Regional Monitoring of Fall Armyworm (FAW) Using Early Warning Systems
Abstract
:1. Introduction
2. Materials and Methods
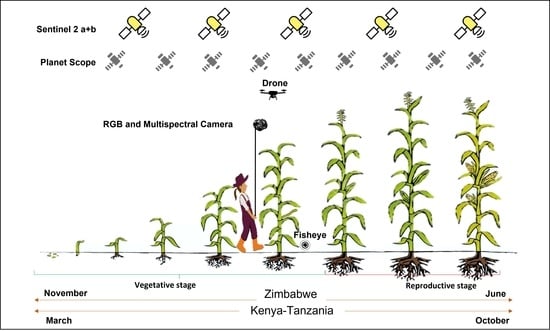
2.1. Study Sites
2.2. Cloud-based Process Satellite Data: Sentinel 2 a+b
2.3. Manually Processed Satellite Data: Sentinel 2 a+b and PlanetScope
2.4. Unnamed Airborne Vehicle (UAV) Data Collection and Analysis
2.5. Multispectral Data Collection and Analysis
2.6. Hemispherical (Fisheye) Lens Image Processing in CAN-EYE
3. Results
3.1. Sentinel 2 a+b Time Series Analyses
3.2. Description of the Maize Fields That We Visited in Zimbabwe, Tanzania, and Kenya
3.3. Comparison of the NDVI, GA Index, and LAI at Different Spatial Resolution
3.4. Vegetation Growth Curves Based on Manually Processed Sentinel 2a+b and PlanetScope Image Data
4. Discussion
4.1. Sentinel 2 a+b Time Series Analyses
4.2. Comparison of the NDVI, GA Index, and LAI at Different Spatial Resolutions
4.3. Vegetation Growth Curves Based on Manually Processed Sentinel 2a+b and PlanetScope Image Data
5. Conclusions and Future
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, M.E. Metrics to accelerate private sector investment in sustainable development goal 2—Zero hunger. Sustainability 2021, 13, 5967. [Google Scholar] [CrossRef]
- Tanumihardjo, S.A.; McCulley, L.; Roh, R.; Lopez-Ridaura, S.; Palacios-Rojas, N.; Gunaratna, N.S. Maize agro-food systems to ensure food and nutrition security in reference to the Sustainable Development Goals. Glob. Food Secur. 2020, 25, 100327. [Google Scholar] [CrossRef]
- Prasanna, B.; Huesing, J.E.; Eddy, R.; Peschke, V.M. Fall Armyworm in Africa: A Guide for Integrated Pest Management, 1st ed.; CIMMYT: Mexico City, Mexico, 2018; pp. 45–62. [Google Scholar]
- FAOSTAT. The State of Food and Agriculture 2016 (SOFA): Climate Change, Agriculture and Food Security; FAO: Rome, Italy, 2016; ISBN 9789251062159. [Google Scholar]
- Hruska, A.J. Fall armyworm (Spodoptera frugiperda) management by smallholders. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2019, 14, 1–11. [Google Scholar] [CrossRef] [Green Version]
- FAO. The Global Action for Fall Armyworm Control: Action Framework 2020–2022; FAO: Rome, Italy, 2020; ISBN 9789251326961. [Google Scholar]
- Balla, A.; Bhaskar, M.; Bagade, P.; Rawal, N. Yield losses in maize (Zea mays) due to fall armyworm infestation and potential IoT-based interventions for its control. J. Entomol. Zool. Stud. 2019, 7, 920–927. [Google Scholar]
- Williams, W.P.; Davis, F.M.; Buckley, P.M.; Hedin, P.A.; Baker, G.T.; Luthe, D.S. Factors Associated with Resistance to Fall Armyworm (Lepidoptera: Noctuidae) and Southwestern Corn Borer (Lepidoptera: Crambidae) in Corn at Different Vegetative Stages. J. Econ. Entomol. 1998, 91, 1471–1480. [Google Scholar] [CrossRef]
- Sisay, B.; Simiyu, J.; Mendesil, E.; Likhayo, P.; Ayalew, G.; Mohamed, S.; Subramanian, S.; Tefera, T. Fall armyworm, (spodoptera frugiperda) infestations in East Africa: Assessment of damage and parasitism. Insects 2019, 10, 195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Day, R.; Abrahams, P.; Bateman, M.; Beale, T.; Clottey, V.; Cock, M.; Colmenarez, Y.; Corniani, N.; Early, R.; Godwin, J.; et al. Fall armyworm: Impacts and implications for Africa. Outlooks Pest Manag. 2017, 28, 196–201. [Google Scholar] [CrossRef] [Green Version]
- Gebreziher, H.G. Review on management methods of fall armyworm (Spodoptera frugiperda J.E. Smith) in Sub- Saharan Africa. Int. J. Entomol. Res. 2020, 5, 09–14. [Google Scholar]
