The Use of Sentinel-2 for Chlorophyll-a Spatial Dynamics Assessment: A Comparative Study on Different Lakes in Northern Germany
Abstract
:1. Introduction
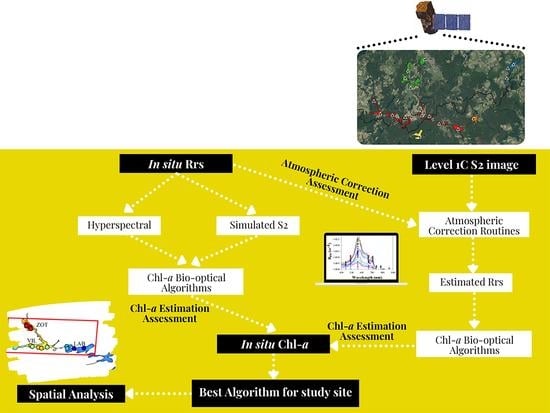
2. Materials and Methods
2.1. Study Site
2.2. In Situ Data Set
2.2.1. Remote Sensing Reflectance
2.2.2. Chl-a Concentration
2.3. Bio-Optical Models for Chl-a
2.4. Image Processing
2.5. Performance Evaluation
3. Results
3.1. Atmospheric Correction Routine Comparison
3.2. Comparison of Chl-a Concentration Estimations for the Entire Data Set
3.3. Comparison of Chl-a Concentration Estimations for the Deep Müritz-Havel Lakes
4. Discussion
4.1. Performance of Algorithms
4.2. Bio-Optical Algorithms Transferability to Different Sensors
4.3. Chl-a Retrieval from the Sentinel-2/MSI Image and Spatial Patterns
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Statistical Metric | 2BDA/ M09A | 2BDA/ G11A | 2BDA/ A14A | 2BDA/ B16A | 3BDA/ M09B | 3BDA/ G11B | 3BDA/ A14B | 3BDA/ B16B | NDCI/ M12A | NDCI/ M12B | NDCI/ A14C | NDCI/ B16C | YA10/ YA10 | GO08/ GO08 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | 0.42 | 0.32 | 0.42 | 0.42 | 0.001 | 0.007 | 0.001 | 0.001 | 0.14 | 0.15 | 0.48 | 0.48 | 0.47 | 0.44 |
| bias | 13.21 | 8.55 | 6.04 | −0.66 | −6.75 | −27.70 | −21.47 | −13.44 | 10.97 | 11.38 | 8.65 | 10.27 | 43.88 | 14.17 |
| MAE | 18.59 | 16.14 | 12.40 | 10.19 | 29.34 | 44.24 | 55.52 | 23.39 | 17.33 | 17.42 | 13.43 | 18.00 | 63.54 | 17.86 |
| MSE | 485.53 | 483.76 | 293.55 | 287.80 | 3742.71 | 28,720.80 | 21,043.93 | 1227.24 | 505.51 | 516.01 | 321.31 | 508.12 | 8655.17 | 451.39 |
| RMSE | 22.03 | 21.99 | 17.13 | 16.96 | 61.18 | 169.47 | 145.06 | 35.03 | 22.48 | 22.71 | 17.92 | 22.54 | 93.03 | 21.24 |
| Statistical Metric | 2BDA/ M09A | 2BDA/ G11A | 2BDA/ A14A | 2BDA/ B16A | 3BDA/ M09B | 3BDA/ G11B | 3BDA/ A14B | 3BDA/ B16B | NDCI/ M12A | NDCI/ M12B | NDCI/ A14C | NDCI/ B16C | YA10/ YA10 | GO08/ GO08 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | 0.40 | 0.32 | 0.40 | 0.40 | 0.002 | 0.01 | 0.002 | 0.002 | 0.24 | 0.25 | 0.46 | 0.46 | 0.38 | 0.43 |
| bias | 10.16 | 6.42 | 3.96 | −3.46 | −1.75 | −14.51 | −8.97 | −11.31 | 11.76 | 12.19 | 6.49 | 4.65 | 48.81 | 11.93 |
| MAE | 15.56 | 13.87 | 11.73 | 10.88 | 22.97 | 30.48 | 36.23 | 21.47 | 16.10 | 16.27 | 12.69 | 13.14 | 72.24 | 15.60 |
| MSE | 392.05 | 403.57 | 279.96 | 287.20 | 2304.78 | 12,981.36 | 11,894.81 | 924.42 | 474.60 | 482.69 | 310.44 | 335.84 | 11,712.69 | 394.83 |
| RMSE | 19.80 | 20.09 | 16.73 | 16.95 | 48.01 | 113.93 | 109.06 | 30.40 | 21.78 | 21.97 | 17.62 | 18.33 | 108.22 | 19.87 |
| Statistical Metric | 2BDA/ M09A | 2BDA/ G11A | 2BDA/ G10A | 2BDA/ A14A | 2BDA/ B16A | 3BDA/ M09B | 3BDA/ G11B | 3BDA/ A14B | 3BDA/ B16B | NDCI/ M12A | NDCI/ M12B | NDCI/ A14C | NDCI/ B16C | YA10/ YA10 | GO08/ GO08 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | 0.93 | 0.94 | 0.93 | 0.93 | 0.93 | 0.94 | 0.93 | 0.93 | 0.72 | 0.85 | 0.90 | 0.90 | 0.87 | 0.94 | |
| bias | −7.60 | −4.60 | −4.97 | −0.03 | −3.85 | −7.29 | −2.03 | −26.00 | 8.53 | 1847.14 | 17.91 | 15.12 | −30.48 | −75.29 | 452.97 |
| MAE | 7.82 | 4.91 | 5.20 | 3.54 | 6.55 | 7.51 | 3.13 | 26.91 | 8.98 | 1848.33 | 28.31 | 21.08 | 30.48 | 83.77 | 460.71 |
| MSE | 91.66 | 42.63 | 56.06 | 32.62 | 65.73 | 85.52 | 27.05 | 1118.88 | 108.74 | 6,214,128.10 | 1205.85 | 645.09 | 1605.96 | 11,403.78 | 899,341.67 |
