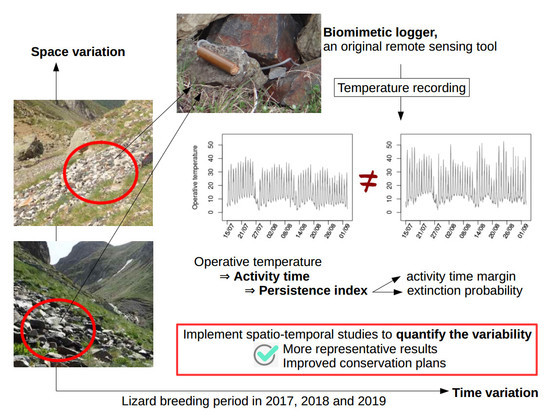
Multi-Site and Multi-Year Remote Records of Operative Temperatures with Biomimetic Loggers Reveal Spatio-Temporal Variability in Mountain Lizard Activity and Persistence Proxy Estimates
Abstract
:1. Introduction
1.1. Climate Change and Spatio-Temporal Variability
1.2. Spatio-Temporal Studies Using Biomimetic Logger, an Original Remote Sensing Tool
1.3. Integration of Spatio-Temporal Variability in Mountain Biodiversity Monitoring Still an Overlooked Issue
1.4. Activity Time and Persistence of Lizards
1.5. Study Aim
2. Materials and Methods
2.1. The Pyrenean Rock Lizard (Iberolacerta Bonnali) as a Study Case
2.2. Biomimetic Logger Deployment on Study Sites
2.3. Activity Time Computing from Te Measurements
2.4. Persistence Index Computing from Overall Activity Time during the Breeding Period
2.5. Statistical Analysis
3. Results
3.1. Intra-Site Variability
3.2. Inter-Slope and Inter-Altitude Variability
3.3. Inter-Site Variability
3.4. Inter-Year Variability
4. Discussion
4.1. Why Should Multi-Site Studies Be Favored?
4.1.1. To Assess the Spatial Variability Due to Micro-Habitat
4.1.2. To Assess the Spatial Variability Due to Habitat, Slope, Altitude and Hydrography
4.2. Why Should Multi-Year Studies Be Favored?
4.3. Remote Sensing for Biodiversity Conservation
4.3.1. Biomimetic Loggers Development to be Even More Similar to Remote Sensing Tools
4.3.2. Linking Ecological and Remote Sensing Communities
5. Conclusions
6. A Quick User Guide for Biomimetic Loggers in Mountain Landscapes
6.1. Why Use a Biomimetic Logger/Sensor?
6.2. Materials
6.3. Prerequisites for the Installation of the Equipment in the Mountains
- Time settings: The internal time of all temperature data-loggers must be set to GMT/UTC. To do this, check your computer’s time to GMT/UTC when starting the loggers.
- Start Time: When starting the loggers, choose “Delayed Start” and set the start time of all data-loggers to the same time. If all the temperature data-loggers used in your program start recording at the same time, you will allow direct comparisons and make data processing much easier. In our study, the recordings all started at 06:00 p.m. with a 10 min frequency, which allowed to characterize the time series with the same argument “start=c(1,109)”, since 06:00 p.m. corresponds to the 109th value recorded over the day (6 × 18 + 1).
- Recording interval: The recording interval must be consistent with that corresponding to the ecophysiological quantity of interest. Allow for a safety zone of a few days to a few weeks and make sure that the recorder configuration is set to stop recording after this time or when its memory is full.
6.4. Installation
6.5. Field Recording and Its Constraints
6.6. Data Processing
6.7. Maintenance and Storage of Equipment
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Te | Operative temperature |
| Ha | Activity time |
| HaDaily | Reference daily activity time |
| PI | Persistence Indice |
| CTmin and CTmax | Minimum and Maximum Critical lethal Temperature |
| VTmin and VTmax | Minimum and Maximum Voluntary Temperature |
| VTR | Voluntary Temperature Range |
Appendix A


References
- Leitão, P.J.; Santos, M.J. Improving Models of Species Ecological Niches: A Remote Sensing Overview. Front. Ecol. Evol. 2019, 7, 9. [Google Scholar] [CrossRef] [Green Version]
- Rummukainen, M. Changes in climate and weather extremes in the 21st century: Changes in climate and weather extremes. Wiley Interdiscip. Rev. Clim. Chang. 2012, 3, 115–129. [Google Scholar] [CrossRef]
- Cattiaux, J.; Douville, H.; Schoetter, R.; Parey, S.; Yiou, P. Projected increase in diurnal and interdiurnal variations of European summer temperatures. Geophys. Res. Lett. 2015, 42, 899–907. [Google Scholar] [CrossRef]
- Suarez-Gutierrez, L.; Müller, W.A.; Li, C.; Marotzke, J. Dynamical and thermodynamical drivers of variability in European summer heat extremes. Clim. Dyn. 2020. [Google Scholar] [CrossRef]
- Vincze, M.; Borcia, I.D.; Harlander, U. Temperature fluctuations in a changing climate: An ensemble-based experimental approach. Sci. Rep. 2017, 7, 254. [Google Scholar] [CrossRef] [PubMed]
- Meteo France. Bulletin Climatique Aquitaine Février 2019; Technical Report; Meteo France, CM Tarbes: Paris, France, 2019; Available online: https://donneespubliques.meteofrance.fr/donnees_libres/bulletins/BCMR/BCMR_02_201902.pdf (accessed on 27 August 2020).