- Hailu, G.; Niassy, S.; Zeyaur, K.R.; Ochatum, N.; Subramanian, S. Maize–legume intercropping and push–pull for management of fall armyworm, stemborers, and striga in Uganda. Agron. J. 2018, 110, 2513–2522. [Google Scholar] [CrossRef] [Green Version]
- Harrison, R.D.; Thierfelder, C.; Baudron, F.; Chinwada, P.; Midega, C.; Schaffner, U.; van den Berg, J. Agro-ecological options for fall armyworm (Spodoptera frugiperda J.E. Smith) management: Providing low-cost, smallholder friendly solutions to an invasive pest. J. Environ. Manag. 2019, 243, 318–330. [Google Scholar] [CrossRef]
- Abate, T.; van Huis, A.; Ampofo, K.O. Pest Management Strategies in Traditional Agriculture: An African Perspective. Annu. Rev. Entomol. 2000, 45, 631–659. [Google Scholar] [CrossRef] [PubMed]
- Wyckhuys, K.A.G.; O’Neil, R.J. Social and ecological facets of pest management in Honduran subsistence agriculture: Implications for IPM extension and natural resource management. Environ. Dev. Sustain. 2010, 12, 297–311. [Google Scholar] [CrossRef]
- Yigezu, G.; Wakgari, M. Local and indigenous knowledge of farmers management practice against fall armyworm (Spodoptera frugiperda) (J.E. Smith) (Lepidoptera: Noctuidae): A review. J. Entomol. Zool. Stud. 2020, 8, 765–770. [Google Scholar]
- Ayra-Pardo, C.; Huang, S.; Kan, Y.; Wright, D.J. Impact of invasive fall armyworm on plant and arthropod communities and implications for crop protection. Int. J. Pest Manag. 2021, 1–12. [Google Scholar] [CrossRef]
- Gross, H.R.; Young, J.R.; Wiseman, B.R. Relative Susceptibility of a Summer-Planted Dent and Tropical Flint Corn Variety to Whorl Stage Damage by the Fall Armyworm (Lepidoptera: Noctuidae). J. Econ. Entomol. 1982, 75, 1153–1156. [Google Scholar] [CrossRef]
- Jonckheere, I.; Fleck, S.; Nackaerts, K.; Muys, B.; Coppin, P.; Weiss, M.; Baret, F. Review of methods for in situ leaf area index determination Part I. Theories, sensors and hemispherical photography. Agric. For. Meteorol. 2004, 121, 19–35. [Google Scholar] [CrossRef]
- Van Gardingen, P.R.; Jackson, G.E.; Hernandez-Daumas, S.; Russell, G.; Sharp, L. Leaf area index estimates obtained for clumped canopies using hemispherical photography. Agric. For. Meteorol. 1999, 94, 243–257. [Google Scholar] [CrossRef]
- Garrigues, S.; Shabanov, N.V.; Swanson, K.; Morisette, J.T.; Baret, F.; Myneni, R.B. Intercomparison and sensitivity analysis of Leaf Area Index retrievals from LAI-2000, AccuPAR, and digital hemispherical photography over croplands. Agric. For. Meteorol. 2008, 148, 1193–1209. [Google Scholar] [CrossRef] [Green Version]
- Tucker, C.J. Red and photographic infrared linear combinations for monitoring vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef] [Green Version]
- Van Hoek, M.; Jia, L.; Zhou, J.; Zheng, C.; Menenti, M. Early drought detection by spectral analysis of satellite time series of precipitation and Normalized Difference Vegetation Index (NDVI). Remote Sens. 2016, 8, 422. [Google Scholar] [CrossRef] [Green Version]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring Vegetation Systems in the Great Plains with ERTS. In Proceedings of the Third ERTS Symposium, Washington, DC, USA, 10–14 December 1973; pp. 309–317. [Google Scholar]
- Thenkabail, P.S.; Smith, R.B.; De Pauw, E. Evaluation of Narrowband and Broadband Vegetation Indices for Determining Optimal Hyperspectral Wavebands for Agricultural Crop Characterization. Photogramm. Eng. Remote Sens. 2002, 68, 607–621. [Google Scholar]
