. Nat. 1994, 143, 192–199. [Google Scholar] [CrossRef]
- Morellato, L.P.C.; Talora, D.C.; Takahasi, A.; Bencke, C.C.; Romera, E.C.; Ziparro, V.B. Phenology of Atlantic Rain Forest Trees: A Comparative Study. Biotropica 2000, 32, 811–823. [Google Scholar] [CrossRef]
- Rivera, G.; Elliott, S.; Caldas, L.S.; Nicolossi, G.; Coradin, V.T.; Borchert, R. Increasing day-length induces spring flushing of tropical dry forest trees in the absence of rain. Trees 2002, 16, 445–456. [Google Scholar] [CrossRef]
- Borchert, R.; Calle, Z.; Strahler, A.H.; Baertschi, A.; Magill, R.E.; Broadhead, J.S.; Kamau, J.; Njoroge, J.; Muthuri, C. Insolation and photoperiodic control of tree development near the equator. New Phytol. 2015, 205, 7–13. [Google Scholar] [CrossRef]
- Murphy, P.G.; Lugo, A.E. Ecology of Tropical Dry Forest. Rev. Ecol. Syst. 1986, 17, 67–88. [Google Scholar] [CrossRef]
- Williams, R.J.; Myers, B.A.; Muller, W.J.; Duff, G.A.; Eamus, D. Leaf phenology of woody species in a north Australian tropical savanna. Ecology 1997, 78, 2542–2558. [Google Scholar] [CrossRef]
- Guan, K.; Pan, M.; Li, H.; Wolf, A.; Wu, J.; Medvigy, D.; Caylor, K.K.; Sheffield, J.; Wood, E.F.; Malhi, Y.; et al. Photosynthetic seasonality of global tropical forests constrained by hydroclimate. Nat. Geosci. 2015, 8, 284–289. [Google Scholar] [CrossRef]
- Reich, P.B.; Borchert, R. Water Stress and Tree Phenology in a Tropical Dry Forest in the Lowlands of Costa Rica. J. Ecol. 1984, 72, 61–74. [Google Scholar] [CrossRef]
- Quesada, M.; Sanchez-Azofeifa, G.A.; Alvarez-Añorve, M.; Stoner, K.E.; Avila-Cabadilla, L.; Calvo-Alvarado, J.; Castillo, A.; Espírito-Santo, M.M.; Fagundes, M.; Fernandes, G.W.; et al. Succession and management of tropical dry forests in the Americas: Review and new perspectives. For. Ecol. Manag. 2009, 258, 1014–1024. [Google Scholar] [CrossRef]
- Singh, K.; Singh, D.V. Effect of rates and sources of nitrogen application on yield and nutrient uptake of Citronella Java (Cymbopogon winterianus Jowitt). Fertil. Res. 1992, 33, 187–191. [Google Scholar] [CrossRef]
- Borchert, R. Soil and stem water storage determine phenology and distribution of tropical dry forest trees. Ecology 1994, 75, 1437–1449. [Google Scholar] [CrossRef]
- Morellato, L.P.C.; Rodriguez, R.R.; Leitão-Filho, H.F.; Joly, C.A. Estudo comparativo da fenologia de espécies arbóreas de floresta de altitude e floresta mesófila semidecídua na Serra do Japi, Jundiaí, São Paulo. Rev. Bras. De Botânica 1989, 12, 85–98. [Google Scholar]
- Singh, K.P.; Kushwaha, C.P. Emerging paradigms of tree phenology in dry tropics. Curr. Sci. 2005, 89, 964–975. [Google Scholar]
- Higgins, S.I.; Delgado-Cartay, M.D.; February, E.C.; Combrink, H.J. Is there a temporal niche separation in the leaf phenology of savanna trees and grasses? J. Biogeogr. 2011, 38, 2165–2175. [Google Scholar] [CrossRef]
- Archibald, S.; Scholes, R.J. Leaf green-up in a semi-arid African savanna –separating tree and grass responses to environmental cues. J. Veg. Sci. 2007, 18, 583–594. [Google Scholar] [CrossRef]
- Whitecross, M.A.; Witkowski, E.T.F.; Archibald, S. Savanna tree-grass interactions: A phenological investigation of green-up in relation to water availability over three seasons. S. Afr. J. Bot. 2017, 108, 29–40. [Google Scholar] [CrossRef]
- Elliott, S.; Baker, P.J.; Borchert, R. Leaf flushing during the dry season: The paradox of Asian monsoon forests. Glob. Ecol. Biogeogr. 2006, 15, 248–257. [Google Scholar] [CrossRef]
- Eamus, D. Ecophysiological traits of deciduous and evergreen woody species in the seasonally dry tropics. Trends Ecol. Evol. 1999, 14, 11–16. [Google Scholar] [CrossRef] [Green Version]