- Meteo France. Bulletin climatologique Nouvelle Aquitaine Février 2020; Technical Report; Meteo France: Paris, France, 2020. [Google Scholar]
- Bozinovic, F.; Bastías, D.A.; Boher, F.; Clavijo-Baquet, S.; Estay, S.A.; Angilletta, M.J. The Mean and Variance of Environmental Temperature Interact to Determine Physiological Tolerance and Fitness. Physiol. Biochem. Zool. 2011, 84, 543–552. [Google Scholar] [CrossRef] [Green Version]
- Estay, S.A.; Clavijo-Baquet, S.; Lima, M.; Bozinovic, F. Beyond average: An experimental test of temperature variability on the population dynamics of Tribolium confusum. Popul. Ecol. 2011, 53, 53–58. [Google Scholar] [CrossRef]
- Vasseur, D.A.; DeLong, J.P.; Gilbert, B.; Greig, H.S.; Harley, C.D.G.; McCann, K.S.; Savage, V.; Tunney, T.D.; O’Connor, M.I. Increased temperature variation poses a greater risk to species than climate warming. Proc. R. Soc. B Biol. Sci. 2014, 281. [Google Scholar] [CrossRef] [Green Version]
- Sinervo, B.; Mendez-de-la Cruz, F.; Miles, D.B.; Heulin, B.; Bastiaans, E.; Villagran-Santa Cruz, M.; Lara-Resendiz, R.; Martinez-Mendez, N.; Calderon-Espinosa, M.L.; Meza-Lazaro, R.N.; et al. Erosion of Lizard Diversity by Climate Change and Altered Thermal Niches. Science 2010, 328, 894–899. [Google Scholar] [CrossRef] [Green Version]
- Arribas, O.J. Habitat selection, thermoregulation and activity of the Pyrenean Rock Lizard Iberolacerta bonnali (LANTZ, 1927). Herpetozoa 2009, 22, 145–166. [Google Scholar]
- Cahill, A.E.; Aiello-Lammens, M.E.; Fisher-Reid, M.C.; Hua, X.; Karanewsky, C.J.; Yeong Ryu, H.; Sbeglia, G.C.; Spagnolo, F.; Waldron, J.B.; Warsi, O.; et al. How does climate change cause extinction? Proc. R. Soc. B Biol. Sci. 2012, 280, 20121890. [Google Scholar] [CrossRef] [PubMed]
- Campbell-Staton, S.C.; Cheviron, Z.A.; Rochette, N.; Catchen, J.; Losos, J.B.; Edwards, S.V. Winter storms drive rapid phenotypic, regulatory, and genomic shifts in the green anole lizard. Science 2017, 357, 495–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buckley, L.B.; Huey, R.B. How Extreme Temperatures Impact Organisms and the Evolution of their Thermal Tolerance. Integr. Comp. Biol. 2016, 56, 98–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrade, D.; Bevier, C.; de Carvalho, J. (Eds.) Amphibian and Reptile Adaptations to the Environment: Interplay Between Physiology and Behavior; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Tourneur, J.C.; Meunier, J. The successful invasion of the European earwig across North America reflects adaptations to thermal regimes but not mean temperatures. bioRxiv 2019. [Google Scholar] [CrossRef]
- Paaijmans, K.P.; Heinig, R.L.; Seliga, R.A.; Blanford, J.I.; Blanford, S.; Murdock, C.C.; Thomas, M.B. Temperature variation makes ectotherms more sensitive to climate change. Glob. Chang. Biol. 2013, 19, 2373–2380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kingsolver, J.G.; Diamond, S.E.; Buckley, L.B. Heat stress and the fitness consequences of climate change for terrestrial ectotherms. Funct. Ecol. 2013, 27, 1415–1423. [Google Scholar] [CrossRef]
- Clusella-Trullas, S.; Chown, S.L. Comment on “Erosion of Lizard Diversity by Climate Change and Altered Thermal Niches”. Science 2011, 332, 537. [Google Scholar] [CrossRef] [Green Version]
- Vicente Liz, A.; Santos, V.; Ribeiro, T.; Guimarães, M.; Verrastro, L. Are lizards sensitive to anomalous seasonal temperatures? Long-term thermobiological variability in a subtropical species. PLoS ONE 2019, 14, e0226399. [Google Scholar] [CrossRef]
- Wang, R.; Gamon, J.A. Remote sensing of terrestrial plant biodiversity. Remote Sens. Environ. 2019, 231, 111218. [Google Scholar] [CrossRef]
- Abd Allah, O.; Abdalla, M.; Abdalla, S.; Babiker, A.; Awad Allah, A. Universal Data Logger System for Environmental Monitoring Applications. Indones. J. Electr. Eng. Inform. 2017, 5, 131–136. [Google Scholar] [CrossRef]
- Campbell, J.B.; Wynne, R.H. Introduction to Remote Sensing, 5th ed.; Guilford Press: New York, NY, USA, 2011. [Google Scholar]
- Wang, K.; Franklin, S.E.; Guo, X.; Cattet, M. Remote Sensing of Ecology, Biodiversity and Conservation: A Review from the Perspective of Remote Sensing Specialists. Sensors 2010, 10, 9647–9667. [Google Scholar] [CrossRef] [PubMed]
- Rocchini, D.; Marcantonio, M.; Da Re, D.; Chirici, G.; Galluzzi, M.; Lenoir, J.; Ricotta, C.; Torresani, M.; Ziv, G. Time-lapsing biodiversity: An open source method for measuring diversity changes by remote sensing. Remote Sens. Environ. 2019, 231, 111192. [Google Scholar] [CrossRef] [Green Version]
- Reinisch, E.C.; Ziemann, A.K.; Flynn, E.B.; Theiler, J. Combining multispectral imagery and synthetic aperture radar for detecting deforestation. In Algorithms, Technologies, and Applications for Multispectral and Hyperspectral Imagery XXVI; Messinger, D.W., Velez-Reyes, M., Eds.; SPIE: Bellingham, WA, USA, 2020; p. 7. [Google Scholar]
- Rocchini, D.; Luque, S.; Pettorelli, N.; Bastin, L.; Doktor, D.; Faedi, N.; Feilhauer, H.; Féret, J.; Foody, G.M.; Gavish, Y.; et al. Measuring β-diversity by remote sensing: A challenge for biodiversity monitoring. Methods Ecol. Evol. 2018, 9, 1787–1798. [Google Scholar] [CrossRef] [Green Version]
- Munafò, M.R.; Nosek, B.A.; Bishop, D.V.M.; Button, K.S.; Chambers, C.D.; Percie du Sert, N.; Simonsohn, U.; Wagenmakers, E.J.; Ware, J.J.; Ioannidis, J.P.A. A manifesto for reproducible science. Nat. Hum. Behav. 2017. [Google Scholar] [CrossRef] [Green Version]
- Pettorelli, N.; Safi, K.; Turner, W. Satellite remote sensing, biodiversity research and conservation of the future. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130190. [Google Scholar] [CrossRef]