- Casadesús, J.; Villegas, D. Conventional digital cameras as a tool for assessing leaf area index and biomass for cereal breeding. J. Integr. Plant Biol. 2014, 56, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Segarra, J.; Buchaillot, M.L.; Araus, J.L.; Kefauver, S.C. Remote sensing for precision agriculture: Sentinel-2 improved features and applications. Agronomy 2020, 10, 641. [Google Scholar] [CrossRef]
- Cheng, Y.; Vrieling, A.; Fava, F.; Meroni, M.; Marshall, M.; Gachoki, S. Phenology of short vegetation cycles in a Kenyan rangeland from PlanetScope and Sentinel-2. Remote Sens. Environ. 2020, 248, 112004. [Google Scholar] [CrossRef]
- Pettorelli, N.; Vik, J.O.; Mysterud, A.; Gaillard, J.M.; Tucker, C.J.; Stenseth, N.C. Using the satellite-derived NDVI to assess ecological responses to environmental change. Trends Ecol. Evol. 2005, 20, 503–510. [Google Scholar] [CrossRef]
- White, M.A.; De Beurs, K.M.; Didan, K.; Inouye, D.W.; Richardson, A.D.; Jensen, O.P.; O’keefe, J.O.; Zhang, G.; Nemani, R.R.; Leeuwen, W.J.D.V.; et al. Intercomparison, interpretation, and assessment of spring phenology in North America estimated from remote sensing for 1982–2006. Glob. Chang. Biol. 2009, 15, 2335–2359. [Google Scholar] [CrossRef]
- Tottrup, C.; Rasmussen, M.S. Mapping long-term changes in savannah crop productivity in Senegal through trend analysis of time series of remote sensing data. Agric. Ecosyst. Environ. 2004, 103, 545–560. [Google Scholar] [CrossRef]
- Qader, S.H.; Dash, J.; Alegana, V.A.; Khwarahm, N.R.; Tatem, A.J.; Atkinson, P.M. The role of earth observation in achieving sustainable agricultural production in arid and semi-arid regions of the world. Remote Sens. 2021, 13, 3382. [Google Scholar] [CrossRef]
- Hüttich, C.; Herold, M.; Schmullius, C.; Egorov, V.; Bartalev, S.A. Indicators of Northern Eurasia’s land-cover change trends from SPOT-VEGETATION time-series analysis 1998–2005. Int. J. Remote Sens. 2007, 28, 4199–4206. [Google Scholar] [CrossRef]
- Funk, C.; Budde, M.E. Phenologically-tuned MODIS NDVI-based production anomaly estimates for Zimbabwe. Remote Sens. Environ. 2009, 113, 115–125. [Google Scholar] [CrossRef]
- Eerens, H.; Haesen, D.; Rembold, F.; Urbano, F.; Tote, C.; Bydekerke, L. Image time series processing for agriculture monitoring. Environ. Model. Softw. 2014, 53, 154–162. [Google Scholar] [CrossRef]
- Reinermann, S.; Gessner, U.; Asam, S.; Kuenzer, C.; Dech, S. The effect of droughts on vegetation condition in Germany: An analysis based on two decades of satellite earth observation time series and crop yield statistics. Remote Sens. 2019, 11, 1783. [Google Scholar] [CrossRef] [Green Version]
- Rembold, F.; Atzberger, C.; Savin, I.; Rojas, O. Using low resolution satellite imagery for yield prediction and yield anomaly detection. Remote Sens. 2013, 5, 1704–1733. [Google Scholar] [CrossRef] [Green Version]
- Serra, P.; Pons, X. Monitoring farmers’ decisions on Mediterranean irrigated crops using satellite image time series. Int. J. Remote Sens. 2008, 29, 2293–2316. [Google Scholar] [CrossRef]
- Meroni, M.; Atzberger, C.; Vancutsem, C.; Gobron, N.; Baret, F.; Lacaze, R.; Eerens, H.; Leo, O. Evaluation of agreement between space remote sensing SPOT-VEGETATION fAPAR Time Series. IEEE Trans. Geosci. Remote Sens. 2013, 51, 1951–1962. [Google Scholar] [CrossRef]
- Winkler, K.; Gessner, U.; Hochschild, V. Identifying droughts affecting agriculture in Africa based on remote sensing time series between 2000-2016: Rainfall anomalies and vegetation condition in the context of ENSO. Remote Sens. 2017, 9, 831. [Google Scholar] [CrossRef] [Green Version]
