Detection of Defoliation Injury in Peanut with Hyperspectral Proximal Remote Sensing
Abstract
:1. Introduction
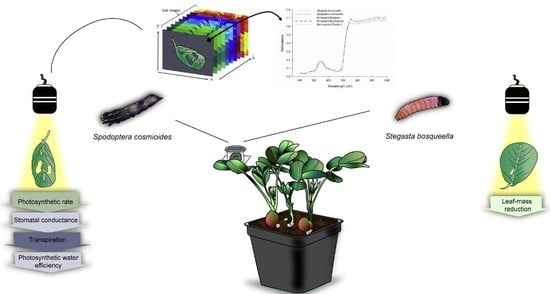
2. Materials and Methods
2.1. Experimental Design
2.2. Test Plants and Insects
2.3. Injury Treatments
2.4. Gas Exchange Measurements
2.5. Hyperspectral Remote Sensing
2.6. Data Analysis
3. Results
3.1. Hyperspectral Images
3.2. Gas Exchange Responses to Herbivory Measurements
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO—Food and Agricultural Organization of the United Nations. Global agriculture towards. In Expert Forum-How Feed World 2050; FAO—Food and Agricultural Organization of the United Nations: Rome, Italy, 2009; Volume 12, pp. 1–4. [Google Scholar]
- EMBRAPA— Brazilian Agricultural Research Corporation. Vision 2014–2034 The Future of Technological Development in Brazilian Agriculture; Embrapa: Brasilia, Brazil, 2014; pp. 1–194. [Google Scholar]
- Deutsch, C.A.; Tewksbury, J.J.; Tigchelaar, M.; Battisti, D.S.; Merrill, S.C.; Huey, R.B.; Naylor, R.L. Increase in crop losses to insect pests in a warming climate. Science 2018, 361, 916–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gebbers, R.; Adamchuk, V.I. Precision Agriculture and Food Security. Science 2010, 327, 828–831. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lee, S.-H.; Chahl, J.S. A review of recent sensing technologies to detect invertebrates on crops. Precis. Agric. 2017, 18, 635–666. [Google Scholar] [CrossRef]
- Nansen, C.; Elliott, N.E. Remote Sensing and Reflectance Profiling in Entomology. Annu. Rev. Entomol. 2016, 61, 139–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behmann, J.; Mahlein, A.-K.; Rumpf, T.; Römer, C.; Plümer, L. A review of advanced machine learning methods for the detection of biotic stress in precision crop protection. Precis. Agric. 2015, 16, 239–260. [Google Scholar] [CrossRef]
- Pedigo, L.P.; Rice, M.E. Entomology and Pest Management: Sixth Edition; Waveland Press, Inc.: Long Grove, IL, USA, 2014; pp. 255–286. [Google Scholar]
- Pedigo, L.P.; Hutchins, S.H.; Higley, L.G. Economic Injury Levels in Theory and Practice. Annu. Rev. Entomol. 1986, 31, 341–368. [Google Scholar] [CrossRef]
- Liu, Z.-Y.; Qi, J.-G.; Wang, N.-N.; Zhu, Z.-R.; Luo, J.; Liu, L.-J.; Tang, J.; Cheng, J.-A. Hyperspectral discrimination of foliar biotic damages in rice using principal component analysis and probabilistic neural network. Precis. Agric. 2018, 19, 973–991. [Google Scholar] [CrossRef]
- El-Ghany, N.M.A.; El-Aziz, S.E.A.; Marei, S.S. A review: Application of remote sensing as a promising strategy for insect pests and diseases management. Environ. Sci. Pollut. Res. 2020, 27, 33503–33515. [Google Scholar] [CrossRef]
- Hunt, E.R.; Rondon, S.I. Detection of potato beetle damage using remote sensing from small unmanned aircraft systems. J. Appl. Remote. Sens. 2017, 11, 11. [Google Scholar] [CrossRef] [Green Version]
- Hatfield, P.; Pinter, P. Remote sensing for crop protection. Crop. Prot. 1993, 12, 403–413. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Gitelson, A.A.; Schepers, J.S.; Walthall, C.L. Application of Spectral Remote Sensing for Agronomic Decisions. Agron. J. 2008, 100, S-117. [Google Scholar] [CrossRef] [Green Version]
- Prabhakar, M.; Prasad, Y.G.; Rao, M.N. Remote Sensing of Biotic Stress in Crop Plants and Its Applications for Pest Management. In Crop Stress and its Management: Perspectives and Strategies; Springer: Berlin/Heidelberg, Germany, 2011; pp. 517–545. [Google Scholar]