- Batalha, M.A.; Mantovani, W. Reproducive Phenological Patterns of Cerrado Plant Species at the Pé-De-Gigante Reserve (Santa Rita Do Passa Quatro, Sp, Brazil): A Comparision Between the Herbaceous and Woody Floras. Rev. Bras. Biol. 2000, 60, 129–145. [Google Scholar] [CrossRef] [PubMed]
- Caldararu, S.; Purves, D.W.; Palmer, P.I. Phenology as a strategy for carbon optimality: A global model. Biogeosciences 2014, 11, 763–778. [Google Scholar] [CrossRef]
- Smith, P.; House, J.I.; Bustamante, M.; Sobocká, J.; Harper, R.; Pan, G.; West, P.; Clark, J.; Adhya, T.; Rumpel, C.; et al. Global change pressures on soils from land use and management. Glob. Chang. Biol. 2016, 22, 1008–1028. [Google Scholar] [CrossRef] [PubMed]
- Chavana-Bryant, C.; Malhi, Y.; Wu, J.; Asner, G.P.; Anastasiou, A.; Enquist, B.J.; Caravasi, E.G.C.; Doughty, C.E.; Saleska, S.R.; Martin, R.E.; et al. Leaf aging of Amazonian canopy trees as revealed by spectral and physiochemical measurements. New Phytol. 2017, 214, 1049–1063. [Google Scholar] [CrossRef] [PubMed]
- Morellato, L.P.C.; Alberton, B.; Alvarado, S.T.; Borges, B.; Buisson, E.; Camargo, M.G.G.; Cancian, L.F.; Carstensen, D.W.; Escobar, D.F.F.; Leite, P.T.P.; et al. Linking plant phenology to conservation biology. Biol. Conserv. 2016, 195, 60–72. [Google Scholar] [CrossRef] [Green Version]
- Abernethy, K.; Busch, E.R.; Forget, P.M.; Mendoza, I.; Morellato, L.P.C. Current issues in tropical phenology: A synthesis. Biotropica 2018, 50, 477–482. [Google Scholar] [CrossRef]
- Richardson, A.D.; Jenkins, J.P.; Braswell, B.H.; Hollinger, D.Y.; Ollinger, S.V.; Smith, M.L. Use of digital webcam images to track spring green-up in a deciduous broadleaf forest. Oecologia 2007, 152, 323–334. [Google Scholar] [CrossRef]
- Morisette, J.T.; Richardson, A.D.; Knapp, A.K.; Fisher, J.I.; Graham, E.A.; Abatzoglou, J.; Wilson, B.E.; Breshears, D.D.; Henebry, G.M.; Hanes, J.M.; et al. Tracking the rhythm of the seasons in the face of global change: Phenological research in the 21st century. Front. Ecol. Environ. 2009, 7, 253–260. [Google Scholar] [CrossRef]
- Alberton, B.; Almeida, J.; Helm, R.; Torres, R.S.; Menzel, A.; Morellato, L.P.C. Using phenological cameras to track the green up in a cerrado savanna and its on-the-ground validation. Ecol. Inform. 2014, 19, 62–70. [Google Scholar] [CrossRef]
- Alberton, B.; Torres, R.S.; Cancian, L.F.; Borges, B.D.; Almeida, J.; Mariano, G.C.; Santos, J.; Morellato, L.P.C. Introducing digital cameras to monitor plant phenology in the tropics: Applications for conservation. Perspect. Ecol. Conserv. 2017, 15, 82–90. [Google Scholar] [CrossRef]
- Crimmins, M.; Crimmins, T.M. Monitoring plant phenology using digital repeat photography. Environ. Manag. 2008, 41, 949–958. [Google Scholar] [CrossRef]
- Brown, T.B.; Hultine, K.R.; Stelzer, H.; Denny, E.G.; Denslow, M.W.; Granados, J.; Henderson, S.; Moore, D.; Nagai, S.; SanClements, M.; et al. Using phenocams to monitor our changing earth: Toward a global phenocam network. Front. Ecol. Environ. 2016, 14, 84–93. [Google Scholar] [CrossRef]
- Scholes, R.J.; Walker, B.H. An African Savanna: Synthesis of the Nylsvley Study; Cambridge University Press: Cambridge, UK, 1993; p. 320. ISBN 0521612101. [Google Scholar]
- Veloso, H.P.; Filho, A.L.R.R.; Lima, J.C.A. Classificação da Vegetação Brasileira, Adaptada a um Sistema Universal; Instituto Brasileiro de Geografia e Estatística, Departamento de Recursos Naturais e Estudos Ambientais: Rio de Janeiro, Brazil, 1991; p. 124. ISBN 85-240-0384-7.
