Synbiotic Supplementation Containing Whole Plant Sugar Cane Fibre and Probiotic Spores Potentiates Protective Synergistic Effects in Mouse Model of IBD
Abstract
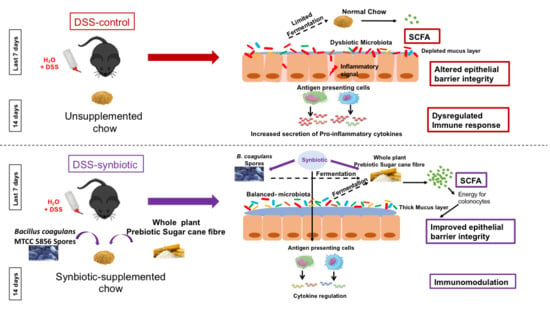
:1. Introduction
2. Materials and Methods
2.1. Probiotic Bacteria and Prebiotic Dietary Fibre
2.2. Animals
2.3. Study Design and Treatments
2.4. Clinical Scoring and Histological Analysis
2.5. Alcian Blue Staining
2.6. Immunohistochemical Detection of Tight Junction Proteins
2.7. Myeloperoxidase Activity
2.8. Tissue Explant Culture and Cytokine Measurements
2.9. iNOS Activity
2.10. Serum C-Reactive Protein Analysis
2.11. Volatile SCFA Analysis
2.12. Metabolic Phenotyping Analysis
2.13. Statistical Analysis
3. Results
3.1. Effects of B. Coagulans, PSCF and Synbiotic Supplementation on DAI and Macroscopic Inflammatory Markers in DSS-Induced Mice
3.2. Effects of B. Coagulans, PSCF and Synbiotic Supplementation on Histological Alterations in DSS-Induced Mice
3.3. Effects of B. Coagulans, PSCF and Synbiotic Supplementation on Goblet Cells and Colonic Tight Junction Barrier
3.4. Immunomodulatory Effects of B. Coagulans, PSCF, and Synbiotic Supplementation on Immune Markers in DSS-Induced Mice
3.5. Effects of B. coagulans, PSCF and Synbiotic Supplementation on Altered Fecal Metabolic Profile in DSS-Induced Mice
3.6. Effects of B. Coagulans, PSCF and Synbiotic Supplementation SCFA Levels in DSS-Induced Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vindigni, S.M.; Zisman, T.L.; Suskind, D.L.; Damman, C.J. The intestinal microbiome, barrier function, and immune system in inflammatory bowel disease: A tripartite pathophysiological circuit with implications for new therapeutic directions. Ther. Adv. Gastroenterol. 2016, 9, 606–625. [Google Scholar] [CrossRef]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.; Chan, F.K. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Hou, J.K.; Lee, D.; Lewis, J. Diet and inflammatory bowel disease: Review of patient-targeted recommendations. Clin. Gastroenterol. Hepatol. 2014, 12, 1592–1600. [Google Scholar] [CrossRef]
- Palumbo, V.; Romeo, M.; Marino Gammazza, A.; Carini, F.; Damiani, P.; Damiano, G.; Buscemi, S.; Lo Monte, A.; Gerges Geagea, A.; Jurjus, A. The long-term effects of probiotics in the therapy of ulcerative colitis: A clinical study. Biomed. Pap. 2016, 160, 372–377. [Google Scholar] [CrossRef] [Green Version]
- Abruzzo, A.; Damiano, G.; Altomare, R.; Palumbo, V.; Tomasello, G.; Buscemi, S.; Lo Monte, G.; Maione, C.; Buscemi, G.; Lo Monte, A. The influence of some dietary components on intestinal microbiota. Prog. Nutr. 2016, 18, 205–212. [Google Scholar]
- Steed, H.; Macfarlane, G.T.; Blackett, K.L.; Bahrami, B.; Reynolds, N.; Walsh, S.V.; Cummings, J.H.; Macfarlane, S. Clinical trial: The microbiological and immunological effects of synbiotic consumption—A randomized double-blind placebo-controlled study in active crohn’s disease. Aliment. Pharmacol. Ther. 2010, 32, 872–883. [Google Scholar] [CrossRef]
- Wasilewski, A.; Zielińska, M.; Storr, M.; Fichna, J. Beneficial effects of probiotics, prebiotics, synbiotics, and psychobiotics in inflammatory bowel disease. Inflamm. Bowel Dis. 2015, 21, 1674–1682. [Google Scholar] [CrossRef]