- Lillesand, T.; Kiefer, R.W.; Chipman, J. Remote Sensing and Image Interpretation; John Wiley and Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Carter, G.A.; Miller, R.L. Early detection of plant stress by digital imaging within narrow stress-sensitive wavebands. Remote Sens. Environ. 1994, 50, 295–302. [Google Scholar] [CrossRef]
- Peterson, R.K.D. Photosynthesis, yield loss, and injury guilds. In Biotic Stress and Yield Loss; CRC Press: Boca Raton, FL, USA, 2001; pp. 83–97. [Google Scholar]
- Schwachtje, J.; Baldwin, I.T. Why Does Herbivore Attack Reconfigure Primary Metabolism? Plant Physiol. 2008, 146, 845–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto, J.R.L.; Boiça, A.L.; Fernandes, O.A. Biology, Ecology, and Management of Rednecked Peanutworm (Lepidoptera: Gelechiidae). J. Integr. Pest Manag. 2020, 11, 9. [Google Scholar] [CrossRef]
- War, A.R.; Paulraj, M.G.; War, M.Y.; Ignacimuthu, S. Differential defensive response of groundnut germplasms to Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae). J. Plant Interact. 2012, 7, 45–55. [Google Scholar] [CrossRef]
- Abbott, C.C.; Sarver, J.M.; Gore, J.; Cook, D.; Catchot, A.; Henn, R.A.; Krutz, L.J. Establishing Defoliation Thresholds for Insect Pest of Peanut in Mississippi. Peanut Sci. 2019, 46, 1–7. [Google Scholar] [CrossRef]
- Almeida, R.P. Technical Recommendations for Peanut Pest Insect Management; Embrapa Algodão: Campina Grande, Brazil, 2015; pp. 1–15. [Google Scholar]
- Akram, N.A.; Shafiq, F.; Ashraf, M. Peanut (Arachis hypogaea L.): A Prospective Legume Crop to Offer Multiple Health Benefits Under Changing Climate. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1325–1338. [Google Scholar] [CrossRef] [Green Version]
- De Campos, A.P.; Boiça, A.L.; Jesus, F.G.; Godoy, I.J. Evaluation of peanut cultivars for Spodoptera frugiperda resistance. Bragantia 2011, 70, 349–355. [Google Scholar] [CrossRef] [Green Version]
- Junior, A.L.B.; Ferrarezi, R.; Rodrigues, N.E.L.; De Souza, B.H.S.; Bottega, D.B.; Da Silva, A.G. Resistance of peanut cultivars of upright and creeping habits to Spodoptera cosmioides in laboratory. Rev. Agro@mbiente On-Line 2013, 7, 80–88. [Google Scholar] [CrossRef]
- Pinto, J.R.L.; Fernandes, O.A. Parasitism capacity of Telenomus remus and Trichogramma pretiosum on eggs of moth pests of peanut. Bull. Insectol. 2020, 73, 71–78. [Google Scholar]
- Peterson, R.K.D.; Danielson, S.D.; Higley, L.G. Photosynthetic Responses of Alfalfa to Actual and Simulated Alfalfa Weevil (Coleoptera: Curculionidae) Injury. Environ. Entomol. 1992, 21, 501–507. [Google Scholar] [CrossRef]
- Macedo, T.B.; Peterson, R.K.D.; Weaver, D.K.; Morrill, W.L. Wheat Stem Sawfly, Cephus cinctus Norton, Impact on Wheat Primary Metabolism: An Ecophysiological Approach. Environ. Entomol. 2005, 34, 719–726. [Google Scholar] [CrossRef] [Green Version]
- Macedo, T.B.; Peterson, R.K.D.; Dausz, C.L.; Weaver, D.K. Photosynthetic responses of wheat, Triticum aestivum L., to defoliation patterns on individual leaves. Environ. Entomol. 2007, 36, 602–608. [Google Scholar] [CrossRef] [Green Version]
- Godoy, I.J.; Moraes, A.R.A.; Santos, J.F.; Michelotto, M.D.; Bolonhezi, D.; Freitas, R.S.; Cavichioli, J.C.; Carvalho, C.R.L.C.; Martins, A.L.M. High Oleic Peanut Cultivars: An Innovation for the Brazilian Producer and Consumer Market. Available online: http://oagronomico.iac.sp.gov.br/?p=1148 (accessed on 11 October 2020).
