Identification of the Best Hyperspectral Indices in Estimating Plant Species Richness in Sandy Grasslands
Abstract
:1. Introduction
- Within the thousands of hyperspectral indices, which ones perform best in estimating species richness?
- Does the hyperspectral data conform to predictions based on the Spectral Variations Hypothesis?
- How well do the investigated hyperspectral spectral indices perform as a proxy for plant species richness under different vegetation cover and structural complexity?
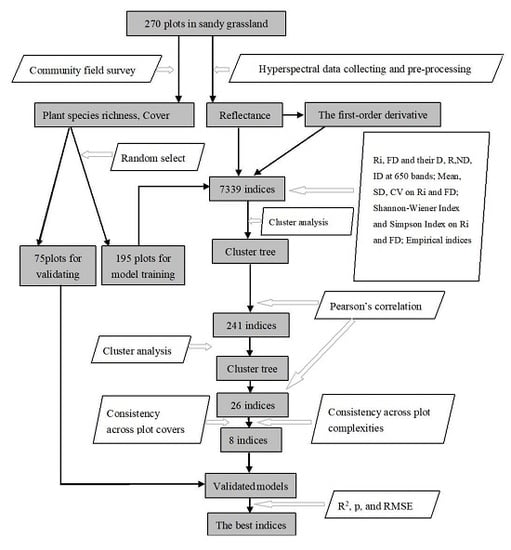
2. Materials and Methods
2.1. Study Area
2.2. Plant Diversity Survey and Analysis
2.3. Hyperspectral Data Measurement and Analysis
2.4. Development Hyperspectral Indices
2.5. The Method for Identification of the Best Indices
3. Results
3.1. Cluster trees in two stages
3.2. Performance Under Different Community Conditions
3.3. Validation of Proposed Models
4. Discussion
4.1. Fit. to SVH
4.2. Methods Used to Identify the Best Indices
4.3. Accuracy, Stability and Complexity
4.4. Fine Spatial Scale
4.5. Limitations of the Approach
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Index1 | Index62 | ||
| Index2 | Index63 | ||
| Index3 | Index64 | ||
| Index4 | Index65 | ||
| Index5 | Index66 | ||
| Index6 | Index67 | ||
| Index7 | Index68 | ||
| Index8 | Index69 | ||
| Index9 | Index70 | ||
| Index10 | Index71 | ||
| Index11 | Index72 | ||
| Index12 | Index73 | ||
| Index13 | Index74 | ||
| Index14 | Index75 | ||
| Index15 | Index76 | ||
| Index16 | Index77 | ||
| Index17 | Index78 | ||
| Index18 | Index79 | ||
| Index19 | Index80 | ||
| Index20 | Index81 | [(R700 − R672) − 0.2 ∗ (R700 − R553)] | |
| Index21 | Index82 | [(R700 − R672) − 0.2 ∗ (R700 − R553)] ∗ (R700/R672) | |
| Index22 | Index83 | ((R782 − R675)/(R782 + R675 + 0.2)) (1.2) | |
| Index23 | Index84 | 0.5(R782 − 0.5R675 − 0.2)/(0.5R782 + 0.5R675 − 0.1) | |
| Index24 | Index85 | ||
| Index25 | Index86 | ()/1.019 | |
| Index26 | Index87 | 2.5 × ((R865 − R672)/(R865 + 6 × R672 − 7.5 × R465 + 1)) | |
| Index27 | Index88 | ||
| Index28 | Index89 | R700 + 40[(R670 + R780)/2 − R700 ]/(R740 − R700) | |
| Index29 RDVI | (R800 − R670)/sqrt(R800 + R670) | Index90 | (1 + 0.16)( R800 − R670)/( R800 + R670 + 0.16) |
| Index30 MSR | (R800/R670 − 1)/sqrt(R800/R670 + 1) | Index91 MCARI | |
| Index31 MCARI1 | 1.2[2.5(R800 − R670) − 1.3(R800 − R550)] | Index92 | |
| Index32 MTVI1 | 1.2[1.2(R800 − R550) − 2.5(R670 − R550)] | Index93 | |
| Index33 TVI | 0.5(120*(R750 − R550) − 200(R670 − R550)) | Index94 | |
| Index34 | Index95 | ||
| Index35 | Index96 | ||
| Index36 | Index97 | ||
| Index37 | Index98 | ||
| Index38 | Index99 | ||
| Index39 | Index100 | ||
| Index40 | Index101 | Mean (R500 to R600) | |
| Index41 | Index102 | MeanRed (600–699 nm)/mean Green (500–599 nm) | |
| Index42 | Index103 | (Max first derivative: 680–750 nm) | |
| Index43 | Index104 | The highest first derivative value between 490–530 nm (Db) | |
| Index44 | Index105 | The highest first derivative value between 550–580 nm (Dg) | |
| Index45 | Index106 | The highest first derivative value between 680–780 nm (DR) | |
| Index46 | Index107 | The highest first derivative between 510–580 nm (Rg) | |
| Index47 | Index108 | The smallest reflectance value between 640–700 nm (Ro) | |