| RMSE | 9.57 | 6.53 | 7.49 | 5.71 | 8.11 | 9.25 | 5.20 | 33.45 | 10.43 | 2492.82 | 34.73 | 25.40 | 40.07 | 106.79 | 948.34 |
| Statistical Metric | 2BDA/ M09A | 2BDA/ G11A | 2BDA/ A14A | 2BDA/ B16A | 3BDA/ M09B | 3BDA/ G11B | 3BDA/ A14B | 3BDA/ B16B | NDCI/ M12A | NDCI/ M12B | NDCI/ A14C | NDCI/ B16C | YA10/ YA10 | GO08/ GO08 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | 0.69 | 0.59 | 0.69 | 0.69 | 0.000 | 0.001 | 0.000 | 0.000 | 0.20 | 0.21 | 0.71 | 0.71 | 0.66 | 0.68 |
| bias | 17.45 | 12.86 | 8.98 | 3.12 | −4.01 | −14.74 | −15.02 | −12.24 | 12.79 | 13.34 | 10.92 | 15.66 | 44.45 | 17.54 |
| MAE | 18.96 | 15.29 | 10.55 | 7.04 | 25.08 | 31.05 | 44.22 | 21.04 | 16.64 | 16.84 | 11.75 | 17.85 | 53.93 | 18.39 |
| MSE | 423.47 | 328.85 | 193.48 | 120.25 | 1777.25 | 5899.48 | 9552.48 | 741.46 | 435.94 | 447.71 | 247.49 | 456.33 | 5306.31 | 419.30 |
| RMSE | 20.58 | 18.13 | 13.91 | 10.97 | 42.16 | 76.81 | 97.74 | 27.23 | 20.88 | 21.16 | 15.73 | 21.36 | 72.84 | 20.48 |
| Statistical Metric | 2BDA/ M09A | 2BDA/ G11A | 2BDA/ A14A | 2BDA/ B16A | 3BDA/ M09B | 3BDA/ G11B | 3BDA/ A14B | 3BDA/ B16B | NDCI/ M12A | NDCI/ M12B | NDCI/ A14C | NDCI/ B16C | YA10/ YA10 | GO08/ GO08 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | 0.63 | 0.56 | 0.63 | 0.63 | 0.03 | 0.02 | 0.03 | 0.03 | 0.40 | 0.41 | 0.65 | 0.65 | 0.51 | 0.62 |
| bias | 8.54 | 6.34 | 4.00 | −0.19 | 1.36 | −0.98 | 0.28 | −5.98 | 8.59 | 8.95 | 5.15 | 5.46 | 30.03 | 9.23 |
| MAE | 9.51 | 7.80 | 5.93 | 4.88 | 11.41 | 11.14 | 13.81 | 11.84 | 9.56 | 9.77 | 6.63 | 7.19 | 40.55 | 9.75 |
| MSE | 198.43 | 163.51 | 113.37 | 79.84 | 411.49 | 341.63 | 1200.57 | 321.15 | 255.03 | 261.26 | 144.96 | 149.02 | 5304.52 | 222.90 |
| RMSE | 14.09 | 12.79 | 10.65 | 8.93 | 20.28 | 18.48 | 34.65 | 17.92 | 15.97 | 16.16 | 12.04 | 12.21 | 72.83 | 14.93 |

References
- Cole, J.J.; Praire, Y.T.; Caraco, N.F.; McDowell, W.H.; Tranvik, L.J.; Striegl, R.G.; Duarte, C.M.; Kortelainen, P.; Downing, J.A.; Middelburg, J.J.; et al. Plumbing the Global Carbon Cycle: Integrating Inland Waters into the Terrestrial Carbon Budget. Ecosystems 2007, 10, 171–184. [Google Scholar] [CrossRef] [Green Version]
- Williamson, C.E.; Dodds, W.; Kratz, T.K.; Palmer, M.A. Lakes and streams as sentinels of environmental change in terrestrial and atmospheric processes. Front. Ecol. Environ. 2008, 6, 247–254. [Google Scholar] [CrossRef]
- Tundisi, J.G.; Matsumura-Tundisi, T.; Tundisi, J.E.M. Reservoirs and human well being: New challenges for evaluating impacts and benefits in the neotropics. Braz. J. Biol. 2008, 68, 1133–1135. [Google Scholar] [CrossRef]
- Häder, D.-P.; Banaszak, A.T.; Villafañe, V.E.; Narvarte, M.A.; González, R.A.; Helbling, E.W. Anthropogenic pollution of aquatic ecosystems: Emerging problems with global implications. Sci. Total Environ. 2020, 713, 136586. [Google Scholar] [CrossRef]
- Wurtsbaugh, W.A.; Paerl, H.W.; Dodds, W.K. Nutrients, eutrophication and harmful algal blooms along the freshwater to marine continuum. Wiley Interdiscip. Rev. Water 2019, 6, e1373. [Google Scholar] [CrossRef]
- UNEP International Environmental Technology Centre. Planning and Management of Lakes and Reservoirs: An Integrated Approach to Eutrophication, 1st ed.; UNEP International Environmental Technology Centre: Shiga, Japan, 1999. [Google Scholar]