- Boiça Junior, A.L.; Ribeiro, Z.A.; Campos, A.P.; Chagas Filho, N.R. Breeding technique and biological parameters of Stegasta bosquella in peanut. Rev. Caatinga 2011, 24, 192–196. [Google Scholar]
- Delaney, K.J.; Haile, F.J.; Peterson, R.K.D.; Higley, L.G. Impairment of Leaf Photosynthesis After Insect Herbivory or Mechanical Injury on Common Milkweed, Asclepias syriaca. Environ. Entomol. 2008, 37, 1332–1343. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine Learning in Python Gaël Varoquaux Bertrand Thirion Vincent Dubourg Alexandre Passos Pedregosa, Varoquaux, Gramfort et al. Matthieu Perrot. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- SAS Institute Inc. SAS/IML® 14.1 User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2015; pp. 1–524. [Google Scholar]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Hawkins, D.M. The problem of overfitting. J. Chem. Inf. Comput. Sci. 2004, 44, 1–12. [Google Scholar] [CrossRef]
- Adelabu, S.; Mutanga, O.; Adam, E. Testing the reliability and stability of the internal accuracy assessment of random forest for classifying tree defoliation levels using different validation methods. Geocarto Int. 2015, 30, 810–821. [Google Scholar] [CrossRef]
- James, G.; Witten, D.; Hastie, T.; Tibshirani, R. An Introduction to Statistical Learning with Applications in R.; Springer: New York, NY, USA, 2013; pp. 303–332. [Google Scholar]
- Higley, L.G.; Browde, J.A.; Higley, P.M. Moving Towards New Understandings of Biotic Stress and Stress Interactions. In Humic Substances and Chemical Contaminants; Wiley: Hoboken, NJ, USA, 2015; pp. 749–754. [Google Scholar]
- Peterson, R.K.D.; Higley, L.G. Illuminating the black box: The relationship between injury and yield. In Biotic Stress and Yield Loss; CRC Press: Boca Raton, FL, USA, 2001; pp. 1–12. [Google Scholar]
- Carter, G.A.; Knapp, A.K. Leaf optical properties in higher plants: Linking spectral characteristics to stress and chlorophyll concentration. Am. J. Bot. 2001, 88, 677–684. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhang, C.; Chen, K.; Kovacs, J.M.; Flores-Verdugo, F.; De Santiago, F.J.F. Spectral response to varying levels of leaf pigments collected from a degraded mangrove forest. J. Appl. Remote Sens. 2012, 6, 63501. [Google Scholar] [CrossRef] [Green Version]
- Abdullah, H.; Darvishzadeh, R.; Wang, T.; Groen, T.A.; Heurich, M. European spruce bark beetle (Ips typographus, L.) green attack affects foliar reflectance and biochemical properties. Int. J. Appl. Earth Obs. Geoinf. 2018, 64, 199–209. [Google Scholar] [CrossRef] [Green Version]
- Slaton, M.R.; Hunt, E.R.; Smith, W.K. Estimating near-infrared leaf reflectance from leaf structural characteristics. Am. J. Bot. 2001, 88, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liao, H.; Zhu, Y.; Sun, J.; Sun, Q.; Liu, X.-D. Hyperspectral detection of rice damaged by rice leaf folder (Cnaphalocrocis medinalis). Comput. Electron. Agric. 2012, 82, 100–107. [Google Scholar] [CrossRef]