| Index48 | Index109 | The sum of first derivative values between 490–530 nm (SDb) | |
| Index49 | Index110 | The sum of first derivative values between 550–580 nm (SDg) | |
| Index50 | Index111 | The sum of first derivative values between 680–780 nm (SDR) | |
| Index51 | Index112 | Rg/Ro | |
| Index52 | Index113 | (Rg − Ro)/(Rg + Ro) | |
| Index53 | Index114 | SDR/SDb | |
| Index54 | Index115 | SDR/SDg | |
| Index55 | Index116 | (SDR − SDb)/(SDR + SDb) | |
| Index56 | Index117 MCARI2 | 1.5*(2.5(R800 − R670) − 1.3(R800 − R550))/sqrt((2R800 + 1)^2 − (6R800 − 5*sqrtR670) − 0.5) | |
| Index57 | Index118 MTVI2 | 1.5*(1.2*(R800 − R550) − 2.5*(R670 − R550))/sqrt((2*R800 + 1)^2 − (6*R800 − 5*sqrtR670)) − 0.5) | |
| Index58 | Index119 CARI | (|670*(R700 − R550)/150 + R670 + R550 − 3.67*(R700 − R550)|/sqrt(((R700 − R550)/150)^2 + 1))*R700/R670 | |
| Index59 | Index120 MSAVI | ||
| Index60 | Index121 NDVI | (mean() − mean(R660~R760))/(mean() + mean()) | |
| Index61 | Index122 SAVI | 1.5*(mean() − mean(R660~R760))/(mean(R760~R960) + mean()+0.5) |
References
- Hector, A.; Bagchi, R.; Hector, A.; Bagchi, R. Biodiversity and ecosystem multifunctionality. Nature 2007, 448, 188–190. [Google Scholar] [CrossRef] [PubMed]
- Duffy, J.E. Why biodiversity is important to the functioning of real-world ecosystems. Front. Ecol. Environ. 2009, 7, 437–444. [Google Scholar] [CrossRef]
- Oldeland, J.; Wesuls, D.; Rocchini, D.; Schmidt, M.; Jürgens, N. Does using species abundance data improve estimates of species diversity from remotely sensed spectral heterogeneity? Ecol. Indic. 2010, 10, 390–396. [Google Scholar] [CrossRef]
- Jetz, W.; Cavender-Bares, J.; Pavlick, R.; Schimel, D.; Davis, F.W.; Asner, G.P.; Guralnick, R.; Kattge, J.; Latimer, A.M.; Moorcroft, P.; et al. Monitoring plant functional diversity from space. Nat. Plants 2016, 2, 16024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavender-Bares, J.; Gamon, J.A.; Hobbie, S.E.; Madritch, M.D.; Meireles, J.E.; Schweiger, A.K.; Townsend, P.A. Harnessing plant spectra to integrate the biodiversity sciences across biological and spatial scales. Am. J. Bot. 2017, 104, 966–969. [Google Scholar] [CrossRef] [PubMed]
- Palmer, M.W.; Earls, P.G.; Hoagland, B.W.; White, P.S.; Wohlgemuth, T. Quantitative tools for predicting species lists. Environmetrics 2002, 13, 121–137. [Google Scholar] [CrossRef]
- Rocchini, D.; Chiarucci, A.; Loiselle, S.A. Testing the spectral variation hypothesis by using satellite multispectral images. Acta Oecol. 2004, 26, 117–120. [Google Scholar] [CrossRef]
- Rocchini, D. Effects of spatial and spectral resolution in estimating ecosystem α-diversity by satellite imagery. Remote Sens. Environ. 2007, 111, 423–434. [Google Scholar] [CrossRef]
- Gillespie, T.W.; Foody, G.M.; Rocchini, D.; Giorgi, A.P.; Saatchi, S. Measuring and modeling biodiversity from space. Prog. Phys. Geogr. 2008, 32, 203–221. [Google Scholar] [CrossRef]
- Gould, W. Remote sensing of vegetation, plant species richness, and regional biodiversity hotspots. Ecol. Appl. 2000, 10, 1861–1870. [Google Scholar] [CrossRef]
- Oindo, B.O.; Skidmore, A.K. Interannual variability of NDVI and species richness in Kenya. Int. J. Remote Sens. 2002, 23, 285–298. [Google Scholar] [CrossRef] [Green Version]