- Moroeanu, V.I.; Vamanu, E.; Paun, G.; Neagu, E.; Ungureanu, O.R.; Eremia, S.A.; Radu, G.-L.; Ionescu, R.; Pelinescu, D.R. Probiotic strains influence on infant microbiota in the in vitro colonic fermentation model gis1. Indian J. Microbiol. 2015, 55, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.; Harris, P.J.; Ferguson, L.R. Potential benefits of dietary fibre intervention in inflammatory bowel disease. Int. J. Mol. Sci. 2016, 17, 919. [Google Scholar] [CrossRef]
- Pituch-Zdanowska, A.; Banaszkiewicz, A.; Albrecht, P. The role of dietary fibre in inflammatory bowel disease. Prz. Gastroenterol. 2015, 10, 135. [Google Scholar] [CrossRef]
- Galvez, J.; Rodríguez-Cabezas, M.E.; Zarzuelo, A. Effects of dietary fiber on inflammatory bowel disease. Mol. Nutr. Food Res. 2005, 49, 601–608. [Google Scholar] [CrossRef]
- Williams, B.A.; Grant, L.J.; Gidley, M.J.; Mikkelsen, D. Gut fermentation of dietary fibres: Physico-chemistry of plant cell walls and implications for health. Int. J. Mol. Sci. 2017, 18, 2203. [Google Scholar] [CrossRef]
- Tuohy, K.M.; Conterno, L.; Gasperotti, M.; Viola, R. Up-regulating the human intestinal microbiome using whole plant foods, polyphenols, and/or fiber. J. Agric. Food Chem. 2012, 60, 8776–8782. [Google Scholar] [CrossRef]
- Liu, R.H. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am. J. Clin. Nutr. 2003, 78, 517S–520S. [Google Scholar] [CrossRef]
- Edwards, G.; Ball, M. Dietary Supplement for the Treatment of Acid Reflux and Gastro-Oesophageal Reflux Disease (Gord/Gerd). U.S. Patent US20160287657A, 2016. [Google Scholar]
- Ball, M.; Edwards, G. Use of Dietary Fibre Supplement in a Food Formulation. U.S. Patent WO2014162303A1, 2016. [Google Scholar]
- Gamage, H.K.; Tetu, S.G.; Chong, R.W.; Bucio-Noble, D.; Rosewarne, C.P.; Kautto, L.; Ball, M.S.; Molloy, M.; Packer, N.H.; Paulsen, I.T. Fibre supplements derived from sugarcane stem, wheat dextrin and psyllium husk have different in vitro effects on the human gut microbiota. Front. Microbiol. 2018, 9, 1618. [Google Scholar] [CrossRef]
- Walton, S.L.; Bischoff, K.M.; van Heiningen, A.R.; van Walsum, G.P. Production of lactic acid from hemicellulose extracts by bacillus coagulans mxl-9. J. Ind. Microbiol. Biotechnol. 2010, 37, 823–830. [Google Scholar] [CrossRef]
- Konuray, G.; Erginkaya, Z. Potential use of bacillus coagulans in the food industry. Foods 2018, 7, 92. [Google Scholar] [CrossRef]
- Slavin, J.L.; Brauer, P.M.; Marlett, J.A. Neutral detergent fiber, hemicellulose and cellulose digestibility in human subjects. J. Nutr. 1981, 111, 287–297. [Google Scholar] [CrossRef]
- Leschine, S.B. Cellulose degradation in anaerobic environments. Annu. Rev. Microbiol. 1995, 49, 399–426. [Google Scholar] [CrossRef]
- Shinde, T.; Vemuri, R.; Shastri, M.D.; Perera, A.P.; Tristram, S.; Stanley, R.; Eri, R. Probiotic bacillus coagulans mtcc 5856 spores exhibit excellent in-vitro functional efficacy in simulated gastric survival, mucosal adhesion and immunomodulation. J. Funct. Foods 2019, 52, 100–108. [Google Scholar] [CrossRef]