- Sudbrink, D.L.; Harris, F.A.; Robbins, J.T.; English, P.J.; Willers, J.L. Evaluation of Remote Sensing to Identify Variability in Cotton Plant Growth and Correlation with Larval Densities of Beet Armyworm and Cabbage Looper (Lepidoptera: Noctuidae). Fla. Entomol. 2003, 86, 290–294. [Google Scholar] [CrossRef]
- Xue, J.; Su, B. Significant Remote Sensing Vegetation Indices: A Review of Developments and Applications. J. Sens. 2017, 2017, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [Green Version]
- Fletcher, R.S.; Reddy, K.N. Random forest and leaf multispectral reflectance data to differentiate three soybean varieties from two pigweeds. Comput. Electron. Agric. 2016, 128, 199–206. [Google Scholar] [CrossRef]
- Bellante, G.; Powell, S.L.; Lawrence, R.L.; Repasky, K.S.; Dougher, T. Hyperspectral Detection of a Subsurface CO2 Leak in the Presence of Water Stressed Vegetation. PLoS ONE 2014, 9, e108299. [Google Scholar] [CrossRef]
- Vitrack-Tamam, S.; Holtzman, L.; Dagan, R.; Levi, S.; Tadmor, Y.; Azizi, T.; Rabinovitz, O.; Naor, A.; Liran, O. Random Forest Algorithm Improves Detection of Physiological Activity Embedded within Reflectance Spectra Using Stomatal Conductance as a Test Case. Remote Sens. 2020, 12, 2213. [Google Scholar] [CrossRef]
- Belgiu, M.; Drăguţ, L. Random forest in remote sensing: A review of applications and future directions. ISPRS J. Photogramm. Remote Sens. 2016, 114, 24–31. [Google Scholar] [CrossRef]
- Du, P.; Samat, A.; Waske, B.; Liu, S.; Li, Z. Random Forest and Rotation Forest for fully polarized SAR image classification using polarimetric and spatial features. ISPRS J. Photogramm. Remote Sens. 2015, 105, 38–53. [Google Scholar] [CrossRef]
- Rodriguez-Galiano, V.; Ghimire, B.; Rogan, J.; Chicaolmo, M.; Rigol-Sanchez, J. An assessment of the effectiveness of a random forest classifier for land-cover classification. ISPRS J. Photogramm. Remote Sens. 2012, 67, 93–104. [Google Scholar] [CrossRef]
- Campbell, J.B.; Wynne, R.H. Introduction to Remote Sensing Fifth Edition; The Guilford Press: New York, NY, USA, 2011; pp. 31–57. [Google Scholar]
- Yang, G.; Liu, J.; Zhao, C.; Li, Z.; Huang, Y.; Yu, H.; Xu, B.; Yang, X.; Zhu, D.; Zhang, X.; et al. Unmanned Aerial Vehicle Remote Sensing for Field-Based Crop Phenotyping: Current Status and Perspectives. Front. Plant Sci. 2017, 8, 1111. [Google Scholar] [CrossRef] [PubMed]
- Hall, F.R.; Ferree, D.C. Effects of Insect Injury Simulation on Photosynthesis of Apple Leaves. J. Econ. Entomol. 1976, 69, 245–248. [Google Scholar] [CrossRef]
- Welter, S.C. Responses of Tomato to Simulated and Real Herbivory by Tobacco Hornworm (Lepidoptera: Sphingidae). Environ. Entomol. 1991, 20, 1537–1541. [Google Scholar] [CrossRef]
- Higley, L.G. New Understandings of Soybean Defoliation and their Implication for Pest Management. In Pest Management in Soybean; Copping, L.G., Green, M.B., Rees, R.T., Eds.; Elsevier: London, UK, 1992; pp. 56–68. [Google Scholar]