- Foody, G.M.; Cutler, M.E.J. Mapping the species richness and composition of tropical forests from remotely sensed data with neural networks. Ecol. Model. 2006, 195, 37–42. [Google Scholar] [CrossRef] [Green Version]
- Christiand, C.; Selmas, D.C. Relationships between floristic diversity and vegetation indices, forest structure and landscape metrics of fragments in Brazilian Cerrado. Forest. Ecol. Manag. 2009, 257, 2157–2165. [Google Scholar]
- Bawa, K.; Rose, J.; Ganeshaiah, K.N.; Barve, N. Assessing biodiversity from space: an example from the western Ghats, India. Conserv. Ecol. 2002, 76, 1662–1663. [Google Scholar] [CrossRef]
- Nagendra, H.; Rocchini, D.; Ghate, R.; Sharma, B.; Pareeth, S. Assessing plant diversity in a dry tropical forest: Comparing the utility of Landsat and Ikonos satellite images. Remote Sens. 2010, 2, 478–496. [Google Scholar] [CrossRef]
- Culbert, P.D.; Radeloff, V.C.; St-Louis, V.; Flather, C.H.; Rittenhouse, C.D.; Albright, T.P.; Pidgeona, A.M. Modeling broad-scale patterns of avian species richness across the midwestern United States with measures of satellite image texture. Remote Sens. Environ. 2012, 118, 140–150. [Google Scholar] [CrossRef]
- Viedma, O.; Torres, I.; Pérez, B.; Moreno, J.M. Modeling plant species richness using reflectance and texture data derived from QuickBird in a recently burned area of Central Spain. Remote Sens. Environ. 2012, 119, 208–221. [Google Scholar] [CrossRef]
- Laurin, V.G.; Chan, J.W.; Chen, Q.; Lindsell, J.A.; Coomes, D.A.; Guerriero, L.; Del Frate, F.; Miglietta, F.; Valentini, R. Biodiversity mapping in a tropical west African forest with airborne hyperspectral data. PLoS ONE 2014, 9, e97910. [Google Scholar]
- Tuanmu, M.N.; Jetz, M. A global, remote sensing-based characterization of terrestrial habitat heterogeneity for biodiversity and ecosystem modeling. Glob. Ecol. Biogeogr. 2015, 24, 1329–1339. [Google Scholar] [CrossRef]
- Rocchini, D.; Balkenhol, N.; Carter, G.A.; Foody, G.M.; Gillespie, T.W.; He, K. Remotely sensed spectral heterogeneity as a proxy of species diversity: recent advances and open challenges. Ecol. Inf. 2010, 5, 318–329. [Google Scholar] [CrossRef]
- Schmidtlein, S.; Fassnacht, F.E. The spectral variability hypothesis does not hold across landscapes. Remote Sens. Environ. 2017, 192, 114–125. [Google Scholar] [CrossRef]
- Plourde, L.C.; Ollinger, S.V.; Smith, M.L.; Martin, M.E. Estimating species abundance in a northern temperate forest using spectral mixture analysis. Photogramm. Eng. Remote Sens. 2007, 73, 829–840. [Google Scholar] [CrossRef]
- Carlson, K.M.; Asner, G.P.; Hughes, R.F.; Ostertag, R.; Martin, R.E. Hyperspectral remote sensing of canopy biodiversity in Hawaiian lowland rainforests. Ecosystems 2007, 10, 536–549. [Google Scholar] [CrossRef]
- Ghiyamat, A.; Shafri, H.Z.M. A review on hyperspectral remote sensing for homogeneous and heterogeneous forest biodiversity assessment. Int. J. Remote Sens. 2010, 31, 1837–1856. [Google Scholar] [CrossRef] [Green Version]
- Asner, G.P.; Martin, R.E.; Carranzajiménez, L.; Sinca, F.; Tupayachi, R.; Anderson, C.B.; Martinez, P. Functional and biological diversity of foliar spectra in tree canopies throughout the Andes to Amazon region. New Phytol. 2014, 204, 127–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feilhauer, H.; Faude, U.; Schmidtlein, S. Combining ISO map ordination and imaging spectroscopy to map continuous floristic gradients in a heterogeneous landscape. Remote Sens. Environ. 2011, 115, 2513–2524. [Google Scholar] [CrossRef]