- Olson, D.M.; Dinerstein, E.; Wikramanayake, E.D.; Burgess, N.D.; Powell, G.V.N.; Underwood, E.C.; D’amico, J.A.; Itoua, I.; Strand, H.E.; Morrison, J.C.; et al. Terrestrial Ecoregions of the World: A New Map of Life on Earth: A new global map of terrestrial ecoregions provides an innovative tool for conserving biodiversity. BioScience 2001, 51, 933–938. [Google Scholar] [CrossRef]
- Silva, J.M.C.; Leal, I.R.; Tabarelli, M. Caatinga: The Largest Tropical Dry Forest Region in South America; Springer International Publishing AG: Cham, Switzerland, 2017; pp. 1–487. ISBN 978-3-319-68338-6. [Google Scholar]
- Kill, L.H.P. Caracterização da vegetação da Reserva Legal da Embrapa Semiárido. Embrapa Semiárido Pet. 2017, 1, 1–27. [Google Scholar]
- Köppen, W.P. Grundriss der Klimakunde, 2nd ed.; Walter de Gruyter: Berlin, Germany, 1931; pp. 1–369. ISBN 311112514. [Google Scholar]
- Oliveira-Filho, A.T.; Ratter, J.A. Vegetation physiognomies and wood flora of the bioma Cerrado. In The Cerrados of Brazil: Ecology and Natural History of a Neotropical Savanna; Oliveira, P.S., Marquis, R.J., Eds.; Columbia University Press: New York, NY, USA, 2002; pp. 91–120, ASIN B0092WWFNC. [Google Scholar]
- Tannus, J.L.S.; Assis, M.A.; Morellato, L.P.C. Fenologia reprodutiva em campo sujo e campo úmido numa área de Cerrado no sudeste do Brasil, Itirapina—SP. Biota Neotrop. 2006, 6, 1–27. [Google Scholar] [CrossRef]
- Reys, P.; Camargo, M.G.G.; Grombone-Guaratini, M.T.; Teixeira, A.P.; Assis, M.A.; Morellato, L.P.C. Estrutura e composição florística de um Cerrado sensu stricto e sua importância para propostas de restauração ecológica. Hoehnea 2013, 40, 449–464. [Google Scholar] [CrossRef]
- Ribeiro, J.F.; Walter, B.M.T. Fitofisionomia do Bioma Cerrado. In Cerrado: Ambiente e Flora; Sano, S.M., Almeida, S.P., Eds.; Embrapa: Brasília, Brazil, 1998; pp. 89–166. ISBN 8570750080. [Google Scholar]
- Pivello, V.R.; Bitencourt, M.D.; Mantovani, W.; Mesquita, H.N., Jr.; Batalha, M.A.; Shida, C.N. Proposta de Zoneamento Ecológico para a Reserva de Cerrado Pé-de-Gigante (Santa Rita do Passa Quatro, SP). Braz. J. Ecol. 1998, 2, 108–118. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Richardson, A.D.; Braswell, B.H.; Hollinger, D.Y.; Jenkins, J.P.; Ollinger, S.V. Near-surface remote sensing of spatial and temporal variation. Ecol. Appl. 2009, 19, 1417–1428. [Google Scholar] [CrossRef] [PubMed]
- Ahrends, H.E.; Etzold, S.; Kutsch, W.L.; Stoeckli, R.; Bruegger, R.; Jeanneret, F.; Wanner, H.; Buchmann, N.; Eugster, W. Tree phenology and carbon dioxide fluxes: Use of digital photography for process-based interpretation at the ecosystem scale. Clim. Res. 2009, 39, 261–274. [Google Scholar] [CrossRef]
- Woebbecke, D.M.; Meyer, G.E.; von Bargen, K.; Mortensen, D.A. Color indices for weed identification under various soil, residue, and lighting conditions. Trans. ASAE 1995, 38, 259–269. [Google Scholar] [CrossRef]