- Peterson, R.K.D.; Higley, L.G.; Spomer, S.M. Injury by Hyalaphora cecropia (Lepidoptera: Saturniidae) and Photosynthetic Responses of Apple and Crabapple. Environ. Entomol. 1996, 25, 416–422. [Google Scholar] [CrossRef]
- Peterson, R.K.D.; Shannon, C.L.; Lenssen, A.W. Photosynthetic Responses of Legume Species to Leaf-Mass Consumption Injury. Environ. Entomol. 2004, 33, 450–456. [Google Scholar] [CrossRef] [Green Version]
- Davidson, J.L.; Milthorpe, F.L. The Effect of Defoliation on the Carbon Balance in Dactylis glomerata. Ann. Bot. 1966, 30, 185–198. [Google Scholar] [CrossRef]
- Poston, F.L.; Pedigo, L.P.; Pearce, R.B.; Hammond, R.B. Effects of Artificial and Insect Defoliation on Soybean Net Photosynthesis. J. Econ. Entomol. 1976, 69, 109–112. [Google Scholar] [CrossRef]
- Burkness, E.C.; Hutchison, W.D.; Higley, L.G. Photosynthesis Response of ‘Carolina’ Cucumber to Simulated and Actual Striped Cucumber Beetle (Coleoptera: Chrysomeli-Dae) Defoliation. Insect Sci. 1999, 6, 29–38. [Google Scholar] [CrossRef]
- Wall, R.G.; Berberet, R.C. Reduction in Leaf Area of Spanish Peanuts by the Rednecked Peanutworm. J. Econ. Entomol. 1979, 72, 671–673. [Google Scholar] [CrossRef]
- Higley, L.G.; Pedigo, L.P. Economic Thresholds for Integrated Pest Management; University of Nebraska Press: Lincoln, NE, USA, 1996; pp. 1–327. [Google Scholar]
- Hutchins, S.H.; Higley, L.G.; Pedigo, L.P. Injury Equivalency as a Basis for Developing Multiple-Species Economic Injury Levels. J. Econ. Entomol. 1988, 81, 1–8. [Google Scholar] [CrossRef]
- Baldwin, I.T. Herbivory simulations in ecological research. Trends Ecol. Evol. 1990, 5, 91–93. [Google Scholar] [CrossRef]





| Treatments | Predicted Class | |||||
|---|---|---|---|---|---|---|
| Stegasta bosqueella | Spodoptera cosmioides | Non-Injured (Control) | Classification Overall | User Accuracy | Overall Accuracy | |
| Stegasta bosqueella | 3 | 0 | 0 | 3 | 100% | 92% |
| Spodoptera cosmioides | 0 | 2 | 1 | 3 | 75% | |
| Non-Injured | 0 | 0 | 3 | 3 | 100% | |
| Truth Overall | 3 | 2 | 4 | 9 | ||
| Kappa Statistic | 0.83 | |||||
| 24 h after Infestation | |||||||
|---|---|---|---|---|---|---|---|
| Treatments | 1 A (μmol CO2 m−2 s−1) | 2 Ci (μmol CO2 mol−1) | 3 E (mmol H2O m−2 s−1) | 4 GS (mmol H2O m−2 s−1) | 5 Tleaf (°C) | 6 VPD (kPa) | 7 WUE (mmol CO2 mol−1 H2O) |
| Stegasta bosqueella | 21.06 ± 2.67 a | 843.50 ± 15.06 a | 4.63 ± 0.33 a | 494.80 ± 76.17 a | 25.85 ± 0.22 a | 1.20 ± 0.09 a | 4.43 ± 0.40 a |
| Spodoptera cosmioides | 19.25 ± 1.53 a | 846.40 ± 10.12 a | 4.53 ± 0.27 a | 427.20 ± 54.21 a | 26.02 ± 0.18 a | 1.27 ± 0.07 a | 4.22 ± 0.17 a |
| Simulated Stegasta bosqueella | 19.46 ± 2.11 a | 860.90 ± 8.45 a | 5.03 ± 0.43 a | 611.30 ± 92.76 a | 25.88 ± 0.19 a | 1.14 ± 0.11 a | 3.89 ± 0.25 a |
| Simulated Spodoptera cosmioides | 18.67 ± 1.76 a | 852.70 ± 18.24 a | 4.57 ± 0.26 a | 459.80 ± 57.47 a | 25.79 ± 0.18 a | 1.20 ± 0.07 a | 4.11 ± 0.37 a |