- Gao, P.; Cheng, C.; Song, C. Satellite Remote Sensing for Biodiversity Conservation: Exemplary Practices and Lessons Learned; Leidner, A.K., Buchanan, G.M., Eds.; Satellite Remote Sensing for Conservation Action: Case Studies from Aquatic and Terrestrial Ecosystems; Cambridge University Press: Cambridge, UK, 2018; 372p. [Google Scholar]
- Ortega Adarme, M.; Queiroz Feitosa, R.; Nigri Happ, P.; Aparecido De Almeida, C.; Rodrigues Gomes, A. Evaluation of Deep Learning Techniques for Deforestation Detection in the Brazilian Amazon and Cerrado Biomes From Remote Sensing Imagery. Remote Sens. 2020, 12, 910. [Google Scholar] [CrossRef] [Green Version]
- Sagan, V.; Maimaitijiang, M.; Sidike, P.; Eblimit, K.; Peterson, K.; Hartling, S.; Esposito, F.; Khanal, K.; Newcomb, M.; Pauli, D.; et al. UAV-Based High Resolution Thermal Imaging for Vegetation Monitoring, and Plant Phenotyping Using ICI 8640 P, FLIR Vue Pro R 640, and thermoMap Cameras. Remote Sens. 2019, 11, 330. [Google Scholar] [CrossRef] [Green Version]
- Song, W.; Song, W.; Gu, H.; Li, F. Progress in the Remote Sensing Monitoring of the Ecological Environment in Mining Areas. Int. J. Environ. Res. Public Health 2020, 17, 1846. [Google Scholar] [CrossRef] [Green Version]
- Das, S.; Angadi, D.P. Land use-land cover (LULC) transformation and its relation with land surface temperature changes: A case study of Barrackpore Subdivision, West Bengal, India. Remote Sens. Appl. Soc. Environ. 2020, 19, 100322. [Google Scholar] [CrossRef]
- Dou, J.; Yunus, A.P.; Tien Bui, D.; Sahana, M.; Chen, C.W.; Zhu, Z.; Wang, W.; Pham, B.T. Evaluating GIS-Based Multiple Statistical Models and Data Mining for Earthquake and Rainfall-Induced Landslide Susceptibility Using the LiDAR DEM. Remote Sens. 2019, 11, 638. [Google Scholar] [CrossRef] [Green Version]
- Chan, B.K.K.; Lima, F.P.; Williams, G.A.; Seabra, R.; Wang, H.Y. A simplified biomimetic temperature logger for recording intertidal barnacle body temperatures: Simplified Biomimetic Temperature Logger. Limnol. Oceanogr. Methods 2016, 14, 448–455. [Google Scholar] [CrossRef]
- Lathlean, J.A.; Ayre, D.J.; Coleman, R.A.; Minchinton, T.E. Using biomimetic loggers to measure interspecific and microhabitat variation in body temperatures of rocky intertidal invertebrates. Mar. Freshw. Res. 2015, 66, 86. [Google Scholar] [CrossRef]
- Lathlean, J.A.; McQuaid, C.D. Biogeographic Variability in the Value of Mussel Beds as Ecosystem Engineers on South African Rocky Shores. Ecosystems 2017, 20, 568–582. [Google Scholar] [CrossRef]
- Chan, S.H.; Loke, L.H.; Crickenberger, S.; Todd, P.A. Robonerite: A low-cost biomimetic temperature logger to monitor operative temperatures of a common gastropod (Nerita spp.) in tropical urban seascapes. HardwareX 2019, 6, e00075. [Google Scholar] [CrossRef]
- Wethey, D.S.; Brin, L.D.; Helmuth, B.; Mislan, K. Predicting intertidal organism temperatures with modified land surface models. Ecol. Model. 2011, 222, 3568–3576. [Google Scholar] [CrossRef]
- Erbakanov, L.; Atanassov, K.; Sotirov, S. Generalized Net Model of a Body Temperature Data Logger Embedded System. Int. J. Bioautomotion 2015, 19, 237–244. [Google Scholar]
- Shipley, J.R.; Kapoor, J.; Dreelin, R.A.; Winkler, D.W. An open-source sensor-logger for recording vertical movement in free-living organisms. Methods Ecol. Evol. 2018, 9, 465–471. [Google Scholar] [CrossRef]
- Nishiumi, N.; Matsuo, A.; Kawabe, R.; Payne, N.; Huveneers, C.; Watanabe, Y.Y.; Kawabata, Y. A miniaturized threshold-triggered acceleration data-logger for recording burst movements of aquatic animals. J. Exp. Biol. 2018, 221, jeb172346. [Google Scholar] [CrossRef] [Green Version]
- Bedi, A.; Dalle, J.; Perrot, M.; Benoit, M. Mangrove restauration: Biomimetic artificial mangrove roots and ecosystem-based management models. In Proceedings of the 5th International Mangrove Macrobenthos and Management Meeting (MMM5), Singapore, 1–5 July 2019. [Google Scholar]
- Helmuth, B.; Choi, F.; Matzelle, A.; Torossian, J.L.; Morello, S.L.; Mislan, K.; Yamane, L.; Strickland, D.; Szathmary, P.L.; Gilman, S.E.; et al. Long-term, high frequency in situ measurements of intertidal mussel bed temperatures using biomimetic sensors. Sci. Data 2016, 3, 160087. [Google Scholar] [CrossRef] [Green Version]
- Wild, J.; Kopecký, M.; Macek, M.; Šanda, M.; Jankovec, J.; Haase, T. Climate at ecologically relevant scales: A new temperature and soil moisture logger for long-term microclimate measurement. Agric. For. Meteorol. 2019, 268, 40–47. [Google Scholar] [CrossRef]
- Upton, G.; Cook, I. A Dictionary of Statistics, 2nd ed.; Oxford University Press: Oxford, UK, 2008. [Google Scholar]
- McMunn, M.S. A time-sorting pitfall trap and temperature datalogger for the sampling of surface-active arthropods. HardwareX 2017, 1, 38–45. [Google Scholar] [CrossRef]
- Pasquali, V.; D’Alessandro, G.; Gualtieri, R.; Leccese, F. A new data logger based on Raspberry-Pi for Arctic Notostraca locomotion investigations. Measurement 2017, 110, 249–256. [Google Scholar] [CrossRef]
- García, M.B.; Domingo, D.; Pizarro, M.; Font, X.; Gómez, D.; Ehrlén, J. Rocky habitats as microclimatic refuges for biodiversity. A close-up thermal approach. Environ. Exp. Bot. 2020, 170, 103886. [Google Scholar] [CrossRef]
- Lamb, J.S.; Satgé, Y.G.; Fiorello, C.V.; Jodice, P.G.R. Behavioral and reproductive effects of bird-borne data logger attachment on Brown Pelicans (Pelecanus occidentalis) on three temporal scales. J. Ornithol. 2017, 158, 617–627. [Google Scholar] [CrossRef] [Green Version]
- Beddows, P.A.; Mallon, E.K. Cave Pearl Data Logger: A Flexible Arduino-Based Logging Platform for Long-Term Monitoring in Harsh Environments. Sensors 2018, 18, 530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morley, P.J.; Donoghue, D.N.; Chen, J.C.; Jump, A.S. Quantifying structural diversity to better estimate change at mountain forest margins. Remote Sens. Environ. 2019, 223, 291–306. [Google Scholar] [CrossRef]