- Pottier, J.; Malenovský, Z.; Psomas, A.; Homolová, L.; Schaepman, M.E.; Choler, P. Modelling plant species distribution in alpine grasslands using airborne imaging spectroscopy. Biol. Lett. 2014, 10, 20140347. [Google Scholar] [CrossRef] [PubMed]
- Möckel, T.; Dalmayne, J.; Schmid, B.C.; Prentice, H.C.; Hall, K. Airborne hyperspectral data predict fine-scale plant species diversity in grazed dry grasslands. Remote Sens. 2016, 8, 133. [Google Scholar] [CrossRef]
- Peng, Y.; Fan, M.; Song, J.; Cui, T.; Li, R. Assessment of plant species diversity based on hyperspectral indices at a fine scale. Sci. Rep. 2018, 8, 4776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castilloriffart, I.; Galleguillos, M.; Lopatin, J.; Quezada, P.; Jorge, F. Predicting vascular plant diversity in anthropogenic peatlands: comparison of modeling methods with free satellite data. Remote Sens. 2017, 9, 681. [Google Scholar] [CrossRef]
- Gholizadeh, H.; Gamon, J.A.; Zygielbaum, A.I.; Wang, R.; Schweiger, A.K.; Cavender-Bares, J. Remote sensing of biodiversity: soil correction and data dimension reduction methods improve assessment of α-diversity (species richness) in prairie ecosystems. Remote Sens. Environ. 2018, 206, 240–253. [Google Scholar] [CrossRef]
- Peng, Y.; Mi, K.; Qin, Y.; Qing, F.T.; Liu, W.C.; Xue, D.Y. Spectral reflectance characteristics of dominant plant species at different eco-restoring stages in the semi-arid grassland. Spectrosc. Spect. Anal. 2014, 34, 3090–3094. [Google Scholar]
- Ramoelo, A.; Skidmore, A.K.; Schlerf, M.; Mathieu, R.; Heitkönig, I.M.A. Water-removed spectra increase the retrieval accuracy when estimating savanna grass nitrogen and phosphorus concentrations. ISPS J. Photogramm. Remote Sens. 2011, 66, 408–417. [Google Scholar] [CrossRef]
- Stratoulias, D.; Balzter, H.; Zlinszky, A.; Tóth, V.R. Assessment of ecophysiology of lake shore reed vegetation based on chlorophyll fluorescence, field spectroscopy and hyperspectral airborne imagery. Remote Sens. Environ. 2015, 157, 72–84. [Google Scholar] [CrossRef] [Green Version]
- Curran, P.J. Remote sensing of foliar chemistry. Remote Sens. Environ. 1989, 30, 271–278. [Google Scholar] [CrossRef]
- Ceccato, P.; Gobron, N.; Flasse, S.; Pinty, B.; Tarantola, S. Designing a spectral index to estimate vegetation water content from remote sensing data: Part I: theoretical approach. Remote Sens. Environ. 2002, 82, 188–197. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Pushnik, J.C.; Dobrowski, S. Steady-state chlorophyll a fluorescence detection from canopy derivative reflectance and double-peak red-edge effects. Remote Sens. Environ. 2003, 84, 283–294. [Google Scholar] [CrossRef]
- Rocchini, D.; Dadalt, L.; Delucchi, L.; Neteler, M.; Palmer, M.W. Disentangling the role of remotely sensed spectral heterogeneity as a proxy for North American plant species richness. Community Ecol. 2014, 15, 37–43. [Google Scholar] [CrossRef]
- Wang, P.; Huang, F.; Liu, X.N. A simple interpretation of the rice spectral indices space for assessment of heavy metal stress. ISPRS J. Photogramm. 2016, XLI-B7, 129–135. [Google Scholar]