- Reinart, A.; Kutser, T. Comparison of different satellite sensors in detecting cyanobacterial bloom events in the Baltic Sea. Remote Sens. Environ. 2006, 102, 74–85. [Google Scholar] [CrossRef]
- Moses, W.J.; Gitelson, A.A.; Berdnikov, S.; Povazhnyy, V. Estimation of chlorophyll-a concentration in case II waters using MODIS and MERIS data—Successes and challenges. Environ. Res. Lett. 2009, 4, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Duan, H.; Ma, R.; Xu, J.; Zhang, Y.; Zhang, B. Comparison of different semi-empirical algorithms to estimate chlorophyll-a concentration in inland lake water. Environ. Monit. Assess. 2010, 170, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Stech, J.L.; Lima, I.B.T.; Novo, E.M.L.M.; Silva, C.M.; Assireu, A.T.; Lorenzzetti, J.A. Telemetric monitoring system for meteorological and limnological data acquisition. Int. Ver. Theor. Angew. Limnol. 2006, 29, 747–1750. [Google Scholar] [CrossRef]
- Marcé, R.; George, G.; Buscarinu, P.; Deidda, M.; Dunalska, J.; de Eyto, E.; Flaim, G.; Grossart, H.-P.; Istvanovics, V.; Lenhardt, M.; et al. Automatic High Frequency Monitoring for Improved Lake and Reservoir Management. Environ. Sci. Technol. 2016, 50, 10780–10794. [Google Scholar] [CrossRef]
- Gons, H.J.; Auer, M.T.; Effler, S.W. MERIS satellite chlorophyll mapping of oligotrophic and eutrophic waters in the Laurentian Great Lakes. Remote Sens. Environ. 2008, 112, 4098–4106. [Google Scholar] [CrossRef]
- Dall’Olmo, G.; Gitelson, A.A. Effect of bio-optical parameter variability on the remote estimation of chlorophyll-a concentration in turbid productive waters: Experimental results. Appl. Opt. 2005, 44, 412–422. [Google Scholar] [CrossRef] [Green Version]
- Mishra, S.; Mishra, D.R. Normalized difference chlorophyll index: A novel model for remote estimation of chlorophyll-a concentration in turbid productive waters. Remote Sens. Environ. 2012, 117, 394–406. [Google Scholar] [CrossRef]
- Olmanson, L.G.; Bauer, M.E.; Brezonik, P.L. A 20-year Landsat water clarity census of Minnesota’s 10,000 lakes. Remote Sens. Environ. 2008, 112, 4086–4097. [Google Scholar] [CrossRef]
- Alikas, K.; Kratzer, S. Improved retrieval of Secchi depth for optically-complex waters using remote sensing data. Ecol. Indic. 2017, 77, 218–227. [Google Scholar] [CrossRef]
- Hadijimitsis, D.G.; Clayton, C. Assessment of temporal variations of water quality in inland water bodies using atmospheric corrected satellite remotely sensed image data. Environ. Monit. Assess. 2009, 159, 281–292. [Google Scholar] [CrossRef]
- Verpoorter, C.; Kutser, T.; Seekell, D.A.; Tranvik, L.J. A global inventory of lakes based on high-resolution satellite imagery. Geophys. Res. Lett. 2014, 41, 6396–6402. [Google Scholar] [CrossRef]
- Cael, B.B.; Seekell, D.A. The size-distribution of Earth’s lakes. Sci. Rep. 2016, 6, 29633. [Google Scholar] [CrossRef] [Green Version]
- Toming, K.; Kutser, T.; Laas, A.; Sepp, M.; Paavel, B.; Nõges, T. First experiences in mapping lake water quality parameters with Sentinel-2 MSI imagery. Remote Sens. 2016, 8, 640. [Google Scholar] [CrossRef] [Green Version]
- Gitelson, A. The peak near 700 nm on radiance spectra of algae and water: Relationships of its magnitude and position with chlorophyll concentration. Int. J. Remote Sens. 1992, 13, 3367–3373. [Google Scholar] [CrossRef]
