Natural and Artificial Sweeteners and High Fat Diet Modify Differential Taste Receptors, Insulin, and TLR4-Mediated Inflammatory Pathways in Adipose Tissues of Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Biochemical Parameters
2.3. White and Brown Adipose Tissue Gene Expression
2.4. Western Blot Analysis
2.5. Histological Analysis
2.6. Statistical Analysis
3. Results
3.1. Body Weight and Biochemical Parameters
3.2. Tongue and Intestine TIR2, TIR3, SGLT-1, GLUT-2 and Incretins After the Consumption of Natural and Artificial Sweeteners With and without a High Fat Diet
3.3. Inflammatory Pathways are Differentially Regulated by the Type of Sweeteners and High Fat
3.4. Insulin Signaling after the Consumption of Sweeteners
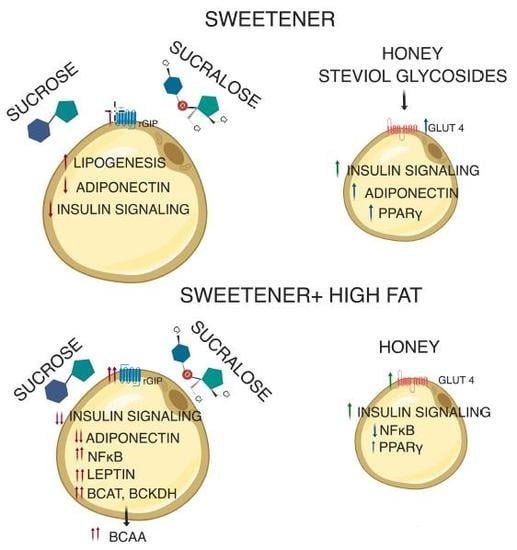
3.5. The Type of Sweetener and High Fat Diet Differentially Modify the Functionality of the Adipocyte in White Adipose Tissue
3.6. Sweeteeners Differentially Regulate Gene Expression of Adiponectin and UCP-1 in BAT
3.7. High Fat Diet and Specific Sweetners Increased the Concentrations of Branched Chain Amino Acids
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- USDA Sugar and Sweeteners Yearbook Tables. Available online: https://www.ers.usda.gov/data-products/sugar-and-sweeteners-yearbook-tables.aspx (accessed on 29 January 2019).
- Pereira, M.A. Sugar-sweetened and artificially-sweetened beverages in relation to obesity risk. Adv. Nutr. 2014, 5, 797–808. [Google Scholar] [CrossRef]
- Fernstrom, J.D. Non-nutritive sweeteners and obesity. Annu. Rev. Food Sci. Technol. 2015, 6, 119–136. [Google Scholar] [CrossRef]
- Tappy, L. Fructose-containing caloric sweeteners as a cause of obesity and metabolic disorders. J. Exp. Biol. 2018, 221. [Google Scholar] [CrossRef]
- Bornemann, V.; Werness, S.C.; Buslinger, L.; Schiffman, S.S. Intestinal Metabolism and Bioaccumulation of Sucralose In Adipose Tissue In The Rat. J.Toxicol. Environ. Health A 2018, 81, 913–923. [Google Scholar] [CrossRef]
- Simon, B.R.; Parlee, S.D.; Learman, B.S.; Mori, H.; Scheller, E.L.; Cawthorn, W.P.; Ning, X.; Gallagher, K.; Tyrberg, B.; Assadi-Porter, F.M.; et al. Artificial sweeteners stimulate adipogenesis and suppress lipolysis independently of sweet taste receptors. J. Biol. Chem. 2013, 288, 32475–32489. [Google Scholar] [CrossRef]
- Masubuchi, Y.; Nakagawa, Y.; Ma, J.; Sasaki, T.; Kitamura, T.; Yamamoto, Y.; Kurose, H.; Kojima, I.; Shibata, H. A novel regulatory function of sweet taste-sensing receptor in adipogenic differentiation of 3T3-L1 cells. PLoS ONE 2013, 8, e54500. [Google Scholar] [CrossRef]
- Masubuchi, Y.; Nakagawa, Y.; Medina, J.; Nagasawa, M.; Kojima, I.; Rasenick, M.M.; Inagaki, T.; Shibata, H. T1R3 homomeric sweet taste receptor regulates adipogenesis through Galphas-mediated microtubules disassembly and Rho activation in 3T3-L1 cells. PLoS ONE 2017, 12, e0176841. [Google Scholar]
- Avau, B.; Bauters, D.; Steensels, S.; Vancleef, L.; Laermans, J.; Lesuisse, J.; Buyse, J.; Lijnen, H.R.; Tack, J.; Depoortere, I. The Gustatory Signaling Pathway and Bitter Taste Receptors Affect the Development of Obesity and Adipocyte Metabolism in Mice. PLoS ONE 2015, 10, e0145538. [Google Scholar] [CrossRef]