- Gillespie, A.R.; Kahle, A.B.; Walker, R.E. Color enhancement of highly correlated images. II. Channel ratio and “chromaticity” transformation techniques. Remote Sens. Environ. 1987, 22, 343–365. [Google Scholar] [CrossRef]
- Migliavacca, M.; Galvagno, M.; Cremonese, E.; Rossini, M.; Meroni, M.; Sonnentag, O.; Cogliati, S.; Manca, G.; Diotri, F.; Busetto, L.; et al. Using digital repeat photography and eddy covariance data to model grassland phenology and photosynthetic CO2 uptake. Agric. For. Meteorol. 2011, 151, 1325–1337. [Google Scholar] [CrossRef]
- Moore, C.E.; Beringer, J.; Evans, B.; Hutley, L.B.; Tapper, N.J. Tree–grass phenology information improves light use efficiency modelling of gross primary productivity for an Australian tropical savanna. Biogeosciences 2017, 14, 111–129. [Google Scholar] [CrossRef]
- Richardson, A.D.; Hufkens, K.; Milliman, T.; Aubrecht, D.M.; Chen, M.; Gray, J.M.; Johnston, M.R.; Keenan, T.F.; Klosterman, S.T.; Kosmala, M.; et al. Tracking vegetation phenology across diverse North American biomes using PhenoCam imagery. Sci. Data 2018, 5, 180028. [Google Scholar] [CrossRef] [PubMed]
- Sonnentag, O.; Hufkens, K.; Teshera-Sterne, C.; Young, A.M.; Friedl, M.; Braswell, B.H.; Milliman, T.; O’Keefe, J.; Richardson, A.D. Digital repeat photography for phenological research in forest ecosystems. Agric. For. Meteorol. 2012, 152, 159–177. [Google Scholar] [CrossRef]
- De Beurs, K.; Henebry, G.M. Spatial-Temporal statistical methods for modelling land surface phenology. In Phenological Research: Methods for Environmental and Climate Change Analysis; Hudson, I.L., Keatley, M.R., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 177–208. ISBN 978-90-481-3335-2. [Google Scholar]
- Zhang, X.; Friedl, M.A.; Schaaf, C.B.; Strahler, A.H.; Hodges, J.C.F.; Gao, F.; Reed, B.C.; Huete, A. Monitoring vegetation phenology using MODIS. Remote Sens. Environ. 2003, 84, 471–475. [Google Scholar] [CrossRef]
- Jonsson, P.; Eklundh, L. TIMESAT—A program for analyzing time- series of satellite sensor data. Comput. Geosci. 2004, 30, 833–845. [Google Scholar] [CrossRef]
- Stephenson, N. Actual evapotranspiration and deficit: Biologically meaningful correlates of vegetation distribution across spatial scales. J. Biogeogr. 1998, 25, 855–870. [Google Scholar] [CrossRef]
- James, R.; Washington, R.; Rowell, D.P. Implications of global warming for the climate of African rainforests. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013, 368, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Murray-Tortarolo, G.; Friedlingstein, P.; Stich, S.; Seneviratne, S.I.; Fletcher, I.; Mueller, B.; Greve, P.; Anav, A.; Lu, Y.; Ahlström, A.; et al. The dry season intensity as a key driver of NPP trends. Geophys. Res. Lett. 2016, 43, 2632–2639. [Google Scholar] [CrossRef] [Green Version]
- Frescino, T.S.; Edwards, T.C., Jr.; Moisen, G.G. Modeling spatially explicit forest structural attributes using Generalized Additive Models. J. Veg. Sci. 2001, 12, 15–26. [Google Scholar] [CrossRef]
- Wood, S.N. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. R. Stat. Soc. Ser. B Stat. Methodol. 2011, 73, 3–36. [Google Scholar] [CrossRef]