- Mouw, C.B.; Greb, S.; Aurin, D.; DiGiacomo, P.M.; Lee, Z.; Twardowski, M.; Binding, C.; Hu, C.; Ma, R.; Moore, T.; et al. Aquatic color radiometry remote sensing of coastal and inland waters: Challenges and recommendations for future satellite missions. Remote Sens. Environ. 2015, 160, 15–30. [Google Scholar] [CrossRef]
- Moses, W.J.; Sterckx, S.; Montes, M.J.; Keukelaere, L.D.; Knaeps, E. Atmospheric Correction for Inland Waters. In Bio-Optical Modeling and Remote Sensing of Inland Waters, 1st ed.; Mishra, D.R., Ogashawara, I., Gitelson, A.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 69–100. [Google Scholar]
- Ruddick, K.G.; Ovidio, F.; Rijkeboer, M. Atmospheric correction of SeaWiFS imagery for turbid coastal and inland waters. Appl. Opt. 2000, 39, 897–912. [Google Scholar] [CrossRef] [Green Version]
- Ha, N.T.T.; Thao, N.T.P.; Koike, K.; Nhuan, M.T. Selecting the best band ratio to estimate chlorophyll-a concentration in a tropical freshwater lake using sentinel 2A images from a case study of Lake Ba Be (Northern Vietnam). ISPRS Int. J. Geo-Inf. 2017, 6, 290. [Google Scholar] [CrossRef]
- Bresciani, M.; Cazzaniga, I.; Austoni, M.; Sforzi, T.; Buzzi, F.; Morabito, G.; Giardino, C. Mapping phytoplankton blooms in deep subalpine lakes from Sentinel-2A and Landsat-8. Hydrobiologia 2018, 824, 197–214. [Google Scholar] [CrossRef] [Green Version]
- Giardino, C.; Candiani, G.; Bresciani, M.; Lee, Z.; Gagliano, S.; Pepe, M. BOMBER: A tool for estimating water quality and bottom properties from remote sensing images. Comput. Geosci. 2012, 45, 313–318. [Google Scholar] [CrossRef]
- Li, S.; Song, K.; Wang, S.; Liu, G.; Wen, Z.; Shang, Y.; Lyu, L.; Chen, F.; Xu, S.; Tao, H.; et al. Quantification of chlorophyll-a in typical lakes across China using Sentinel-2 MSI imagery with machine learning algorithm. Sci. Total Environ. 2021, 778, 146271. [Google Scholar] [CrossRef]
- Ansper, A.; Alikas, K. Retrieval of chlorophyll a from Sentinel-2 MSI data for the European Union water framework directive reporting purposes. Remote Sens. 2019, 11, 64. [Google Scholar] [CrossRef] [Green Version]
- Thiemann, S.; Kaufmann, H. Determination of chlorophyll content and trophic state of lakes using field spectrometer and IRS-1C satellite data in the Mecklenburg Lake District, Germany. Remote Sens. Environ. 2000, 73, 227–235. [Google Scholar] [CrossRef]
- Mantzouki, E.; Campbell, J.; Van Loon, E.; Visser, P.; Konstantinou, I.; Antoniou, M.; Giuliani, G.; Machado-Vieira, D.; De Oliveira, A.G.; Maronić, D.Š.; et al. A European Multi Lake Survey dataset of environmental variables, phytoplankton pigments and cyanotoxins. Sci. Data 2018, 5, 180226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LAWA. Trophieklassifikation von Seen. Richtlinie zur Ermittlung des Trophie-Index nach LAWA für natürliche Seen, Baggerseen, Talsperren und Speicherseen. Empfehlungen Oberirdische Gewässer. In Hrsg. LAWA—Bund/Länder Arbeitsgemeinschaft Wasser. 34 S. zzgl. Access-Auswertetool; Riedmüller, U., Hoehn, E., Mischke, U., Eds.; LAWA: Munich, Germany, 2014. [Google Scholar]
- Seensteckbriefe des Landes Brandenburg. Available online: https://lfu.brandenburg.de/lfu/de/aufgaben/wasser/fliessgewaesser-und-seen/gewaesserzustandsbewertung/seensteckbriefe/ (accessed on 8 February 2021).