- Baron, M. Original research: A patented strain of bacillus coagulans increased immune response to viral challenge. Postgrad. Med. 2009, 121, 114–118. [Google Scholar] [CrossRef]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran sulfate sodium (dss)-induced colitis in mice. Curr. Protoc. Immunol. 2014, 104, 15.25. 1–15.25. 14. [Google Scholar]
- Murthy, S.; Cooper, H.S.; Shim, H.; Shah, R.S.; Ibrahim, S.A.; Sedergran, D.J. Treatment of dextran sulfate sodium-induced murine colitis by intracolonic cyclosporin. Dig. Dis. Sci. 1993, 38, 1722–1734. [Google Scholar] [CrossRef]
- Perera, A.P.; Fernando, R.; Shinde, T.; Gundamaraju, R.; Southam, B.; Sohal, S.S.; Robertson, A.A.; Schroder, K.; Kunde, D.; Eri, R. Mcc950, a specific small molecule inhibitor of nlrp3 inflammasome attenuates colonic inflammation in spontaneous colitis mice. Sci. Rep. 2018, 8, 8618. [Google Scholar] [CrossRef]
- Demon, D.; Kuchmiy, A.; Fossoul, A.; Zhu, Q.; Kanneganti, T.-D.; Lamkanfi, M. Caspase-11 is expressed in the colonic mucosa and protects against dextran sodium sulfate-induced colitis. Mucosal Immunol. 2014, 7, 1480. [Google Scholar] [CrossRef]
- Eri, R.; McGuckin, M.A.; Wadley, R. T cell transfer model of colitis: A great tool to assess the contribution of t cells in chronic intestinal inflammation. In Leucocytes; Springer: Berlin/Heidelberg, Germany, 2012; pp. 261–275. [Google Scholar]
- Koelink, P.J.; Wildenberg, M.E.; Stitt, L.W.; Feagan, B.G.; Koldijk, M.; van’t Wout, A.B.; Atreya, R.; Vieth, M.; Brandse, J.F.; Duijst, S. Development of reliable, valid and responsive scoring systems for endoscopy and histology in animal models for inflammatory bowel disease. J. Crohn’s Colitis 2018, 1, 10. [Google Scholar] [CrossRef]
- Sovran, B.; Lu, P.; Loonen, L.M.; Hugenholtz, F.; Belzer, C.; Stolte, E.H.; Boekschoten, M.V.; Van Baarlen, P.; Smidt, H.; Kleerebezem, M. Identification of commensal species positively correlated with early stress responses to a compromised mucus barrier. Inflamm. Bowel Dis. 2016, 22, 826–840. [Google Scholar] [CrossRef]
- Li, P.; Zhang, R.; Wang, L.; Gan, Y.; Xu, Y.; Song, L.; Luo, L.; Zhao, C.; Zhang, C.; Ouyang, B. Long-term load duration induces n-cadherin down-regulation and loss of cell phenotype of nucleus pulposus cells in a disc bioreactor culture. Biosci. Rep. 2017, 37, BSR20160582. [Google Scholar] [CrossRef]
- Lean, Q.Y.; Eri, R.D.; Randall-Demllo, S.; Sohal, S.S.; Stewart, N.; Peterson, G.M.; Gueven, N.; Patel, R.P. Orally administered enoxaparin ameliorates acute colitis by reducing macrophage-associated inflammatory responses. PLoS ONE 2015, 10, e0134259. [Google Scholar] [CrossRef] [PubMed]
- Mei, Q.; Xu, J.; Xiang, L.; Hu, Y.; Hu, X.; Xu, Z. Change of nitric oxide in experimental colitis and its inhibition by melatonin in vivo and in vitro. Postgrad. Med. J. 2005, 81, 667–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furuhashi, T.; Sugitate, K.; Nakai, T.; Jikumaru, Y.; Ishihara, G. Rapid profiling method for mammalian feces short chain fatty acids by gc-ms. Anal. Biochem. 2018, 543, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Vemuri, R.; Shinde, T.; Gundamaraju, R.; Gondalia, S.; Karpe, A.; Beale, D.; Martoni, C.; Eri, R. Lactobacillus acidophilus dds-1 modulates the gut microbiota and improves metabolic profiles in aging mice. Nutrients 2018, 10, 1255. [Google Scholar] [CrossRef]