- Yang, L.; Qin, G.; Zhao, N.; Wang, C.; Song, G. Using a generalized additive model with autoregressive terms to study the effects of daily temperature on mortality. BMC Med. Res. Methodol. 2012, 12, 1–13. [Google Scholar] [CrossRef]
- Pezzini, F.F.; Ranieri, B.D.; Brandão, D.O.; Fernandes, G.W.; Quesada, M.; Espírito-Santo, M.M.; Jacobi, C.M. Changes in tree phenology along natural regeneration in a seasonally dry tropical forest. Plant Biosyst. 2014, 148, 965–974. [Google Scholar] [CrossRef]
- Marra, G.; Wood, S.N. Practical variable selection for generalized additive models. Comput. Stat. Data Anal. 2011, 55, 2372–2387. [Google Scholar] [CrossRef]
- Morellato, L.P.C.; Camargo, M.G.G.; Gressler, E. A review of plant phenology in South and Central America. In Phenology: An Integrative Environmental Science; Schwartz, M., Ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 91–113. ISBN 978-94-007-0632-3. [Google Scholar]
- Lopes, A.P.; Nelson, B.W.; Wu, J.; Graça, P.M.L.A.; Tavares, J.V.; Prohaska, N.; Martins, G.A.; Saleska, S. Leaf flush drives dry season green-up of the Central Amazon. Remote Sens. Environ. 2016, 182, 90–98. [Google Scholar] [CrossRef]
- Gutierrez, A.P.A.; Engle, N.L.; De Nys, E.; Molejon, C.; Martins, E.S. Drought preparedness in Brazil. Weather Clim. Extrem. 2014, 3, 95–106. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, D.C.A.; Alves, J.L.H.; Prazeres, S.M.; Paiva, A.M.A. Dados fenológicos de 10 espécies arbóreas de uma área de caatinga (Alagoinha-PE). Acta Bot. Bras. 1989, 3, 109–117. [Google Scholar] [CrossRef]
- Araújo, E.L.; Castro, C.C.; Albuquerque, U.P. Dynamics of Brazilian Caatinga—A Review Concerning the Plants, Environment and People. Funct. Ecosyst. Communities 2007, 1, 15–28. [Google Scholar]
- Monasterio, M.; Sarmiento, G. Phenological strategies of plants species in the tropical savanna and semi-deciduous forest of the Venezuelan Lianos. J. Biogeogr. 1976, 3, 325–356. [Google Scholar] [CrossRef]
- Pirani, F.R.; Sanchez, M.; Pedroni, F. Fenologia de uma comunidade arbórea em cerrado sentido restrito, Barra do Garças, MT, Brasil. Acta Bot. Bras. 2009, 23, 1096–1110. [Google Scholar] [CrossRef]
- Munhoz, C.B.R.; Felfili, J.M. Fenologia do estrato herbáceo-subarbustivo de uma comunidade de campo sujo na Fazenda Água Limpa no Distrito Federal, Brasil. Acta Bot. Bras. 2005, 19, 979–988. [Google Scholar] [CrossRef]
- Borchert, R.; Rivera, G.; Hagnauer, W. Modification of vegetative phenology in a tropical semi-deciduous forest by abnormal drought and rain. Biotropica 2002, 34, 27–39. [Google Scholar] [CrossRef]
- Rossatto, D.R.; Hoffmann, W.A.; Silva, L.C.R.; Haridasan, M.; Sternberg, L.S.L.; Franco, A.C. Seasonal variation in leaf traits between congeneric savanna and forest trees in Central Brazil: Implications for forest expansion into savanna. Trees 2013, 27, 1139–1150. [Google Scholar] [CrossRef]