- Ogashawara, I.; Jechow, A.; Kiel, C.; Kohnert, K.; Berger, S.A.; Wollrab, S. Performance of the Landsat 8 provisional aquatic reflectance product for inland waters. Remote Sens. 2020, 12, 2410. [Google Scholar] [CrossRef]
- Mobley, C.D. Estimation of the remote-sensing reflectance from above-surface measurements. Appl. Opt. 1999, 38, 7442–7455. [Google Scholar] [CrossRef]
- Wright, S.W.; Jeffrey, S.W. High-resolution HPLC system for chlorophylls and carotenoids of marine phytoplankton. In Pigments in Oceanography; Jeffrey, S.W., Mantoura, R.F.C., Wright, S.W., Eds.; UNESCO: Paris, France, 1997; pp. 327–341. [Google Scholar]
- Shatwell, T.; Nicklisch, A.; Köhler, J. Temperature and photoperiod effects on phytoplankton growing under simulated mixed layer light fluctuations. Limnol. Oceanogr. 2012, 57, 541–553. [Google Scholar] [CrossRef]
- Barlow, R.G.; Cummings, D.G.; Gibb, S.W. Improved resolution of mono- and divenyl chlorophylls a and b and zeaxanthin and lutein in phytoplankton extracts using reverse phase C-8 HPLC. Mar. Ecol. Prog. Ser. 1997, 161, 303–307. [Google Scholar] [CrossRef]
- Gurlin, D.; Gitelson, A.A.; Moses, W.J. Remote estimation of chl-a concentration in turbid productive waters—Return to a simple two-band NIR-red model? Remote Sens. Environ. 2011, 115, 3479–3490. [Google Scholar] [CrossRef]
- Augusto-Silva, P.B.; Ogashawara, I.; Barbosa, C.C.F.; de Carvalho, L.A.S.; Jorge, D.S.F.; Fornari, C.I.; Stech, J.L. Analysis of MERIS reflectance algorithms for estimating chlorophyll-a concentration in a Brazilian reservoir. Remote Sens. 2014, 6, 11689–11707. [Google Scholar] [CrossRef] [Green Version]
- Beck, R.; Zhan, S.; Liu, H.; Tong, S.; Yang, B.; Xu, M.; Ye, Z.; Huang, Y.; Shu, S.; Wu, Q.; et al. Comparison of satellite reflectance algorithms for estimating chlorophyll-a in a temperate reservoir using coincident hyperspectral aircraft imagery and dense coincident surface observations. Remote Sens. Environ. 2016, 178, 15–30. [Google Scholar] [CrossRef] [Green Version]
- Neil, C.; Spyrakos, E.; Hunter, P.D.; Tyler, A.N. A global approach for chlorophyll-a retrieval across optically complex inland waters based on optical water types. Remote Sens. Environ. 2019, 229, 159–178. [Google Scholar] [CrossRef]
- Yang, W.; Matsushita, B.; Chen, J.; Fukushima, B.; Ma, R. An enhanced three-band index for estimating chlorophyll-a in turbid case-II waters: Case studies of Lake Kasumigaura, Japan, and Lake Dianchi, China. IEEE Geosci. Remote Sens. Lett. 2010, 7, 655–659. [Google Scholar] [CrossRef] [Green Version]
- Gilerson, A.A.; Gitelson, A.A.; Zhou, J.; Gurlin, D.; Moses, W.; Loannou, L.; Ahmed, S.A. Algorithms for remote estimation of chlorophyll-a in coastal and inland waters using red and near infrared bands. Opt. Express 2010, 18, 24109–24125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanhellemont, Q.; Ruddick, K. Atmospheric correction of metre-scale optical satellite data for inland and coastal water applications. Remote Sens. Environ. 2018, 216, 586–597. [Google Scholar] [CrossRef]