- Gilman, S.; Hayford, H.; Craig, C.; Carrington, E. Body temperatures of an intertidal barnacle and two whelk predators in relation to shore height, solar aspect, and microhabitat. Mar. Ecol. Prog. Ser. 2015, 536, 77–88. [Google Scholar] [CrossRef] [Green Version]
- Chapperon, C.; Seuront, L. Space-time variability in environmental thermal properties and snail thermoregulatory behaviour: Variability in snail thermoregulatory behaviour. Funct. Ecol. 2011, 25, 1040–1050. [Google Scholar] [CrossRef]
- Tagliarolo, M.; McQuaid, C.D. Field Measurements Indicate Unexpected, Serious Underestimation of Mussel Heart Rates and Thermal Tolerance by Laboratory Studies. PLoS ONE 2016, 11, e0146341. [Google Scholar] [CrossRef] [Green Version]
- Tito, R.; Vasconcelos, H.L.; Feeley, K.J. Mountain Ecosystems as Natural Laboratories for Climate Change Experiments. Front. For. Glob. Chang. 2020, 3, 38. [Google Scholar] [CrossRef]
- Han, G.; Wang, W.; Dong, Y. Effects of balancing selection and microhabitat temperature variations on heat tolerance of the intertidal black mussel Septifer virgatus. Integr. Zool. 2020. [Google Scholar] [CrossRef] [PubMed]
- Monaco, C.J.; Wethey, D.S.; Helmuth, B. Thermal sensitivity and the role of behavior in driving an intertidal predator-prey interaction. Ecol. Monogr. 2016, 86, 429–447. [Google Scholar] [CrossRef]
- Helmuth, B.; Broitman, B.R.; Yamane, L.; Gilman, S.E.; Mach, K.; Mislan, K.A.S.; Denny, M.W. Organismal climatology: Analyzing environmental variability at scales relevant to physiological stress. J. Exp. Biol. 2010, 213, 995–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiménez-Robles, O.; De la Riva, I. Lizards in the mist: Thermal niches constrained by habitat and microclimates in the Andes of southern Bolivia. J. Biogeogr. 2019, 46, 1676–1686. [Google Scholar] [CrossRef]
- Kubisch, E.; Corbalán, V.; Ibargüengoytía, N.; Sinervo, B. Local extinction risk of three species of lizard from Patagonia as a result of global warming. Can. J. Zool. 2016, 94, 49–59. [Google Scholar] [CrossRef]
- Sinervo, B.; Miles, D.B.; Wu, Y.; Méndez-De La Cruz, F.R.; Kirchhof, S.; Qi, Y. Climate change, thermal niches, extinction risk and maternal-effect rescue of toad-headed lizards, Phrynocephalus Therm. Extrem. Arabian Peninsula Qinghai-Tibetan Plateau. Integr. Zool. 2018, 13, 450–470. [Google Scholar] [CrossRef]
- Pauli, H.; Gottfried, M.; Lamprecht, A.; Niessner, S.; Rumpf, S.; Winkler, M.; Steinbauer, K.; Grabherr, G. The GLORIA Filed Manual, Standard Multi-Summit Approach, Supplementary Methods and Extra Approaches, 5th ed.; Technical Report; Institute for Interdisciplinary Mountain Research: Vienna, Austria, 2015. [Google Scholar]
- Antonelli, A.; Kissling, W.D.; Flantua, S.G.A.; Bermúdez, M.A.; Mulch, A.; Muellner-Riehl, A.N.; Kreft, H.; Linder, H.P.; Badgley, C.; Fjeldså, J.; et al. Geological and climatic influences on mountain biodiversity. Nat. Geosci. 2018, 11, 718–725. [Google Scholar] [CrossRef]
- Körner, C. Mountain Biodiversity, Its Causes and Function. AMBIO J. Hum. Environ. 2004, 33, 11. [Google Scholar] [CrossRef]
- Chemini, C.; Rizzoli, A. Land use change and biodiversity conservation in the Alps. J. Mt. Ecol. 2014, 7, 1–7. [Google Scholar]
- Elsen, P.R.; Tingley, M.W. Global mountain topography and the fate of montane species under climate change. Nat. Clim. Chang. 2015, 5, 772–776. [Google Scholar] [CrossRef]
- Lehikoinen, A.; Brotons, L.; Calladine, J.; Campedelli, T.; Escandell, V.; Flousek, J.; Grueneberg, C.; Haas, F.; Harris, S.; Herrando, S.; et al. Declining population trends of European mountain birds. Glob. Chang. Biol. 2019, 25, 577–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pepin, N.; Bradley, R.S.; Diaz, H.F.; Baraër, M.; Caceres, E.B.; Forsythe, N.; Fowler, H.; Greenwood, G.; Hashmi, M.Z.; Liu, X.D.; et al. Elevation-dependent warming in mountain regions of the world. Nat. Clim. Chang. 2015, 5, 424–430. [Google Scholar] [CrossRef] [Green Version]
- Viterbi, R.; Cerrato, C.; Bassano, B.; Bionda, R.; Hardenberg, A.; Provenzale, A.; Bogliani, G. Patterns of biodiversity in the northwestern Italian Alps: A multi-taxa approach. Community Ecol. 2013, 14, 18–30. [Google Scholar] [CrossRef]
- Mallard, F. Tome I: DéVeloppement D’Indicateurs des Effets du Changement Climatique sur la Biodiversité en Nouvelle-Aquitaine, Programme les Sentinelles du Climat; Technical Report; Cistude Nature: Le Haillan, France, 2016. [Google Scholar]
- Mallard, F.; Couderchet, L. Climate Sentinels Research Program: Developing Indicators of the Effects of Climate Change on Biodiversity in the Region of New Aquitaine (South West, France). In Handbook of Climate Change and Biodiversity; Leal Filho, W., Barbir, J., Preziosi, R., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 223–241. [Google Scholar] [CrossRef]
- Leal, M.; Gunderson, A.R. Rapid Change in the Thermal Tolerance of a Tropical Lizard. Am. Nat. 2012, 180, 815–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubisch, E.L.; Fernández, J.B.; Ibargüengoytía, N.R. Vulnerability to climate warming of Liolaemus pictus (Squamata, Liolaemidae), a lizard from the cold temperate climate in Patagonia, Argentina. J. Comp. Physiol. B 2016, 186, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, A.L.; Miles, D.B. Natural selection on thermal preference, critical thermal maxima and locomotor performance. Proc. R. Soc. B Biol. Sci. 2017, 284, 20170536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huey, R.B.; Deutsch, C.A.; Tewksbury, J.J.; Vitt, L.J.; Hertz, P.E.; Álvarez Pérez, H.J.; Garland, T. Why tropical forest lizards are vulnerable to climate warming. Proc. R. Soc. B Biol. Sci. 2009, 276, 1939–1948. [Google Scholar] [CrossRef]