- Ferreira, M.P.; Zortea, M.; Zanotta, D.C.; Shimabukuro, Y.E.; Filho, C.R. Mapping tree species in tropical seasonal semi-deciduous forests with hyperspectral and multispectral data. Remote Sens. Environ. 2016, 179, 66–78. [Google Scholar] [CrossRef]
- Peón, J.; Recondo, C.; Fernández, S.F.; Calleja, J.; De Miguel, E.; Carretero, L. Prediction of topsoil organic carbon using airborne and satellite hyperspectral imagery. Remote Sens. 2017, 9, 1211. [Google Scholar] [CrossRef]
- Torrecilla, E.; Stramski, D.; Reynolds, R.A.; Millán-Núñez, E.; Piera, J. Cluster analysis of hyperspectral optical data for discriminating phytoplankton pigment assemblages in the open ocean. Remote Sens. Environ. 2011, 115, 2578–2593. [Google Scholar] [CrossRef] [Green Version]
- Uitz, J.; Stramski, D.; Reynolds, R.A.; Dubranna, J. Assessing phytoplankton community composition from hyperspectral measurements of phytoplankton absorption coefficient and remote-sensing reflectance in open-ocean environments. Remote Sens. Environ. 2015, 171, 58–74. [Google Scholar] [CrossRef]
- Lopatin, J.; Dolos, K.; Hernández, H.J.; Galleguillos, M.; Fassnacht, F.E. Comparing Generalized Linear Models and random forest to model vascular plant species richness using LiDAR data in a natural forest in central Chile. Remote Sens. Environ. 2016, 173, 200–210. [Google Scholar] [CrossRef]
- Somers, B.; Asner, G.P.; Martin, R.E.; Anderson, C.B.; Knapp, D.E.; Wright, S.J. Mesoscale assessment of changes in tropical tree species richness across a bioclimatic gradient in panama using airborne imaging spectroscopy. Remote Sens. Environ. 2015, 167, 111–120. [Google Scholar] [CrossRef]
- Fassnacht, F.E.; Hartig, F.; Latifi, H.; Berger, C.; Hernández, J.; Corvalán, P.; Koch, B. Importance of sample size, data type and prediction method for remote sensing-based estimations of aboveground forest biomass. Remote Sens. Environ. 2014, 154, 102–114. [Google Scholar] [CrossRef]
- Kokaly, R.F.; Asner, G.P.; Ollinger, S.V.; Martin, M.E.; Wessman, C.A. Characterizing canopy biochemistry from imaging spectroscopy and its application to ecosystem studies. Remote Sens. Environ. 2009, 113, S78–S91. [Google Scholar] [CrossRef]
- Aneece, I.; Epstein, H. Distinguishing early successional plant communities using ground-level hyperspectral data. Remote Sens. 2015, 7, 16588–16606. [Google Scholar] [CrossRef]
- Huang, C.Y.; Asner, G.P. Applications of remote sensing to alien invasive plant studies. Sensors 2009, 9, 4869–4889. [Google Scholar] [CrossRef] [PubMed]
- Montesinos-López, O.A.; Montesinos-López, A.; Crossa, J.; de los Campos, G.; Alvarado, G.; Suchismita, M.; Rutkoski, J.; González-Pérez, L.; Burgueño, J. Predicting grain yield using canopy hyperspectral reflectance in wheat breeding data. Plant Methods 2017, 13, 4. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Wang, Q.H.; Mi, K.; Peng, Y. Scale-dependent effects of landscape pattern on plant diversity in Hunshandak Sandland. Biodivers. Conserv. 2017, 26, 2169–2185. [Google Scholar] [CrossRef]
- Abdel-Rahman, E.M.; Ahmed, F.B.; Ismail, R. Random forest regression and spectral band selection for estimating sugarcane leaf nitrogen concentration using EO-1 Hyperion hyperspectral data. Int. J. Remote Sens. 2013, 34, 712–728. [Google Scholar] [CrossRef]