- Vanhellemont, Q. Adaptation of the dark spectrum fitting atmospheric correction for aquatic applications of the Landsat and Sentinel-2 archives. Remote Sens. Environ. 2019, 225, 175–192. [Google Scholar] [CrossRef]
- Vanhellemont, Q. Sensitivity analysis of the dark spectrum fitting atmospheric correction for metre-and decametre-scale satellite imagery using autonomous hyperspectral radiometry. Opt. Express 2020, 28, 29948–29965. [Google Scholar] [CrossRef]
- Doerffer, R.; Schiller, H. The MERIS Case 2 water algorithm. Int. J. Remote Sens. 2007, 28, 517–535. [Google Scholar] [CrossRef]
- Doerffer, R.; Schiller, H. The MERIS neural network algorithm. In Remote Sensing of Inherent Optical Properties: Fundamentals, Tests of Algorithms, and Applications; Lee, Z.P., Ed.; IOCCG: Dartmouth, NS, Canada, 2007. [Google Scholar]
- Brockmann, C.; Doerffer, R.; Peters, M.; Stelzer, K.; Embacher, S.; Ruescas, A. Evolution of the C2RCC Neural Network for Sentinel 2 and 3 for the Retrieval of Ocean Colour Products in Normal and Extreme Optically Complex Water; ESA: Prague, Czech Republic, 2016; Volume SP-740, pp. 9–13. [Google Scholar]
- De Keukelaere, L.; Sterckx, S.; Adriaensen, S.; Knaeps, E.; Reusen, I.; Giardino, C. Atmospheric correction of Landsat-8/OLI and Sentinel-2/MSI data using iCOR algorithm: Validation for coastal and inland waters. Eur. J. Remote Sens. 2018, 51, 525–542. [Google Scholar] [CrossRef] [Green Version]
- Page, B.P.; Olmanson, L.G.; Mishra, D.R. A harmonized image processing workflow using Sentinel-2/MSI and Landsat-8/OLI for mapping water clarity in optically variable lake systems. Remote Sens. Environ. 2019, 231, 111284. [Google Scholar] [CrossRef]
- ESA. Sen2Cor Software Release Note. Available online: http://step.esa.int/thirdparties/sen2cor/2.8.0/docs/S2-PDGS-MPC-L2A-SRN-V2.8.pdf (accessed on 24 March 2021).
- Chami, M.; Santer, R.; Dilligeard, E. Radiative transfer model for the computation of radiance and polarization in an ocean–atmosphere system: Polarization properties of suspended matter for remote sensing. Appl. Opt. 2001, 40, 2398–2416. [Google Scholar] [CrossRef] [PubMed]
- Brewin, R.J.; Sathyendranath, S.; Müller, D.; Brockmann, C.; Deschamps, P.-Y.; Devred, E.; Doerffer, R.; Fomferra, N.; Franz, B.; Grant, M. The ocean colour climate change initiative: Iii. A round-robin comparison on in-water bio-optical algorithms. Remote Sens. Environ. 2015, 162, 271–294. [Google Scholar] [CrossRef] [Green Version]
- Warren, M.A.; Simis, S.G.H.; Martinez-Vicente, V.; Poser, K.; Bresciani, M.; Alikas, K.; Spyrakos, E.; Giardino, C.; Ansper, A. Assessment of atmospheric correction algorithms for the Sentinel-2A MultiSpectral Imager over coastal and inland waters. Remote Sens. Environ. 2019, 225, 267–289. [Google Scholar] [CrossRef]