- Theisinger, O. Thermal Limits of Reptiles. Ecological and Environmental Constraints on the Thermal Biology of Malagsy Lizards. Ph.D. Thesis, University of Hamburg, Hamburg, Germany, 2016. [Google Scholar]
- Sinclair, B.J.; Marshall, K.E.; Sewell, M.A.; Levesque, D.L.; Willett, C.S.; Slotsbo, S.; Dong, Y.; Harley, C.D.G.; Marshall, D.J.; Helmuth, B.S.; et al. Can we predict ectotherm responses to climate change using thermal performance curves and body temperatures? Ecol. Lett. 2016, 19, 1372–1385. [Google Scholar] [CrossRef] [Green Version]
- Blouin-Demers, G.; Nadeau, P. The cost-benefit model of thermoregulation does not predict lizard thermoregulatory behaviour. Ecology 2005, 86, 560–566. [Google Scholar] [CrossRef]
- Huey, R.B.; Losos, J.B.; Moritz, C. Are Lizards Toast? Science 2010, 328, 832–833. [Google Scholar] [CrossRef]
- Caetano, G.H.O.; Santos, J.C.; Godinho, L.B.; Cavalcante, V.H.G.L.; Diele-Viegas, L.M.; Campelo, P.H.; Martins, L.F.; Oliveira, A.F.S.; Alvarenga, J.M.; Wiederhecker, H.C.; et al. Time of activity is a better predictor of the distribution of a tropical lizard than pure environmental temperatures. Oikos 2020. [Google Scholar] [CrossRef]
- Gunderson, A.R.; Leal, M. Patterns of Thermal Constraint on Ectotherm Activity. Am. Nat. 2015, 185, 653–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortega, Z.; Mencía, A.; Pérez-Mellado, V. The peak of thermoregulation effectiveness: Thermal biology of the Pyrenean rock lizard, Iberolacerta bonnali (Squamata, Lacertidae). J. Therm. Biol. 2016, 56, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Ortega, Z.; Mencía, A.; Pérez-Mellado, V. Behavioral buffering of global warming in a cold-adapted lizard. Ecol. Evol. 2016, 6, 4582–4590. [Google Scholar] [CrossRef] [Green Version]
- Adolph, S.C.; Porter, W.P. Temperature, Activity, and Lizard Life Histories. Am. Nat. 1993, 142, 273–295. [Google Scholar] [CrossRef] [Green Version]
- Ceia-Hasse, A.; Sinervo, B.; Vicente, L.; Pereira, H.M. Integrating ecophysiological models into species distribution projections of European reptile range shifts in response to climate change. Ecography 2014, 37, 679–688. [Google Scholar] [CrossRef]
- Kearney, M. Activity restriction and the mechanistic basis for extinctions under climate warming. Ecol. Lett. 2013, 16, 1470–1479. [Google Scholar] [CrossRef]
- Keogh, J.S.; Noble, D.W.A.; Wilson, E.E.; Whiting, M.J. Activity Predicts Male Reproductive Success in a Polygynous Lizard. PLoS ONE 2012, 7, e38856. [Google Scholar] [CrossRef] [Green Version]
- Lantz, L. Description of Iberolacerta bonnali, Quelques observations nouvelles sur l’herpétologie des Pyrénées centrales. Rev. D’Histoire Nat. Aplliquée 1927, 8, 58–61. [Google Scholar]
- Pottier, G. Plan National D’Actions en Faveur des LéZards Des PyréNéEs Iberolacerta Aranica, I. Aurelioi Et I. Bonnali, 2013–2017; Technical Report; Nature Midi-Pyrénées: Toulouse, France, 2012. [Google Scholar]
- Mallard, F. Tome IV: Ajustement Des Protocoles D’éChantillonnage et Analyses Exploratoires des Indicateurs du CC en NA, Programme Les Sentinelles du Climat; Technical Report; Cistude Nature: Le Haillan, France, 2017. [Google Scholar]
- Arribas, O.J.; Galan, P. Reproductive characteristics of the Pyrenean high-mountain lizards: Iberolacerta aranica, I. aurelioi and I. bonnali. Anim. Biol. 2005, 55, 163–190. [Google Scholar]
- IUCN. Iberolacerta Bonnali: Valentin Pérez-Mellado, Marc Cheylan, Iñigo Martínez-Solano: The IUCN Red List of Threatened Species 2009; Technical Report; International Union for Conservation of Nature: Gland, Switzerland, 2008. [Google Scholar]
- Le Moigne, C.; Jailloux, A. Liste Rouge Régionale des Amphibiens et Reptiles d’Aquitaine; Technical Report; Observatoire Aquitaine de la Faune Sauvage: Talence, France, 2013. [Google Scholar]
- D’Amico, F. Mountaineous areas. In AcclimaTerra—Anticipating climate change in New Aquitaine. To Act in the Territories—Synthesis, Région Nouvelle Aquitaine; Le Treut, H., Ed.; Éditions Région Nouvelle-Aquitaine: Bordeaux, France, 2018; pp. 76–78. [Google Scholar]
- Grbac, I.; Bauwens, D. Constraints on Temperature Regulation in Two Sympatric Podarcis Lizards during Autumn. Copeia 2001, 2001, 178–186. [Google Scholar] [CrossRef]
- Huey, R.B.; Kearney, M.R.; Krockenberger, A.; Holtum, J.A.M.; Jess, M.; Williams, S.E. Predicting organismal vulnerability to climate warming: Roles of behaviour, physiology and adaptation. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 1665–1679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camacho, A.; Rusch, T.W. Methods and pitfalls of measuring thermal preference and tolerance in lizards. J. Therm. Biol. 2017, 68, 63–72. [Google Scholar] [CrossRef]
- Herrando-Pérez, S.; Ferri-Yáñez, F.; Monasterio, C.; Beukema, W.; Gomes, V.; Belliure, J.; Chown, S.L.; Vieites, D.R.; Araújo, M.B. Intraspecific variation in lizard heat tolerance alters estimates of climate impact. J. Anim. Ecol. 2019, 88, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Castilla, A.M.; Van Damme, R.; Bauwens, D. Field body temperatures, mechanisms of thermoregulation and evolution of thermal characteristics in lacertid lizards. Nat. Croat. 1999, 8, 253–274. [Google Scholar]
- Pérez-Mellado, V. Datos sobre Lacerta monticola en el oeste del Sistema Central. Acta Vertebr. 1982, 9, 107–129. [Google Scholar]
- Fay, M.P. Asht: Applied Statistical Hypothesis Tests. R Package Version 0.9.4. 2018. Available online: https://cran.r-project.org/web/packages/asht/asht.pdf (accessed on 19 June 2020).