- Chen, S.; Li, D.; Wang, Y.; Peng, Z.; Chen, W. Spectral characterization and prediction of nutrient content in winter leaves of litchi during flower bud differentiation in southern China. Precis. Agric. 2011, 12, 682–698. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, B.; Meng, J. Quantifying winter wheat residue biomass with a spectral angle index derived from China environmental satellite data. Int. J. Appl. Earth Obs. Geoinform. 2014, 32, 105–113. [Google Scholar] [CrossRef]
- Lu, S.; Lu, F.; You, W.; Wang, Z.; Liu, Y.; Omasa, K. A robust vegetation index for remotely assessing chlorophyll content of dorsiventral leaves across several species in different seasons. Plant Methods 2018, 14, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jongschaap, R.E.E.; Booij, R. Spectral measurements at different spatial scales in potato: relating leaf, plant and canopy nitrogen status. Int. J. Appl. Earth Obs. Geoinform. 2004, 5, 205–218. [Google Scholar] [CrossRef]
- Sims, D.; Gamon, J. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Fava, F.; Parolo, G.; Colombo, R.; Gusmeroli, F.; Della Marianna, G.; Monteiro, A.T.; Bocchi, S. Fine-scale assessment of hay meadow productivity and plant diversity in the European Alps using field spectrometric data. Agric. Ecosyst. Environ. 2010, 137, 151–157. [Google Scholar] [CrossRef]
- Wang, J.; Wang, T.; Skidmore, A.; Shi, T.; Wu, G. Evaluating different methods for grass nutrient estimation from canopy hyperspectral reflectance. Remote Sens. 2015, 7, 5901–5917. [Google Scholar] [CrossRef]
- Kemper, T.; Sommer, S. Estimate of heavy metal contamination in soils after a mining accident using reflectance spectroscopy. Environ. Sci. Technol. 2002, 36, 2742–2747. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Jiang, G.; Liu, M.; Niu, S.; Yu, S.; Biswas, D.K. Potentials for combating desertification in Hunshandak Sandland through nature reserve. Environ. Manag. 2005, 35, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Jiang, G.M.; Li, Y.G.; Niu, S.L.; Liu, M.Z.; Gao, L.M. Photosynthesis, transpiration and water use efficiency of four typical grass along the gradient of grazing intensity in Hunshandak Sandland, China. J. Arid Environ. 2007, 70, 304–315. [Google Scholar] [CrossRef]
- Peng, J.; Li, Y.; Tian, L.; Liu, Y.H.; Wang, Y.L. Vegetation dynamics and associated driving forces in Eastern China during 1999–2008. Remote Sens. 2015, 7, 13641–13663. [Google Scholar] [CrossRef]
- Zhang, J.; Rivard, B.; Sanchez-Azofeifa, A.; Castro-Esau, K. Intra- and inter-class spectral variability of tropical tree species at La Selva, Costa Rica: Implications for species identification using HYDICE imagery. Remote Sens. Environ. 2006, 105, 129–141. [Google Scholar] [CrossRef]
- Cochrane, M.A. Using vegetation reflectance variability for species level classification of hyperspectral data. Int. J. Remote Sens. 2000, 21, 2075–2087. [Google Scholar] [CrossRef]
- Clark, M.L.; Roberts, D.A.; Clark, D.B. Hyperspectral discrimination of tropical rain forest tree species at leaf to crown scales. Remote Sens. Environ. 2005, 96, 375–398. [Google Scholar] [CrossRef]