- Garcia, L.C.; Barros, F.V.; Lemos-Filho, J.P. Environmental drivers on leaf phenology of ironstone outcrops species under seasonal climate. An. Da Acad. Bras. De Ciências 2017, 89, 131–143. [Google Scholar] [CrossRef] [Green Version]
- Albuquerque, U.P.; Araújo, E.L.; El-Deir, A.C.A.; Lima, A.L.A.; Souto, A.; Bezerra, B.M.; Ferraz, E.M.N.; Freire, E.M.X.; Sampaio, E.V.S.B.; Las-Casas, F.M.G.; et al. Caatinga Revisited: Ecology and Conservation of na Important Seasonal Dry Forest. Sci. World J. 2012, 2012, 1–18. [Google Scholar] [CrossRef]
- Eamus, D.; Prior, L.D. Ecophysiology of trees of seasonally dry tropics: Comparisons among phenologies. Adv. Ecol. Res. 2001, 32, 113–197. [Google Scholar]
- Goldstein, G.; Meinzer, F.C.; Bucci, S.J.; Scholz, F.G.; Franco, A.C.; Hoffmann, W.A. Water economy of Neotropical savanna trees: Six paradigms revisited. Tree Physiol. 2008, 28, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Borchert, R.; Rivera, G. Photoperiodic control of seasonal development and dormancy in tropical stem- succulent trees. Tree Physiol. 2001, 21, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento, G. The Ecology of Neotropical Savannas; Harvard University Press: Cambridge, MA, USA, 1984; p. 235. ISBN 9780674418554. [Google Scholar]
- Rossatto, D.R.; Hoffmann, W.A.; Franco, A.C. Differences in growth patterns between co-occurring forest and savanna trees affect the forest–savanna boundary. Funct. Ecol. 2009, 23, 689–698. [Google Scholar] [CrossRef]
- Wagner, F.H.; Hérault, B.; Bonal, D.; Stahl, C.; Anderson, L.O.; Baker, T.R.; Becker, G.S.; Beeckman, H.; Souza, D.B.; Botosso, P.C.; et al. Climate seasonality limits leaf carbon assimilation and wood productivity in tropical forests. Biogeosciences 2016, 13, 2537–2562. [Google Scholar] [CrossRef] [Green Version]
- Dalmolin, Â.C.; Lobo, F.A.; Vourlitis, G.; Silva, P.R.; Dalmagro, H.J.; Antunes, M.Z., Jr.; Ortíz, C.E.R. Is the dry season an important driver of phenology and growth for two Brazilian savanna tree species with contrasting leaf habits? Plant Ecol. 2015, 216, 407–417. [Google Scholar] [CrossRef]
- Silverio, D.V.; Lenza, E. Fenologia de espécies lenhosas em um cerrado típico no Parque Municipal do Bacaba, Nova Xavantina, Mato Grosso, Brasil. Biota Neotrop. 2010, 10, 205–216. [Google Scholar] [CrossRef]
- Lenza, E.; Klink, C.A. Comportamento fenológico de espécies lenhosas em um cerrado sentido restrito de Brasília, DF. Rev. Bras. Bot. 2006, 29, 627–638. [Google Scholar] [CrossRef]
- Streher, A.S.; Sobreiro, J.F.F.; Morellato, L.P.C.; Silva, T.S.F. Land Surface Phenology in the Tropics: The Role of Climate and Topography in a Snow-Free Mountain. Ecosystems 2017, 20, 1436–1453. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Fu, R.; Juarez, R.I.N.; Fernandes, K. Observed change of the standardized precipitation index, its potential cause and implications to future climate change in the Amazon region. Philos. Trans. R. Soc. B 2008, 363, 1767–1772. [Google Scholar] [CrossRef]
- Costa, M.H.; Pires, G.F. Effects of Amazon and Central Brazil deforestation scenarios on the duration of the dry season in the arc of deforestation. Int. J. Climatol. 2010, 30, 1970–1979. [Google Scholar] [CrossRef]