- Hodges, J.; Lehmann, E. Estimates of Location Based on Rank Tests. Ann. Math. Stat. 1963, 34, 598–611. [Google Scholar] [CrossRef]
- Pike, N. Using false discovery rates for multiple comparisons in ecology and evolution: False discovery rates for multiple comparisons. Methods Ecol. Evol. 2011, 2, 278–282. [Google Scholar] [CrossRef]
- White, T.; van der Ende, J.; Nichols, T.E. Beyond Bonferroni revisited: Concerns over inflated false positive research findings in the fields of conservation genetics, biology, and medicine. Conserv. Genet. 2019, 20, 927–937. [Google Scholar] [CrossRef] [Green Version]
- Soberón, J.; Arroyo-Peña, B. Are fundamental niches larger than the realized? Testing a 50-year-old prediction by Hutchinson. PLoS ONE 2017, 12, e0175138. [Google Scholar] [CrossRef]
- Genna, A. Carte Géologique Harmonisée du Département des Pyrénées Atlantiques, Notice Technique, Rapport Final BRGM/RP 55408 FR; Technical Report; BRGM, Géosciences Pour Une Terre Durable: Orléans, France, 2007. [Google Scholar]
- Barry-Macaulay, D.; Bouazza, A.; Singh, R.; Wang, B.; Ranjith, P. Thermal conductivity of soils and rocks from the Melbourne (Australia) region. Eng. Geol. 2013, 164, 131–138. [Google Scholar] [CrossRef] [Green Version]
- Cho, W.; Kwon, S.; Choi, J. The thermal conductivity for granite with various water contents. Eng. Geol. 2009, 107, 167–171. [Google Scholar] [CrossRef]
- Kearney, M.; Shine, R.; Porter, W.P. The potential for behavioral thermoregulation to buffer “cold-blooded” animals against climate warming. Proc. Natl. Acad. Sci. USA 2009, 106, 3835–3840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pincebourde, S.; Salle, A. On the importance of getting fine-scale temperature records near any surface. Glob. Chang. Biol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Vicenzi, N.; Corbalán, V.; Miles, D.; Sinervo, B.; Ibargüengoytía, N. Range increment or range detriment? Predicting potential changes in distribution caused by climate change for the endemic high-Andean lizard Phymaturus palluma. Biol. Conserv. 2017, 206, 151–160. [Google Scholar] [CrossRef]
- Daly, C.; Conklin, D.R.; Unsworth, M.H. Local atmospheric decoupling in complex topography alters climate change impacts. Int. J. Climatol. 2009. [Google Scholar] [CrossRef]
- Ortega, Z.; Mencía, A.; Giroux, A.; Pérez-Mellado, V. Broad seasonal changes in thermoregulation of Podarcis lilfordi (Squamata, Lacertidae) at Binicodrell islet (Menorca, Spain). Herpetozoa 2019, 32, 57–63. [Google Scholar] [CrossRef]
- Gontijo, A.S.B.; Garcia, C.S.; Righi, A.F.; Galdino, C.A. To warm on the rocks, to cool in the wind: Thermal relations of a small-sized lizard from a mountain environment. J. Therm. Biol. 2018, 76, 52–57. [Google Scholar] [CrossRef]
- Ortega, Z.; Mencía, A.; Pérez-Mellado, V. Wind constraints on the thermoregulation of high mountain lizards. Int. J. Biometeorol. 2017, 61, 565–573. [Google Scholar] [CrossRef]
- Rozen-Rechels, D.; Dupoué, A.; Lourdais, O.; Chamaillé-Jammes, S.; Meylan, S.; Clobert, J.; Le Galliard, J. When water interacts with temperature: Ecological and evolutionary implications of thermo-hydroregulation in terrestrial ectotherms. Ecol. Evol. 2019, 9, 10029–10043. [Google Scholar] [CrossRef] [Green Version]
- Enriquez-Urzelai, U.; Kearney, M.R.; Nicieza, A.G.; Tingley, R. Integrating mechanistic and correlative niche models to unravel range-limiting processes in a temperate amphibian. Glob. Chang. Biol. 2019, 25, 2633–2647. [Google Scholar] [CrossRef] [PubMed]
- Guillon, M.; Lourdais, O.; Astruc, G.; Besnard, A. Etude de la Distribution du Lézard Ocellé (Timon lepidus), Intérêt d’une Approche Corrélative et Mécanistique, Présentation du Projet Dans le Cadre du PNA. Unpublished work. 2016. [Google Scholar]
- Hurlbert, S.H.; Levine, R.A.; Utts, J. Coup de Grâce for a Tough Old Bull: “Statistically Significant” Expires. Am. Stat. 2019, 73, 352–357. [Google Scholar] [CrossRef] [Green Version]
- Greenland, S.; Senn, S.J.; Rothman, K.J.; Carlin, J.B.; Poole, C.; Goodman, S.N.; Altman, D.G. Statistical tests, P values, confidence intervals, and power: A guide to misinterpretations. Eur. J. Epidemiol. 2016, 31, 337–350. [Google Scholar] [CrossRef] [Green Version]
- Meteo France. Bulletin Climatique Aquitaine Juillet 2019; Technical Report; Meteo France, CMIR Bordeaux: Paris, France, 2019; Available online: https://donneespubliques.meteofrance.fr/donnees_libres/bulletins/BCMR/BCMR_02_201907.pdf (accessed on 25 August 2020).