- Lucas, R.M.; Bunting, P.; Paterson, M.; Chisholm, M. Classification of Australian forest communities using aerial photography CASI and HyMap Data. Remote Sens. Environ. 2008, 112, 2088–2103. [Google Scholar] [CrossRef]
- Papes, M.; Tupayachi, R.; Martinez, P.; Peterson, A.T.; Powell, G.V.N. Using hyperspectral imageries for regional inventories: a test with tropical emergent trees in the Amazon Basin. J. Veg. Sci. 2009, 21, 342–354. [Google Scholar] [CrossRef]
- Leutner, B.F.; Reineking, B.; Muller, J.; Bachmann, M.; Beierkuhnlein, C.; Dech, S.; Wegmann, M. Modelling forest a-diversity and floristic composition-on the added value of LiDAR plus hyperspectral remote sensing. Remote Sens. 2012, 4, 2818–2845. [Google Scholar] [CrossRef]
- Kim, D. Modeling spatial and temporal dynamics of plant species richness across tidal creeks in a temperate salt marsh. Ecol. Indic. 2018, 93, 188–195. [Google Scholar] [CrossRef]
- Tian, Y.C.; Yao, X.; Yang, J.; Cao, W.X.; Hannaway, D.B.; Zhu, Y. Assessing newly developed and published vegetation indices for estimating rice leaf nitrogen concentration with ground- and space-based hyperspectral reflectance. Field Crops Res. 2011, 120, 299–310. [Google Scholar] [CrossRef]
- Wang, R.; Gamon, J.A.; Cavender-Bares, J.; Townsend, P.A.; Zygielbaum, A.I. The spatial sensitivity of the spectral diversity-biodiversity relationship: an experimental test in a prairie grassland. Ecol. Appl. 2018, 28, 541–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.M.; Cable, M.L.; Hook, S.J.; Green, R.O.; Ustin, S.L.; Mandl, D.J.; Middleton, E.M. An introduction to the NASA Hyperspectral InfraRed Imager (HyspIRI) mission and preparatory activities. Remote Sens. Environ. 2015, 167, 6–19. [Google Scholar] [CrossRef]
- Castaldi, F.; Chabrillat, S.; Jones, A.; Vreys, K.; Bomans, B.; van Wesemael, B. Soil organic carbon estimation in croplands by hyperspectral remote APEX data using the LUCAS Topsoil Database. Remote Sens. 2018, 10, 153. [Google Scholar] [CrossRef]
- Alison, B.; Nicholas, C.; Sabine, C.; Birgit, H. A phenological approach to spectral differentiation of low-arctic tundra vegetation communities, north slope, Alaska. Remote Sens. 2017, 9, 1200. [Google Scholar]





| Wavelength Ranges (nm) | Plant Bio-Traits | References |
|---|---|---|
| 420–440 | Chlorophyll-a absorption | [35] |
| 420–480 | Chlorophyll absorption | [36] |
| 450–470 | Chlorophyll-b absorption | [35] |
| 490–550 | Pigment reflected peak | [36] |
| 510–570 | Green reflected peak | [36] |
| 630–650 | Chlorophyll-b absorption | [35] |
| 630–690 | Plant species discrimination | [36] |
| 650–670 | Chlorophyll-a absorption | [35] |
| 640–700 | Chlorophyll absorption | [37] |
| 673–683 | Chlorophyll fluorescence | [37] |
| 680–700 | Chlorophyll fluorescence | [37] |
| 720–740 | Chlorophyll fluorescence | [37] |
| 700–750 | Leaf health and shape | [36] |
| 760–800 | Cell structure reflectance peak | [36] |
| 760–900 | Discrimination between plant species | [36] |
| 800–960 | Species biochemical traits | [36] |
| 900–920 | Proteins | [35] |
| 920–940 | Oil | [35] |
| 960–980 | Starch content, water | [35] |
| 980–1000 | Starch content | [35] |
| Hyperspectral Indices | R2 (n = 195) | Cover (n = 65) | Complexity (n = 65) | ||||
|---|---|---|---|---|---|---|---|