- Vico, G.; Thompson, S.E.; Manzoni, S.; Molini, A.; Albertson, J.D.; Almeida-Cortez, J.S.; Fay, P.A.; Feng, X.; Guswa, A.J.; Liu, H.; et al. Climatic, ecophysiological, and phenological controls on plant ecohydrological strategies in seasonally dry ecosystems. Ecohydrology 2015, 8, 660–681. [Google Scholar] [CrossRef]
- Machado, I.C.S.; Barros, L.M.; Sampaio, E.V.S.B. Phenology of Caatinga Species at Serra Talhada, PE, Northeastern Brazil. Biotropica 1997, 29, 57–68. [Google Scholar] [CrossRef]
- Borchert, R. Responses of tropical trees to rainfall seasonality and its long-term changes. Clim. Chang. 1998, 39, 381–393. [Google Scholar] [CrossRef]
- Scholz, F.G.; Bucci, S.J.; Goldstein, G.; Moreira, M.Z.; Meinzer, F.C.; Domec, J.C.; Villalobos-Vega, R.; Franco, A.C.; Miralles-Wilhelm, F. Biophysical and life-history determinants of hydraulic lift in Neotropical savanna trees. Funct. Ecol. 2008, 22, 773–786. [Google Scholar] [CrossRef]
- Scholz, F.G.; Bucci, S.J.; Goldstein, G.; Meinzer, F.C.; Franco, A.C. Hydraulic redistribution of soil water by neotropical savanna trees. Tree Physiol. 2002, 22, 603–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leite, M.B.; Xavier, R.O.; Oliveira, P.T.S.; Silva, F.K.G.; Matos, D.M.S. Groundwater depth as a constraint on the woody cover in a Neotropical Savanna. Plant Soil 2018, 426, 1–15. [Google Scholar] [CrossRef]
- Damasceno, G.; Souza, L.; Pivello, V.R.; Gorgone-Barbosa, E.; Giroldo, P.Z.; Fidelis, A. Impacto f invasive grasses on Cerrado under natural regeneration. Biol. Invasions 2018, 20, 3621–3629. [Google Scholar] [CrossRef]
- Wolkovich, E.M.; Cleland, E.E. The phenology of plant invasions: A community ecology perspective. Front. Ecol. Environ. 2011, 9, 287–294. [Google Scholar] [CrossRef]
- Novy, A.; Flory, S.L.; Hartman, J.M. Evidence for rapid evolution of phenology in an invasive grass. J. Evol. Biol. 2013, 26, 443–450. [Google Scholar] [CrossRef]
- Franco, A.C. Ecophysiology of woody plants. In The Cerrados of Brazil: Ecology and Natural History of a Neotropical Savanna; Oliveira, P.S., Marquis, R.J., Eds.; Columbia University Press: New York, NY, USA, 2002; pp. 178–197, ASIN B0092WWFNC. [Google Scholar]
- Cadule, P.; Friedlingstein, P.; Bopp, L.; Sitch, S.; Jones, C.D.; Ciais, P.; Piao, S.L.; Peylin, P. Benchmarking coupled climate-carbon models against long-term atmospheric CO2 measurements, Global Biogeochem. Cycles 2010, 24, 1–24. [Google Scholar] [CrossRef]






| Name | Study Site Designation Lat/Long. | Location | Vegetation Type | Phenocam Monitoring | Mean Annual Total Precipitation | Length of Dry Season (months) |
|---|---|---|---|---|---|---|
| caatinga | Embrapa Semiárido—9°02′47.5″S/40°19′ W | Petrolina, PE, northeast Brazil | xeric shrubland (Caatinga) | 10/May/2013 to 31/Dec/2015 | 510 mm | 8 |
| cerrado shrubland | Itirapina Ecological Station—22°13′23″S/47°53′02.67″W | Itirapina, SP, southeastern Brazil | grasslands, savannas and shrublands (Cerrado campo sujo) | 28/Mar/2013 to 28/May/2015 | 1524 mm | 6 |