- Cano Ortega, A.; Sánchez Sutil, F.J.; De la Casa Hernández, J. Power Factor Compensation Using Teaching Learning Based Optimization and Monitoring System by Cloud Data Logger. Sensors 2019, 19, 2172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganchev, T.D. Ubiquitous computing and biodiversity monitoring. In Advances in Ubiquitous Computing; Elsevier: Amsterdam, The Netherlands, 2020; pp. 239–259. [Google Scholar]
- Turner, W. Sensing biodiversity. Science 2014, 346, 301–302. [Google Scholar] [CrossRef]
- Randin, C.F.; Ashcroft, M.B.; Bolliger, J.; Cavender-Bares, J.; Coops, N.C.; Dullinger, S.; Dirnböck, T.; Eckert, S.; Ellis, E.; Fernández, N.; et al. Monitoring biodiversity in the Anthropocene using remote sensing in species distribution models. Remote Sens. Environ. 2020, 239, 111626. [Google Scholar] [CrossRef]
- Gholizadeh, H.; Gamon, J.A.; Zygielbaum, A.I.; Wang, R.; Schweiger, A.K.; Cavender-Bares, J. Remote sensing of biodiversity: Soil correction and data dimension reduction methods improve assessment of α-diversity (species richness) in prairie ecosystems. Remote Sens. Environ. 2018, 206, 240–253. [Google Scholar] [CrossRef]
- Dittrich, A.; Roilo, S.; Sonnenschein, R.; Cerrato, C.; Ewald, M.; Viterbi, R.; Cord, A.F. Modelling Distributions of Rove Beetles in Mountainous Areas Using Remote Sensing Data. Remote Sens. 2019, 12, 80. [Google Scholar] [CrossRef] [Green Version]
- Buckley, L.B.; Waaser, S.A.; MacLean, H.J.; Fox, R. Does including physiology improve species distribution model predictions of responses to recent climate change? Ecology 2011, 92, 2214–2221. [Google Scholar] [CrossRef] [Green Version]
- Dormann, C.F.; Schymanski, S.J.; Cabral, J.; Chuine, I.; Graham, C.; Hartig, F.; Kearney, M.; Morin, X.; Römermann, C.; Schröder, B.; et al. Correlation and process in species distribution models: Bridging a dichotomy: Bridging the correlation-process dichotomy. J. Biogeogr. 2012, 39, 2119–2131. [Google Scholar] [CrossRef]
- Evans, T.G.; Diamond, S.E.; Kelly, M.W. Mechanistic species distribution modelling as a link between physiology and conservation. Conserv. Physiol. 2015, 3, cov056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vicenzi, N.; Ibarguengoytia, N.; Corbalán, V. Activity Patterns and Thermoregulation Behavior of the Viviparous Lizard Phymaturus palluma in Aconcagua Provincial Park, Agentine Andes. Herpetol. Conserv. Biol. 2019, 14, 337–348. [Google Scholar]
- Lertzman-Lepofsky, G.F.; Kissel, A.M.; Sinervo, B.; Palen, W.J. Water loss and temperature interact to compound amphibian vulnerability to climate change. Glob. Chang Biol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Sai Ram, K.S.; Gupta, A. IoT based Data Logger System for weather monitoring using Wireless sensor networks. Int. J. Eng. Trends Technol. 2016, 32, 71–75. [Google Scholar] [CrossRef]
- Sawarkar, A.; Bramhe, M.V. Real Time Data-Logger and Cloud based Data Management System. Int. J. Res. Eng. Sci. Manag. 2019, 2, 2581–5792. [Google Scholar]
- Rowley, J.J.; Alford, R.A. Models in field studies of temperature and moisture. In Amphibian Ecology and Conservation: A Handbook of Techniques; Kenneth, D.C., Ed.; Techniques in Ecology and Conservation Series; Oxford University Press: Oxford, NY, USA, 2010; pp. 387–406. [Google Scholar]
- Roznik, E.A.; Alford, R.A. Using pairs of physiological models to estimate temporal variation in amphibian body temperature. J. Therm. Biol. 2014, 45, 22–29. [Google Scholar] [CrossRef]
- Blouin-Demers, G.; Weatherhead, P.J. Thermal Ecology of Black Rat Snakes (Elaphe obsoleta) in a Thermally Challenging Environment. Ecology 2001, 82, 3025. [Google Scholar] [CrossRef] [Green Version]
- Albert, C.H.; Yoccoz, N.G.; Edwards, T.C.; Graham, C.H.; Zimmermann, N.E.; Thuiller, W. Sampling in ecology and evolution—Bridging the gap between theory and practice. Ecography 2010, 33, 1028–1037. [Google Scholar] [CrossRef] [Green Version]
- Hurlbert, S.H. Pseudoreplication and the Design of Ecological Field Experiments. Ecol. Monogr. 1984, 54, 187–211. [Google Scholar] [CrossRef] [Green Version]
- Lohr, S.L. Sampling: Design and Analysis, 2nd ed.; Brooks/Cole: Boston, MA, USA, 2010. [Google Scholar]
- McDonald, T.L. Review of Environmental Monitoring Methods: Survey Designs. Environ. Monit. Assess. 2003, 85, 277–292. [Google Scholar] [CrossRef]
- Kermorvant, C.; D’Amico, F.; Bru, N.; Caill-Milly, N.; Robertson, B. Spatially balanced sampling designs for environmental surveys. Environ. Monit. Assess. 2019, 191, 524. [Google Scholar] [CrossRef] [PubMed]
- Kandel, S.; Paepcke, A.; Hellerstein, J.; Heer, J. Wrangler: Interactive visual specification of data transformation scripts. In Proceedings of the 2011 Annual Conference on Human Factors in Computing Systems—CHI’11, Vancouver, BC, Canada, 7–12 May 2011; ACM Press: Vancouver, BC, Canada, 2011; p. 3363. [Google Scholar]