- Odermatt, D.; Kiselev, S.; Heege, T.; Kneubuhler, M.; Itten, K.I. Adjacency effect consideration and air/water constituent retrieval for Lake Constance. In Proceedings of the 2nd MERIS/(A)ATSR Workshop; Lacoste, H., Ouwehand, L., Eds.; ESA-ESRIN: Frascati, Italy, 2008. [Google Scholar]
- Soomets, T.; Uudeberg, K.; Jakovels, D.; Brauns, A.; Zagars, M.; Kutser, T. Validation and Comparison of Water Quality Products in Baltic Lakes Using Sentinel-2 MSI and Sentinel-3 OLCI Data. Sensors 2020, 20, 742. [Google Scholar] [CrossRef] [Green Version]
- Devlin, M.; Schroeder, T.; McKinna, L.; Brodie, J.; Brando, V.; Dekker, A. Combining in-situ water quality and remotely sensed data across spatial and temporal scales to measure variability in wet season chlorophyll-a: Great Barrier Reef lagoon (Queensland, Australia). Ecol. Process. 2013, 2, 31. [Google Scholar] [CrossRef] [Green Version]









| Group | Lake | Acronym | Latitude | Longitude | Area (km2) | Mean Depth (m) | Retention Time (year) | Trophic Index (LAWA) [32] |
|---|---|---|---|---|---|---|---|---|
| Deep Müritz-Havel Lakes | Ellbogensee | ELL | 53.21 | 13.04 | 1.74 | 7.60 | 0.10 | 2.90 |
| Deep Müritz-Havel Lakes | Großer Pälitzsee | GPA | 53.20 | 12.99 | 2.67 | 7.92 | 0.07 | 2.60 |
| Deep Müritz-Havel Lakes | Kleiner Pälitzsee | KPA | 53.20 | 12.96 | 2.08 | 9.30 | 0.08 | 2.90 |
| Deep Müritz-Havel Lakes | Labussee | LAB | 53.21 | 12.90 | 2.58 | 7.00 | 0.22 | 2.90 |
| Deep Müritz-Havel Lakes | Großer Priepertsee | PRI | 53.22 | 13.03 | 1.05 | 10.80 | 0.16 | 3.20 |
| Deep Müritz-Havel Lakes | Röblinsee | ROB | 53.18 | 13.12 | 0.87 | 3.70 | 7.00 | 3.40 |
| Deep Müritz-Havel Lakes | Stolpsee | STO | 53.17 | 13.21 | 3.71 | 6.64 | 3.00 | 2.35 |
| Deep Müritz-Havel Lakes | Vilzsee | VIL | 53.21 | 12.84 | 2.00 | 7.90 | 0.22 | 3.20 |
| Deep Müritz-Havel Lakes | Ziernsee | ZIE | 53.20 | 13.07 | 1.12 | 6.00 | 0.05 | 2.80 |
| Deep Müritz-Havel Lakes | Zotzensee | ZOT | 53.24 | 12.81 | 1.49 | 6.70 | 0.15 | 3.30 |
| Shallow Havel Lakes | Großer Labussee | GLA | 53.31 | 12.95 | 3.35 | 4.10 | 0.55 | 2.80 |
| Shallow Havel Lakes | Useriner See | USE | 53.33 | 12.97 | 3.76 | 4.63 | 0.72 | 2.80 |
| Shallow Havel Lakes | Woblitzsee | WOB | 53.28 | 12.98 | 5.04 | 3.93 | 0.34 | 3.30 |
| Shallow Havel Lakes | Zierker See | ZIS | 53.36 | 13.04 | 3.50 | 1.62 | 1.12 | 3.70 |
| Individual | Stechlinsee | STE | 53.15 | 13.03 | 4.52 | 23.00 | 65.00 | 1.70 |
| Individual | Großer Lychensee | GLY | 53.20 | 13.28 | 2.94 | 7.20 | 3.00 | 3.20 |
| Deep Feldberg Lakes | Breiter Luzin | BLU | 53.36 | 13.46 | 3.37 | 22.80 | 16.25 | 1.90 |
| Deep Feldberg Lakes | Feldberger Haussee | FEH | 53.35 | 13.44 | 1.32 | 5.83 | 3.05 | 2.10 |
| Deep Feldberg Lakes | Schmaler Luzin | SLU | 53.32 | 13.43 | 1.46 | 14.49 | 3.35 | 1.40 |
| Algorithm 1 | Formula 2 | Measured Chl-a (mg/m3) |
|---|---|---|
| 2BDA | 4.4–217.3 | |
| GO08 | where: | 2–994.0 |
| 3BDA | 4.4–217.3 | |
| YA10 | 0–100.0 | |
| NDCI | 0.9–28.17 |
| Algorithm | Calibration 1 | Coefficients 2 | Retrieved Chl-a Range (mg/m3) |
|---|---|---|---|
| 2BDA | M09A | 0–70 | |