| woody cerrado | Botelho Farm—22°10′49.18″S/47°52′16.54″W | Itirapina, SP, southeastern Brazil | grasslands, savannas and shrublands (Cerrado sensu stricto) | 02/Sept/2011 to 03/Feb/2015 | 1524 mm | 6 |
| dense cerrado | Pé de Gigante—21°37′9″S/47°37′58″W | Santa Rita do Passa Quatro, SP, southeastern Brazil | grasslands, savannas and shrublands (Cerrado sensu stricto denso) | 26/Aug/2013 to 31/Dec/2015 | 1499 mm | 6 |
| SITE/YEAR | SOS | POS | EOS | LOS |
|---|---|---|---|---|
| caatinga | ||||
| 2013/2014 | 296 | 6 | 183 | 252 |
| 2014/2015 | 282 | 360 | 214 | 297 |
| 2015/2016 | 310 | 364 | NS | NS |
| cerrado shrubland | ||||
| 2013/2014 | NS | NS | 230 | NS |
| 2014/2015 | 239 | 317 | 219 | 345 |
| 2015/2016 | 228 | 309 | NS | NS |
| woody cerrado | ||||
| 2011/2012 | NS | NS | 170 | NS |
| 2012/2013 | 185 | 284 | 134 | 314 |
| 2013/2014 | 158 | 254 | 135 | 342 |
| 2014/2015 | 147 | 240 | NS | NS |
| dense cerrado | ||||
| 2013/2014 | NS | 290 | 204 | NS |
| 2014/2015 | 213 | 279 | 223 | 345 |
| 2015/2016 | 229 | 298 | NS | NS |
| Site Location | ROI | Day-Length (Hours) | cwd (mm) | Rainfall_ lag7days (mm) | Rainfall_Cum_30days (mm) | Tamp(oC) | R2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| edf | F-Test | edf | F-Test | edf | F-Test | edf | F-Test | edf | F-Test | |||
| Caatinga | comu | 7.83 | 12.324 | 8.559 | 28.565 | 1.0 | 36.02 | 6.91 | 16.36 | N. S | N. S | 0.86 |
| Cerrado shrubland | comu | 5.726 | 39.535 | 6.678 | 13.189 | N. S | N. S | 3.907 | 4.617 | N. S | N. S | 0.88 |
| grass | 2.114 | 9.535 | N. S | N. S | N. S | N. S | N. S | N. S | 1.00 | 19.531 | 0.56 | |
| Woody cerrado | comu | 7.773 | 36.383 | 7.94 | 8.317 | N. S | N. S | 6.745 | 3.156 | N. S | N. S | 0.79 |
| dec | 7.144 | 30.9 | 8.264 | 28.548 | 2.719 | 6.734 | 5.884 | 3.857 | 1.00 | 6.772 | 0.82 | |
| Dense cerrado | comu | 5.218 | 22.436 | 7.097 | 8.484 | N. S | N. S | N. S | N. S | 3.558 | 17.051 | 0.51 |
| dec | N. S | N. S | 7.665 | 5.904 | N. S | N. S | N. S | N. S | 3.663 | 25.488 | 0.42 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alberton, B.; da Silva Torres, R.; Sanna Freire Silva, T.; Rocha, H.R.d.; S. B. Moura, M.; Morellato, L.P.C. Leafing Patterns and Drivers across Seasonally Dry Tropical Communities. Remote Sens. 2019, 11, 2267. https://0-doi-org.brum.beds.ac.uk/10.3390/rs11192267
Alberton B, da Silva Torres R, Sanna Freire Silva T, Rocha HRd, S. B. Moura M, Morellato LPC. Leafing Patterns and Drivers across Seasonally Dry Tropical Communities. Remote Sensing. 2019; 11(19):2267. https://0-doi-org.brum.beds.ac.uk/10.3390/rs11192267
Chicago/Turabian StyleAlberton, Bruna, Ricardo da Silva Torres, Thiago Sanna Freire Silva, Humberto R. da Rocha, Magna S. B. Moura, and Leonor Patricia Cerdeira Morellato. 2019. "Leafing Patterns and Drivers across Seasonally Dry Tropical Communities" Remote Sensing 11, no. 19: 2267. https://0-doi-org.brum.beds.ac.uk/10.3390/rs11192267