| Non-Injured (Control) | 22.45 ± 2.25 a | 850.60 ± 14.04 a | 5.10 ± 0.31 a | 618.10 ± 88.52 a | 25.82 ± 0.30 a | 1.12 ± 0.11 a | 4.35 ± 0.27 a |
| A (μmol CO2 m−2 s−1) | Ci (μmol CO2 mol−1) | E (mmol H2O m−2 s−1) | GS (mmol H2O m−2 s−1) | Tleaf (°C) | VPD (kPa) | WUE (mmol CO2 mol−1 H2O) | |
| Contrast | p value | p value | p value | p value | p value | p value | p value |
| Stegasta bosqueella × Spodoptera cosmioides | 0.5405 | 0.8424 | 0.8291 | 0.5059 | 0.5886 | 0.6380 | 0.6418 |
| Contrast | A | Ci | E | GS | Tleaf | VPD | WUE |
| p value | p value | p value | p value | p value | p value | p value | |
| Real Injury × Simulated Injury | 0.6019 | 0.2547 | 0.5091 | 0.3016 | 0.6526 | 0.4804 | 0.3043 |
| 48 h after Infestation | |||||||
|---|---|---|---|---|---|---|---|
| Treatments | 1 A (μmol CO2 m−2 s−1) | 2 Ci (μmol CO2 mol−1) | 3 E (mmol H2O m−2 s−1) | 4 GS (mmol H2O m−2 s−1) | 5 Tleaf (°C) | 6 VPD (kPa) | 7 WUE (mmol CO2 mol−1 H2O) |
| Stegasta bosqueella | 24.07 ± 1.35 a | 546.60 ± 16.52 a | 4.53 ± 0.17 a | 405.30 ± 33.78 a | 25.74 ± 0.09 a | 1.27 ± 0.05 a | 5.30 ± 0.22 a |
| Spodoptera cosmioides | 16.57 ± 1.05 b | 563.70 ± 22.71 a | 3.70 ± 0.28 a | 279.80 ± 34.92 a | 25.98 ± 0.11 a | 1.48 ± 0.07 a | 4.53 ± 0.12 a |
| Simulated Stegasta bosqueella | 20.68 ± 1.34 ab | 545.90 ± 18.16 a | 3.99 ± 0.30 a | 322.50 ± 52.98 a | 26.23 ± 0.20 a | 1.49 ± 0.10 a | 5.30 ± 0.27 a |
| Simulated Spodoptera cosmioides | 19.37 ± 2.22 ab | 567.20 ± 16.12 a | 3.84 ± 0.34 a | 308.80 ± 52.07 a | 26.01 ± 0.13 a | 1.48 ± 0.09 a | 4.97 ± 0.31 a |
| Non-Injured (Control) | 20.54 ± 1.86 ab | 542.20 ± 15.12 a | 4.22 ± 0.39 a | 383.90± 66.64 a | 26.04 ± 0.09 a | 1.39 ± 0.10 a | 4.95 ± 0.29 a |
| A (μmol CO2 m−2 s−1) | Ci (μmol CO2 mol−1) | E (mmol H2O m−2 s−1) | GS (mmol H2O m−2 s−1) | Tleaf (°C) | VPD (kPa) | WUE (mmol CO2 mol−1 H2O) | |
| Contrast | p value | p value | p value | p value | p value | p value | p value |
| Stegasta bosqueella × Spodoptera cosmioides | 0.0024 | 0.2956 | 0.0363 | 0.0495 | 0.1835 | 0.0504 | 0.0277 |
| Contrast | A | Ci | E | GS | Tleaf | VPD | WUE |
| p value | p value | p value | p value | p value | p value | p value | |
| Real Injury × Simulated Injury | 0.8570 | 0.9029 | 0.4487 | 0.5416 | 0.0449 | 0.1455 | 0.3582 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto, J.; Powell, S.; Peterson, R.; Rosalen, D.; Fernandes, O. Detection of Defoliation Injury in Peanut with Hyperspectral Proximal Remote Sensing. Remote Sens. 2020, 12, 3828. https://0-doi-org.brum.beds.ac.uk/10.3390/rs12223828
Pinto J, Powell S, Peterson R, Rosalen D, Fernandes O. Detection of Defoliation Injury in Peanut with Hyperspectral Proximal Remote Sensing. Remote Sensing. 2020; 12(22):3828. https://0-doi-org.brum.beds.ac.uk/10.3390/rs12223828
Chicago/Turabian StylePinto, José, Scott Powell, Robert Peterson, David Rosalen, and Odair Fernandes. 2020. "Detection of Defoliation Injury in Peanut with Hyperspectral Proximal Remote Sensing" Remote Sensing 12, no. 22: 3828. https://0-doi-org.brum.beds.ac.uk/10.3390/rs12223828