- Sansone, S.-A.; Fan, T.; Goodacre, R.; Griffin, J.L.; Hardy, N.W.; Kaddurah-Daouk, R.; Kristal, B.S.; Lindon, J.; Mendes, P.; Morrison, N. The metabolomics standards initiative. Nat. Biotechnol. 2007, 25, 846. [Google Scholar]
- Smart, K.F.; Aggio, R.B.; Van Houtte, J.R.; Villas-Bôas, S.G. Analytical platform for metabolome analysis of microbial cells using methyl chloroformate derivatization followed by gas chromatography–mass spectrometry. Nat. Protoc. 2010, 5, 1709. [Google Scholar] [CrossRef]
- Srutkova, D.; Schwarzer, M.; Hudcovic, T.; Zakostelska, Z.; Drab, V.; Spanova, A.; Rittich, B.; Kozakova, H.; Schabussova, I. Bifidobacterium longum ccm 7952 promotes epithelial barrier function and prevents acute dss-induced colitis in strictly strain-specific manner. PLoS ONE 2015, 10, e0134050. [Google Scholar] [CrossRef]
- Han, F.; Fan, H.; Yao, M.; Yang, S.; Han, J. Oral administration of yeast β-glucan ameliorates inflammation and intestinal barrier in dextran sodium sulfate-induced acute colitis. J. Funct. Foods 2017, 35, 115–126. [Google Scholar] [CrossRef]
- Al Mijan, M.; Lim, B.O. Diets, functional foods, and nutraceuticals as alternative therapies for inflammatory bowel disease: Present status and future trends. World J. Gastroenterol. 2018, 24, 2673. [Google Scholar] [CrossRef]
- Ferguson, L.R.; Shelling, A.N.; Browning, B.L.; Huebner, C.; Petermann, I. Genes, diet and inflammatory bowel disease. Mutat. Res. /Fundam. Mol. Mech. Mutagenesis 2007, 622, 70–83. [Google Scholar] [CrossRef]
- Kanauchi, O.; Serizawa, I.; Araki, Y.; Suzuki, A.; Andoh, A.; Fujiyama, Y.; Mitsuyama, K.; Takaki, K.; Toyonaga, A.; Sata, M. Germinated barley foodstuff, a prebiotic product, ameliorates inflammation of colitis through modulation of the enteric environment. J. Gastroenterol. 2003, 38, 134–141. [Google Scholar] [CrossRef]
- Mitsuyama, K.; Saiki, T.; Kanauchi, O.; Iwanaga, T.; Tomiyasu, N.; Nishiyama, T.; Tateishi, H.; Shirachi, A.; Ide, M.; Suzuki, A. Treatment of ulcerative colitis with germinated barley foodstuff feeding: A pilot study. Aliment. Pharmacol. Ther. 1998, 12, 1225–1230. [Google Scholar] [CrossRef]
- Majeed, M.; Nagabhushanam, K.; Natarajan, S.; Sivakumar, A.; Ali, F.; Pande, A.; Majeed, S.; Karri, S.K. Bacillus coagulans mtcc 5856 supplementation in the management of diarrhea predominant irritable bowel syndrome: A double blind randomized placebo controlled pilot clinical study. Nutr. J. 2016, 15, 21. [Google Scholar] [CrossRef]
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799. [Google Scholar] [CrossRef]
- Gong, Y.; Li, H.; Li, Y. Effects of bacillus subtilis on epithelial tight junctions of mice with inflammatory bowel disease. J. Interferon Cytokine Res. 2016, 36, 75–85. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, Y.-H.; Zhou, D.; Wu, Q.; Song, D.; Dicksved, J.; Wang, J.-F. Oral administration of a select mixture of bacillus probiotics affects the gut microbiota and goblet cell function following escherichia coli challenge in newly weaned pigs of genotype muc4 that are supposed to be enterotoxigenic e. Coli f4ab/ac receptor negative. Appl. Environ. Microbiol. 2017, 83, e02747-16. [Google Scholar] [CrossRef]