- Rembold, F.; Meroni, M.; Urbano, F.; Royer, A.; Atzberger, C.; Lemoine, G.; Eerens, H.; Haesen, D. Remote sensing time series analysis for crop monitoring with the SPIRITS software: New functionalities and use examples. Front. Environ. Sci. 2015, 3, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ramos, A.P.M.; Gomes, F.D.G.; Pinheiro, M.M.F.; Furuya, D.E.G.; Gonçalvez, W.N.; Marcato, J., Jr.; Michereff, M.F.F.; Blassioli-Moraes, M.C.; Borges, M.; Alaumann, R.A.; et al. Detecting the attack of the fall armyworm (Spodoptera frugiperda) in cotton plants with machine learning and spectral measurements. Precis. Agric. 2021, 23, 470–491. [Google Scholar] [CrossRef]
- Brown, M.E.; Mugo, S.; Petersen, S.; Klauser, D. Designing a Pest and Disease Outbreak Warning System for Farmers, Agronomists and Agricultural Input Distributors in East Africa. Insects 2022, 13, 232. [Google Scholar] [CrossRef]
- Pisman, T.I.; Erunova, M.G.; Botvich, I.Y.; Shevyrnogov, A.P. Spatial Distribution of NDVI Seeds of Cereal Crops with Different Levels of Weediness According to PlanetScope Satellite Data. J. Sib. Fed. Univ. Eng. Technol. 2020, 13, 578–585. [Google Scholar] [CrossRef]
- Moyroud, N.; Portet, F. Introduction to QGIS. In QGIS Generic Tools; Wiley: Hoboken, NJ, USA, 2018; pp. 1–17. [Google Scholar]
- Casadesus, J.; Kaya, Y.; Bort, J.; Nachit, M.M.; Araus, J.L.; Amor, S.; Ferrazzano, G.; Maalouf, F.; Maccaferri, M.; Martos, V.; et al. Using vegetation indices derived from conventional digital cameras as selection criteria for wheat breeding in water-limited environments. Ann. Appl. Biol. 2007, 150, 227–236. [Google Scholar] [CrossRef]
- Buchaillot, M.L.; Gracia-Romero, A.; Vergara-Diaz, O.; Zaman-Allah, M.A.; Tarekegne, A.; Cairns, J.E.; Prasanna, B.M.; Araus, J.L.; Kefauver, S.C. Evaluating maize genotype performance under low nitrogen conditions using RGB UAV phenotyping techniques. Sensors 2019, 19, 1815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, S.; Danielson, B.; Clare, S.; Koenig, S.; Campos-Vargas, C.; Sanchez-Azofeifa, A. Radiometric calibration assessments for UAS-borne multispectral cameras: Laboratory and field protocols. Int. Soc. J. Photogramm. Remote Sens. 2019, 149, 132–145. [Google Scholar] [CrossRef]
- Demarez, V.; Duthoit, S.; Baret, F.; Weiss, M. Dedieu Estimation of leaf area and clumping indexes of crops with hemispherical photographs. Agric. For. Meteorol. 2008, 148, 644–655. [Google Scholar] [CrossRef]
- Baudron, F.; Zaman-Allah, M.A.; Chaipa, I.; Chari, N.; Chinwada, P. Understanding the factors influencing fall armyworm (Spodoptera frugiperda J.E. Smith) damage in African smallholder maize fields and quantifying its impact on yield. A case study in Eastern Zimbabwe. Crop Prot. 2019, 120, 141–150. [Google Scholar] [CrossRef]
- Wang, R.; Cherkauer, K.; Bowling, L. Corn response to climate stress detected with satellite-based NDVI time series. Remote Sens. 2016, 8, 269. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Jia, M.; Zhang, R.; Ren, Y.; Wen, X. Incorporating the plant phenological trajectory into mangrove species mapping with dense time series Sentinel-2 imagery and the Google Earth Engine platform. Remote Sens. 2019, 11, 2479. [Google Scholar] [CrossRef] [Green Version]
- Mahdianpari, M.; Jafarzadeh, H.; Granger, J.E.; Mohammadimanesh, F.; Brisco, B.; Salehi, B.; Homayouni, S.; Weng, Q. A large-scale change monitoring of wetlands using time series Landsat imagery on Google Earth Engine: A case study in Newfoundland. GISci. Remote Sens. 2020, 57, 1102–1124. [Google Scholar] [CrossRef]