- Van den Broeck, J.; Argeseanu Cunningham, S.; Eeckels, R.; Herbst, K. Data Cleaning: Detecting, diagnosing, and editing data abnormalities. PLoS Med. 2005, 2, e267. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| p-Value | Pseudo-Median [IC] | PI | |
|---|---|---|---|
| An vs. An in 2017 | 8.28 × 10 | −2.08 [−3.00; −1.42] | An: 0.933, An: 1.406 |
| An vs. An in 2018 | 4.87 × 10 | −1.67 [−2.42; −0.83] | An: 1.136, An: 1.510 |
| An vs. An in 2019 | 1.04 × 10 | −0.75 [−1.00; −0.33] | An: 0.902, An: 1.073 |
| Ar vs. Ar in 2019 | 2.65 × 10 | −0.67 [−1.00; −0.42] | Ar: 0.730, Ar: 0.897 |
| 2017 | 2018 | 2019 | |
|---|---|---|---|
| NH | PI HaSM = 29.25 h | PI HaSM = 11.25 h | PI HaSM = 68.40 h |
| SH | PI < 1 → extP = 0.125 | PI HaSM = 34.65 h | PI HaSM = 7.20 h |
| NB | PI < 1 → extP = 0.250 | PI HaSM = 10.58 h | PI HaSM = 4.72 h |
| SB | PI HaSM = 33.30 h | PI < 1 → extP = 0.001 | PI < 1 → extP = 0.032 |
| P | PI HaSM = 43.88 h | ||
| An | PI < 1 → extP = 0.067 | PI HaSM = 30.60 h | PI < 1 → extP = 0.098 |
| An | PI HaSM = 91.35 h | PI HaSM = 114.75 h | PI HaSM = 16.42 h |
| Ar | PI < 1 → extP = 0.270 | ||
| Ar | PI < 1 → extP = 0.103 |
| p-Value | Pseudo-Median [IC] | PI | |
|---|---|---|---|
| NH vs. SH in 2017 | 9.43 × 10 | 1.25 [0.50; 2.00] | NH: 1.130, SH: 0.875 |
| NH vs. SH in 2018 | 0.218 | −0.58 [−1.33; 0.25] | NH: 1.050, SH: 1.154 |
| NH vs. SH in 2019 | 1.29 × 10 | 1.25 [0.58; 1.92] | NH: 1.304, SH: 1.032 |
| NL vs. SL in 2017 | 2.23 × 10 | −1.83 [−2.58; −1.00] | NL: 0.750, SL: 1.148 |
| NL vs. SL in 2018 | 0.187 | 0.42 [−0.17; 0.83] | NL: 1.047, SL: 0.999 |
| NL vs. SL in 2019 | 0.476 | 0.25 [−0.33; 0.92] | NL: 1.021, SL: 0.968 |
| NH vs. NL in 2017 | 2.23 × 10 | 1.67 [0.83; 2.58] | NH: 1.130, NL: 0.750 |
| NH vs. NL in 2018 | 0.902 | 0.08 [−0.75; 0.92] | NH: 1.050, NL: 1.047 |
| NH vs. NL in 2019 | 9.49 × 10 | 1.33 [0.42; 2.25] | NH: 1.304, NL: 1.021 |
| SH vs. SL in 2017 | 5.71 × 10 | −1.25 [−2.00; −0.50] | SH: 0.875, SL: 1.148 |
| SH vs. SL in 2018 | 5.71 × 10 | 0.75 [0.33; 1.17] | SH: 1.154, SL: 0.999 |
| SH vs. SL in 2019 | 0.436 | 0.25 [−0.25; 0.83] | SH: 1.032, SL: 0.968 |
| p-Value | Pseudo-Median [IC] | PI | |
|---|---|---|---|
| P vs. An | 1.06 × 10 | 1.42 [0.42; 2.25] | P: 1.195, An: 0.902 |
| P vs. An | 0.187 | −0.58 [−0.25; 1.42] | P: 1.195, An: 1.073 |
| P vs. Ar | 2.23 × 10 | 2.17 [0.92; 3.17] | P: 1.195, Ar: 0.730 |
| P vs. Ar | 9.37 × 10 | 1.25 [0.42; 2.17] | P: 1.195, Ar: 0.897 |
| An vs. Ar | 9.49 × 10 | 0.83 [0.33; 1.33] | An: 0.902, Ar: 0.730 |
| An vs. Ar | 0.975 | 0.00 [−0.50; 0.58] | An: 0.902, Ar: 0.897 |
| An vs. Ar | 2.23 × 10 | 1.67 [0.92; 2.33] | An: 1.073, Ar: 0.730 |
| An vs. Ar | 2.09 × 10 | 0.92 [0.17; 1.42] | An: 1.073, Ar: 0.897 |
| p-Value | Pseudo-Median [IC] | PI in 2017, 2018, 2019, Mean ± SD | |
|---|---|---|---|
| 2017 vs. 2018 for NH | 0.690 | −0.25 [−0.58; 1.25] | 1.130, 1.050, 1.304, 1.161 ± 0.130 |
| 2017 vs. 2019 for NH | 0.125 | −0.92 [−2.08; 0.17] | |
| 2018 vs. 2019 for NH | 7.37 × 10 * | −1.08 [−2.33; −0.08] * | |
| 2017 vs. 2018 for NL | 1.02 × 10 | −1.33 [−2.25; −0.42] | 0.750, 1.047, 1.021, 0.939 ± 0.164 |
| 2017 vs. 2019 for NL | 1.17 × 10 | −1.08 [−1.83; −0.33] | |
| 2018 vs. 2019 for NL | 0.690 | 0.17 [−0.50; 0.83] | |
| 2017 vs. 2018 for SH | 1.94 × 10 | −1.17 [−2.08; −0.42] | 0.875, 1.154, 1.032, 1.020 ± 0.140 |
| 2017 vs. 2019 for SH | 0.177 | −0.75 [−1.58; 0.17] | |
| 2018 vs. 2019 for SH | 0.187 | 0.50 [−0.25; 1.25] | |
| 2017 vs. 2018 for SL | 0.125 | 0.83 [−0.17; 1.67] | 1.148, 0.999, 0.968, 1.038 ± 0.096 |
| 2017 vs. 2019 for SL | 0.125 * | 1.08 [−0.17; 2.17] * | |
| 2018 vs. 2019 for SL | 0.857 | 0.08 [−0.83; 1.08] | |
| 2017 vs. 2018 for An | 0.106 | −0.75 [−1.83; 0.08] | 0.933, 1.136, 0.902, 0.990 ± 0.127 |
| 2017 vs. 2019 for An | 0.690 | 0.25 [−0.67; 1.08] | |
| 2018 vs. 2019 for An | 5.53 × 10 * | 1.25 [0.17; 2.17] * | |
| 2017 vs. 2018 for An | 0.476 | −0.50 [−1.83; 0.83] | 1.406, 1.510, 1.073, 1.330 ± 0.228 |
| 2017 vs. 2019 for An | 2.78 × 10 | 1.83 [0.42; 2.92] | |
| 2018 vs. 2019 for An | 2.78 × 10 | 2.25 [0.50; 3.67] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hugon, F.; Liquet, B.; D’Amico, F. Multi-Site and Multi-Year Remote Records of Operative Temperatures with Biomimetic Loggers Reveal Spatio-Temporal Variability in Mountain Lizard Activity and Persistence Proxy Estimates. Remote Sens. 2020, 12, 2908. https://0-doi-org.brum.beds.ac.uk/10.3390/rs12182908
Hugon F, Liquet B, D’Amico F. Multi-Site and Multi-Year Remote Records of Operative Temperatures with Biomimetic Loggers Reveal Spatio-Temporal Variability in Mountain Lizard Activity and Persistence Proxy Estimates. Remote Sensing. 2020; 12(18):2908. https://0-doi-org.brum.beds.ac.uk/10.3390/rs12182908
Chicago/Turabian StyleHugon, Florèn, Benoit Liquet, and Frank D’Amico. 2020. "Multi-Site and Multi-Year Remote Records of Operative Temperatures with Biomimetic Loggers Reveal Spatio-Temporal Variability in Mountain Lizard Activity and Persistence Proxy Estimates" Remote Sensing 12, no. 18: 2908. https://0-doi-org.brum.beds.ac.uk/10.3390/rs12182908