- Xavier, R.; Podolsky, D. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007, 448, 427. [Google Scholar] [CrossRef]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329. [Google Scholar] [CrossRef]
- Hyams, J.S.; Lerer, T.; Griffiths, A.; Pfefferkorn, M.; Kugathasan, S.; Evans, J.; Otley, A.; Carvalho, R.; Mack, D.; Bousvaros, A. Long-term outcome of maintenance infliximab therapy in children with crohn’s disease. Inflamm. Bowel Dis. 2008, 15, 816–822. [Google Scholar] [CrossRef]
- Atreya, R.; Mudter, J.; Finotto, S.; Müllberg, J.; Jostock, T.; Wirtz, S.; Schütz, M.; Bartsch, B.; Holtmann, M.; Becker, C. Blockade of interleukin 6 trans signaling suppresses t-cell resistance against apoptosis in chronic intestinal inflammation: Evidence in crohn disease and experimental colitis in vivo. Nat. Med. 2000, 6, 583. [Google Scholar] [CrossRef]
- Jobin, C.; Sartor, B.R. Nf-κb signaling proteins as therapeutic targets for inflammatory bowel diseases. Inflamm. Bowel Dis. 2000, 6, 206–213. [Google Scholar] [CrossRef]
- Neuman, M.G. Immune dysfunction in inflammatory bowel disease. Transl. Res. 2007, 149, 173–186. [Google Scholar] [CrossRef]
- Soufli, I.; Toumi, R.; Rafa, H.; Touil-Boukoffa, C. Cytokines and nitric oxide in immunopathogenesis of ibd and potential therapeutic approaches. In New Insights into Inflammatory BOWEL disease; InTech: Vienna, Austria, 2016. [Google Scholar]
- Groschwitz, K.R.; Hogan, S.P. Intestinal barrier function: Molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 2009, 124, 3–20. [Google Scholar] [CrossRef]
- Del Giudice, M.; Gangestad, S.W. Rethinking il-6 and crp: Why they are more than inflammatory biomarkers, and why it matters. Brainbehav. Immun. 2018, 70, 61–75. [Google Scholar] [CrossRef]
- Solem, C.A.; Loftus, E.V., Jr.; Tremaine, W.J.; Harmsen, W.S.; Zinsmeister, A.R.; Sandborn, W.J. Correlation of c-reactive protein with clinical, endoscopic, histologic, and radiographic activity in inflammatory bowel disease. Inflamm. Bowel Dis. 2005, 11, 707–712. [Google Scholar] [CrossRef]
- Kerr, T.; Ciorba, M.; Matsumoto, H.; Davis, V.; Luo, J.; Kennedy, S.; Xie, Y.; Shaker, A.; Dieckgraefe, B.; Davidson, N. Dextran sodium sulfate inhibition of real-time polymerase chain reaction amplification: A poly-a purification solution. Inflamm. Bowel Dis. 2011, 18, 344–348. [Google Scholar] [CrossRef]
- Levy, M.; Thaiss, C.A.; Elinav, E. Metabolites: Messengers between the microbiota and the immune system. Genes Dev. 2016, 30, 1589–1597. [Google Scholar] [CrossRef]
- Vernocchi, P.; Del Chierico, F.; Putignani, L. Gut microbiota profiling: Metabolomics based approach to unravel compounds affecting human health. Front. Microbiol. 2016, 7, 1144. [Google Scholar] [CrossRef]
- Martin, F.-P.; Su, M.-M.; Xie, G.-X.; Guiraud, S.P.; Kussmann, M.; Godin, J.-P.; Jia, W.; Nydegger, A. Urinary metabolic insights into host-gut microbial interactions in healthy and ibd children. World J. Gastroenterol. 2017, 23, 3643. [Google Scholar] [CrossRef]