- Decuyper, M.; Chávez, R.O.; Lohbeck, M.; Lastra, J.A.; Tsendbazar, N.; Hackländer, J.; Herold, M.; Vågen, T.G. Continuous monitoring of forest change dynamics with satellite time series. Remote Sens. Environ. 2022, 269, 112829. [Google Scholar] [CrossRef]
- Nonni, F.; Malacarne, D.; Pappalardo, S.E. Sentinel-2 Data Analysis and Comparison with UAV Multispectral Images for Precision Viticulture Study areas. GI Forum 2018, 1, 105–116. [Google Scholar]
- Berra, E.F.; Gaulton, R.; Barr, S. Assessing spring phenology of a temperate woodland: A multiscale comparison of ground, unmanned aerial vehicle and Landsat satellite observations. Remote Sens. Environ. 2019, 223, 229–242. [Google Scholar] [CrossRef]
- Matese, A.; Toscano, P.; Di Gennaro, F.S.; Genesio, L.; Vaccari, F.P.; Primicerio, J.; Belli, C.; Zaldei, A.; Bianconi, R.; Gioli, B. Intercomparison of UAV, Aircraft and Satellite Remote Sensing Platforms for Precision Viticulture. Remote Sens. 2015, 7, 2971–2990. [Google Scholar] [CrossRef] [Green Version]
- Kovacs, J.M.; Flores-Verdugo, F.; Wang, J.; Aspden, L.P. Estimating leaf area index of a degraded mangrove forest using high spatial resolution satellite data. Aquat. Bot. 2004, 80, 13–22. [Google Scholar] [CrossRef]
- Ihuoma, S.O.; Madramootoo, C.A.; Kalacska, M. Integration of satellite imagery and in situ soil moisture data for estimating irrigation water requirements. Int. J. Appl. Earth Obs. Geoinf. 2021, 102, 102396. [Google Scholar] [CrossRef]
- Salih, A.A.M.; Baraibar, M.; Mwangi, K.K.; Artan, G. Climate change and locust outbreak in East Africa. Nat. Clim. Chang. 2020, 10, 584–585. [Google Scholar] [CrossRef]
- Meynard, C.N.; Lecoq, M.; Chapuis, M.P.; Piou, C. On the relative role of climate change and management in the current desert locust outbreak in East Africa. Glob. Chang. Biol. 2020, 26, 3753–3755. [Google Scholar] [CrossRef] [PubMed]
- Odhiambo, K.; Lewis, J.; Tefera, N.; Thomas, A.; Meroni, M.; Rembold, M. Impacts of COVID-19 and Desert Locusts on Smallholder Farmers Food Systems and Value Chains in Kenya; Publications Office of the EU: Luxembourg, 2021; ISBN 978-92-76-32073-9. [Google Scholar]
- Kehs, A.; Mccloskey, P.; Chelal, J.; Morr, D.; Amakove, S.; Mayieka, J.; Ntango, G.; Nyongesa, K.; Pamba, L.; Mugo, J.; et al. From village to globe: A dynamic real-time map of African fields through PlantVillage. Front. Sustain. Food Syst. 2019, 5, 514785. [Google Scholar] [CrossRef]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buchaillot, M.L.; Cairns, J.; Hamadziripi, E.; Wilson, K.; Hughes, D.; Chelal, J.; McCloskey, P.; Kehs, A.; Clinton, N.; Araus, J.L.; et al. Regional Monitoring of Fall Armyworm (FAW) Using Early Warning Systems. Remote Sens. 2022, 14, 5003. https://0-doi-org.brum.beds.ac.uk/10.3390/rs14195003
Buchaillot ML, Cairns J, Hamadziripi E, Wilson K, Hughes D, Chelal J, McCloskey P, Kehs A, Clinton N, Araus JL, et al. Regional Monitoring of Fall Armyworm (FAW) Using Early Warning Systems. Remote Sensing. 2022; 14(19):5003. https://0-doi-org.brum.beds.ac.uk/10.3390/rs14195003
Chicago/Turabian StyleBuchaillot, Ma. Luisa, Jill Cairns, Esnath Hamadziripi, Kenneth Wilson, David Hughes, John Chelal, Peter McCloskey, Annalyse Kehs, Nicholas Clinton, José Luis Araus, and et al. 2022. "Regional Monitoring of Fall Armyworm (FAW) Using Early Warning Systems" Remote Sensing 14, no. 19: 5003. https://0-doi-org.brum.beds.ac.uk/10.3390/rs14195003