| 2BDA | G10A | 0–1000 | |
| 2BDA | G11A | 2.3–200.8 | |
| 2BDA | A14A | 2.3–306.03 | |
| 2BDA | B16A | 30–80 | |
| GO08 | GO08 | 0–100 | |
| 3BDA | M09B | 0–70 | |
| 3BDA | G10B | 0–1000 | |
| 3BDA | G11B | 2.3–200.8 | |
| 3BDA | A14B | 2.3–306.03 | |
| 3BDA | B16B | 30–80 | |
| YA10 | YA10 | 0–100 | |
| NDCI | M12A | 14.35–28.17 | |
| NDCI | M12B | 0.90–16.06 | |
| NDCI | A14C | 2.3–306.03 | |
| NDCI | B16C | 30–80 |
| Metric | Formula 1,2,3 | Score 4 |
|---|---|---|
| R2 | 0 point: >±2 σ from 1 1 point: ±2 σ from 1 2 points: ±1 σ from 1 | |
| bias | Per metric: 0 point: >±2 σ from 0 1 point: ±2 σ from 0 2 points: ±1 σ from 0 | |
| MAE | ||
| MSE | ||
| RMSE |
| Spectral Band Center | ACOLITE | C2RCC | C2X | iCOR | MAIN | Sen2Cor | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | RMSE | R2 | RMSE | R2 | RMSE | R2 | RMSE | R2 | RMSE | R2 | RMSE | |
| 443 nm | 0.79 | 0.001 | 0.41 | 0.001 | 0.61 | 0.001 | 0.72 | 0.004 | 0.49 | 0.007 | 0.64 | 0.003 |
| 490 nm | 0.90 | 0.002 | 0.70 | 0.002 | 0.76 | 0.003 | 0.87 | 0.006 | 0.81 | 0.012 | 0.87 | 0.005 |
| 560 nm | 0.97 | 0.012 | 0.95 | 0.008 | 0.89 | 0.011 | 0.97 | 0.014 | 0.95 | 0.024 | 0.96 | 0.014 |
| 665 nm | 0.91 | 0.004 | 0.93 | 0.003 | 0.94 | 0.003 | 0.91 | 0.005 | 0.95 | 0.011 | 0.95 | 0.005 |
| 705 nm | 0.93 | 0.004 | 0.95 | 0.002 | 0.97 | 0.004 | 0.94 | 0.005 | 0.97 | 0.010 | 0.95 | 0.005 |
| 740 nm | 0.21 | 0.003 | 0.14 | 0.003 | 0.19 | 0.002 | 0.09 | 0.004 | 0.06 | 0.008 | 0.00 | 0.002 |
| Sensor | Algorithm/Calibration | R2 | RMSE | p-Values |
|---|---|---|---|---|
| Hyperspectral (19 lakes) | 2BDA/B16A | 0.42 | 16.96 | <0.001 |
| Simulates MSI (19 lakes) | 2BDA/A14A | 0.40 | 16.73 | <0.001 |
| Hyperspectral (10 lakes) | 2BDA/B16A | 0.69 | 10.97 | <0.001 |
| Simulates MSI (10 lakes) | 2BDA/B16A | 0.63 | 8.93 | <0.001 |
| MSI (6 lakes) | 2BDA/A14A | 0.93 | 5.71 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogashawara, I.; Kiel, C.; Jechow, A.; Kohnert, K.; Ruhtz, T.; Grossart, H.-P.; Hölker, F.; Nejstgaard, J.C.; Berger, S.A.; Wollrab, S. The Use of Sentinel-2 for Chlorophyll-a Spatial Dynamics Assessment: A Comparative Study on Different Lakes in Northern Germany. Remote Sens. 2021, 13, 1542. https://0-doi-org.brum.beds.ac.uk/10.3390/rs13081542
Ogashawara I, Kiel C, Jechow A, Kohnert K, Ruhtz T, Grossart H-P, Hölker F, Nejstgaard JC, Berger SA, Wollrab S. The Use of Sentinel-2 for Chlorophyll-a Spatial Dynamics Assessment: A Comparative Study on Different Lakes in Northern Germany. Remote Sensing. 2021; 13(8):1542. https://0-doi-org.brum.beds.ac.uk/10.3390/rs13081542
Chicago/Turabian StyleOgashawara, Igor, Christine Kiel, Andreas Jechow, Katrin Kohnert, Thomas Ruhtz, Hans-Peter Grossart, Franz Hölker, Jens C. Nejstgaard, Stella A. Berger, and Sabine Wollrab. 2021. "The Use of Sentinel-2 for Chlorophyll-a Spatial Dynamics Assessment: A Comparative Study on Different Lakes in Northern Germany" Remote Sensing 13, no. 8: 1542. https://0-doi-org.brum.beds.ac.uk/10.3390/rs13081542