- Marchesi, J.R.; Holmes, E.; Khan, F.; Kochhar, S.; Scanlan, P.; Shanahan, F.; Wilson, I.D.; Wang, Y. Rapid and noninvasive metabonomic characterization of inflammatory bowel disease. J. Proteome Res. 2007, 6, 546–551. [Google Scholar] [CrossRef]
- Robinson, A.M.; Gondalia, S.V.; Karpe, A.V.; Eri, R.; Beale, D.J.; Morrison, P.D.; Palombo, E.A.; Nurgali, K. Fecal microbiota and metabolome in a mouse model of spontaneous chronic colitis: Relevance to human inflammatory bowel disease. Inflamm. Bowel Dis. 2016, 22, 2767–2787. [Google Scholar] [CrossRef]
- Sun, M.; Wu, W.; Liu, Z.; Cong, Y. Microbiota metabolite short chain fatty acids, gpcr, and inflammatory bowel diseases. J. Gastroenterol. 2017, 52, 1–8. [Google Scholar] [CrossRef]
- Breuer, R.I.; Buto, S.K.; Christ, M.L.; Bean, J.; Vernia, P.; Paoluzi, P.; Di Paolo, M.; Caprilli, R. Rectal irrigation with short-chain fatty acids for distal ulcerative colitis. Dig. Dis. Sci. 1991, 36, 185–187. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Kaur, A.; Tuncil, Y.E.; Sikaroodi, M.; Gillevet, P.; Patterson, J.A.; Keshavarzian, A.; Hamaker, B.R. Alterations in the amounts of microbial metabolites in different regions of the mouse large intestine using variably fermentable fibres. Bioact. Carbohydr. Diet. Fibre 2018, 13, 7–13. [Google Scholar] [CrossRef]
- Pang, W.; Vogensen, F.K.; Nielsen, D.S.; Hansen, A.K. Faecal and caecal microbiota profiles of mice do not cluster in the same way. Lab. Anim. 2012, 46, 231–236. [Google Scholar] [CrossRef] [Green Version]
- Hirano, A.; Umeno, J.; Okamoto, Y.; Shibata, H.; Ogura, Y.; Moriyama, T.; Torisu, T.; Fujioka, S.; Fuyuno, Y.; Kawarabayasi, Y. Comparison of the microbial community structure between inflamed and non-inflamed sites in patients with ulcerative colitis. J. Gastroenterol. Hepatol. 2018, 33, 1590–1597. [Google Scholar] [CrossRef]
- Majeed, M.; Nagabhushanam, K.; Arumugam, S.; Natarajan, S.; Majeed, S.; Pande, A.; Beede, K.; Ali, F. Cranberry seed fibre: A promising prebiotic fibre and its fermentation by the probiotic bacillus coagulans mtcc 5856. Int. J. Food Sci. Technol. 2018, 53, 1640–1647. [Google Scholar] [CrossRef]
- Majeed, M.; Majeed, S.; Nagabhushanam, K.; Arumugam, S.; Natarajan, S.; Beede, K.; Ali, F. Galactomannan from trigonella foenum-graecum l. Seed: Prebiotic application and its fermentation by the probiotic bacillus coagulans strain mtcc 5856. Food Sci. Nutr. 2018, 6, 666–673. [Google Scholar] [CrossRef]
- Nielsen, D.S.G.; Jensen, B.B.; Theil, P.K.; Nielsen, T.S.; Knudsen, K.E.B.; Purup, S. Effect of butyrate and fermentation products on epithelial integrity in a mucus-secreting human colon cell line. J. Funct. Foods 2018, 40, 9–17. [Google Scholar] [CrossRef]
- Zheng, L.; Kelly, C.J.; Battista, K.D.; Schaefer, R.; Lanis, J.M.; Alexeev, E.E.; Wang, R.X.; Onyiah, J.C.; Kominsky, D.J.; Colgan, S.P. Microbial-derived butyrate promotes epithelial barrier function through il-10 receptor–dependent repression of claudin-2. J. Immunol. 2017, 199, 2976–2984. [Google Scholar] [CrossRef]