| L | M | H | L | M | H | ||
| R583 | 0.284 | −0.611 | −0.509 | −0.424 | −0.552 | −0.222 | 0.181 |
| SDFD420-480 | 0.340 | −0.623 | −0.474 | −0.351 | −0.410 | −0.162 | 0.203 |
| SDFD450-470 | 0.310 | 0.660 | 0.281 | 0.387 | 0.381 | 0.379 | −0.234 |
| D604 | 0.298 | −0.671 | −0.338 | −0.293 | −0.407 | −0.081 | 0.111 |
| FD603 | 0.275 | −0.401 | −0.727 | −0.395 | −0.268 | −0.073 | −0.176 |
| FD606 | 0.275 | −0.335 | −0.580 | −0.582 | −0.561 | −0.333 | −0.319 |
| FD437 | 0.327 | −0.293 | −0.572 | −0.427 | −0.301 | −0.341 | −0.058 |
| FD490 | 0.325 | −0.279 | −0.546 | −0.399 | −0.534 | −0.210 | −0.027 |
| SD-D420-480 | 0.278 | −0.585 | −0.476 | −0.412 | −0.566 | −0.620 | 0.177 |
| SD-D450-470 | 0.267 | −0.532 | −0.413 | −0.382 | −0.581 | −0.310 | −0.090 |
| FD460 | 0.283 | −0.573 | −0.451 | −0.313 | −0.379 | −0.232 | 0.237 |
| FD583 | 0.283 | −0.606 | −0.325 | −0.462 | −0.592 | −0.264 | 0.402 |
| D583 | 0.329 | −0.629 | 0.005 | −0.486 | −0.486 | −0.195 | −0.135 |
| MeanFD450-470 | 0.275 | 0.551 | 0.156 | 0.475 | 0.273 | −0.005 | 0.277 |
| SD450-470 | 0.295 | −0.476 | −0.505 | −0.466 | −0.680 | −0.405 | 0.365 |
| FD497 | 0.292 | −0.396 | −0.544 | −0.317 | −0.436 | −0.087 | 0.040 |
| FD403 | 0.354 | −0.395 | −0.528 | −0.279 | −0.455 | −0.125 | 0.219 |
| FD531 | 0.279 | −0.455 | −0.465 | −0.428 | −0.686 | −0.391 | 0.400 |
| ND528 | 0.264 | −0.598 | −0.464 | −0.499 | −0.513 | −0.051 | 0.132 |
| R528 | 0.263 | −0.525 | −0.553 | −0.612 | −0.484 | −0.058 | −0.170 |
| FDD588 | 0.260 | 0.358 | 0.455 | 0.378 | 0.285 | −0.003 | 0.216 |
| ShannonFD760-900 | 0.258 | 0.409 | 0.358 | 0.313 | 0.474 | 0.333 | −0.390 |
| Index48 | 0.211 | 0.408 | 0.368 | 0.332 | 0.452 | 0.395 | −0.361 |
| Index69 | 0.218 | 0.477 | 0.394 | 0.254 | 0.495 | 0.326 | −0.379 |
| Index78 | 0.231 | 0.505 | 0.399 | 0.267 | 0.502 | 0.307 | −0.413 |
| Index121 | 0.238 | −0.611 | −0.509 | −0.424 | −0.552 | −0.222 | 0.181 |
| The Indices | The Models | R2 | p | RMSE |
|---|---|---|---|---|
| FD606 | Y = 5.691 − 1188.364x | 0.271 | <0.001 | 1.839 |
| FD583 | Y = 5.609 − 1999.106x | 0.279 | <0.001 | 1.828 |
| FD531 | Y = 8.861 − 3241.099x | 0.276 | <0.001 | 1.833 |
| SD450-470 | Y = 7.043 − 857.869x | 0.291 | <0.001 | 1.813 |
| ShannFD760-900 | Y = 2.256x − 3.402 | 0.254 | <0.001 | 1.860 |
| Index48 | Y = 1.776 + 6.17x | 0.207 | <0.001 | 1.918 |
| Index69 | Y = 2.18 + 7.024x | 0.214 | <0.001 | 1.909 |
| Index78 | Y = 3.827x − 4.641 | 0.227 | <0.001 | 1.893 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Y.; Fan, M.; Bai, L.; Sang, W.; Feng, J.; Zhao, Z.; Tao, Z. Identification of the Best Hyperspectral Indices in Estimating Plant Species Richness in Sandy Grasslands. Remote Sens. 2019, 11, 588. https://0-doi-org.brum.beds.ac.uk/10.3390/rs11050588
Peng Y, Fan M, Bai L, Sang W, Feng J, Zhao Z, Tao Z. Identification of the Best Hyperspectral Indices in Estimating Plant Species Richness in Sandy Grasslands. Remote Sensing. 2019; 11(5):588. https://0-doi-org.brum.beds.ac.uk/10.3390/rs11050588
Chicago/Turabian StylePeng, Yu, Min Fan, Lan Bai, Weiguo Sang, Jinchao Feng, Zhixin Zhao, and Ziye Tao. 2019. "Identification of the Best Hyperspectral Indices in Estimating Plant Species Richness in Sandy Grasslands" Remote Sensing 11, no. 5: 588. https://0-doi-org.brum.beds.ac.uk/10.3390/rs11050588