- Simeoli, R.; Mattace Raso, G.; Pirozzi, C.; Lama, A.; Santoro, A.; Russo, R.; Montero-Melendez, T.; Berni Canani, R.; Calignano, A.; Perretti, M. An orally administered butyrate-releasing derivative reduces neutrophil recruitment and inflammation in dextran sulphate sodium-induced murine colitis. Br. J. Pharmacol. 2017, 174, 1484–1496. [Google Scholar] [CrossRef]
- Van Immerseel, F.; Ducatelle, R.; De Vos, M.; Boon, N.; Van De Wiele, T.; Verbeke, K.; Rutgeerts, P.; Sas, B.; Louis, P.; Flint, H.J. Butyric acid-producing anaerobic bacteria as a novel probiotic treatment approach for inflammatory bowel disease. J. Med. Microbiol. 2010, 59, 141–143. [Google Scholar] [CrossRef]
- Tedelind, S.; Westberg, F.; Kjerrulf, M.; Vidal, A. Anti-inflammatory properties of the short-chain fatty acids acetate and propionate: A study with relevance to inflammatory bowel disease. World J. Gastroenterol. WJG 2007, 13, 2826. [Google Scholar] [CrossRef]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor gpr43. Nature 2009, 461, 1282. [Google Scholar] [CrossRef]
- Macia, L.; Tan, J.; Vieira, A.T.; Leach, K.; Stanley, D.; Luong, S.; Maruya, M.; McKenzie, C.I.; Hijikata, A.; Wong, C. Metabolite-sensing receptors gpr43 and gpr109a facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat. Commun. 2015, 6, 6734. [Google Scholar] [CrossRef]
- Yuille, S.; Reichardt, N.; Panda, S.; Dunbar, H.; Mulder, I.E. Human gut bacteria as potent class i histone deacetylase inhibitors in vitro through production of butyric acid and valeric acid. PLoS ONE 2018, 13, e0201073. [Google Scholar] [CrossRef]
- Thorburn, A.N.; Macia, L.; Mackay, C.R. Diet, metabolites, and “western-lifestyle” inflammatory diseases. Immunity 2014, 40, 833–842. [Google Scholar] [CrossRef]
- Nguyen, T.L.A.; Vieira-Silva, S.; Liston, A.; Raes, J. How informative is the mouse for human gut microbiota research? Dis. Models Mech. 2015, 8, 1–16. [Google Scholar] [CrossRef] [Green Version]










© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shinde, T.; Perera, A.P.; Vemuri, R.; Gondalia, S.V.; Karpe, A.V.; Beale, D.J.; Shastri, S.; Southam, B.; Eri, R.; Stanley, R. Synbiotic Supplementation Containing Whole Plant Sugar Cane Fibre and Probiotic Spores Potentiates Protective Synergistic Effects in Mouse Model of IBD. Nutrients 2019, 11, 818. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11040818
Shinde T, Perera AP, Vemuri R, Gondalia SV, Karpe AV, Beale DJ, Shastri S, Southam B, Eri R, Stanley R. Synbiotic Supplementation Containing Whole Plant Sugar Cane Fibre and Probiotic Spores Potentiates Protective Synergistic Effects in Mouse Model of IBD. Nutrients. 2019; 11(4):818. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11040818
Chicago/Turabian StyleShinde, Tanvi, Agampodi Promoda Perera, Ravichandra Vemuri, Shakuntla V. Gondalia, Avinash V. Karpe, David J. Beale, Sonia Shastri, Benjamin Southam, Rajaraman Eri, and Roger Stanley. 2019. "Synbiotic Supplementation Containing Whole Plant Sugar Cane Fibre and Probiotic Spores Potentiates Protective Synergistic Effects in Mouse Model of IBD" Nutrients 11, no. 4: 818. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11040818