- Duca, F.A.; Bauer, P.V.; Hamr, S.C.; Lam, T.K. Glucoregulatory Relevance of Small Intestinal Nutrient Sensing in Physiology, Bariatric Surgery, and Pharmacology. Cell Metab. 2015, 22, 367–380. [Google Scholar] [CrossRef]
- McIntosh, C.H.; Widenmaier, S.; Kim, S.J. Glucose-dependent insulinotropic polypeptide signaling in pancreatic beta-cells and adipocytes. J. Diabetes Investig. 2012, 3, 96–106. [Google Scholar] [CrossRef]
- Ceperuelo-Mallafre, V.; Duran, X.; Pachon, G.; Roche, K.; Garrido-Sanchez, L.; Vilarrasa, N.; Tinahones, F.J.; Vicente, V.; Pujol, J.; Vendrell, J.; et al. Disruption of GIP/GIPR axis in human adipose tissue is linked to obesity and insulin resistance. J. Clin. Endocrinol. Metab. 2014, 99, E908–E919. [Google Scholar] [CrossRef]
- Chen, S.; Okahara, F.; Osaki, N.; Shimotoyodome, A. Increased GIP signaling induces adipose inflammation via a HIF-1alpha-dependent pathway and impairs insulin sensitivity in mice. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E414–E425. [Google Scholar] [CrossRef]
- Schenk, S.; Saberi, M.; Olefsky, J.M. Insulin sensitivity: Modulation by nutrients and inflammation. J. Clin. Investig. 2008, 118, 2992–3002. [Google Scholar] [CrossRef]
- Stern, J.H.; Rutkowski, J.M.; Scherer, P.E. Adiponectin, Leptin, and Fatty Acids in the Maintenance of Metabolic Homeostasis through Adipose Tissue Crosstalk. Cell Metab. 2016, 23, 770–784. [Google Scholar] [CrossRef]
- Palsson-McDermott, E.M.; O’Neill, L.A. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology 2004, 113, 153–162. [Google Scholar] [CrossRef]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- Mace, O.J.; Affleck, J.; Patel, N.; Kellett, G.L. Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2. J. Physiol. 2007, 582, 379–392. [Google Scholar] [CrossRef]
- Jang, H.J.; Kokrashvili, Z.; Theodorakis, M.J.; Carlson, O.D.; Kim, B.J.; Zhou, J.; Kim, H.H.; Xu, X.; Chan, S.L.; Juhaszova, M.; et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc. Natl. Acad. Sci. USA 2007, 104, 15069–15074. [Google Scholar] [CrossRef]
- Konner, A.C.; Bruning, J.C. Toll-like receptors: Linking inflammation to metabolism. Trends Endocrinol. Metab. 2011, 22, 16–23. [Google Scholar] [CrossRef]
- Siri-Tarino, P.W.; Sun, Q.; Hu, F.B.; Krauss, R.M. Saturated fat, carbohydrate, and cardiovascular disease. Am. J. Clin. Nutr. 2010, 91, 502–509. [Google Scholar] [CrossRef]
- Margolskee, R.F.; Dyer, J.; Kokrashvili, Z.; Salmon, K.S.; Ilegems, E.; Daly, K.; Maillet, E.L.; Ninomiya, Y.; Mosinger, B.; Shirazi-Beechey, S.P. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc. Natl. Acad. Sci. USA 2007, 104, 15075–15080. [Google Scholar] [CrossRef]
- Ait-Omar, A.; Monteiro-Sepulveda, M.; Poitou, C.; Le Gall, M.; Cotillard, A.; Gilet, J.; Garbin, K.; Houllier, A.; Chateau, D.; Lacombe, A.; et al. GLUT2 accumulation in enterocyte apical and intracellular membranes: A study in morbidly obese human subjects and ob/ob and high fat-fed mice. Diabetes 2011, 60, 2598–2607. [Google Scholar] [CrossRef]
- Song, D.H.; Getty-Kaushik, L.; Tseng, E.; Simon, J.; Corkey, B.E.; Wolfe, M.M. Glucose-dependent insulinotropic polypeptide enhances adipocyte development and glucose uptake in part through Akt activation. Gastroenterology 2007, 133, 1796–1805. [Google Scholar] [CrossRef]
- Leguisamo, N.M.; Lehnen, A.M.; Machado, U.F.; Okamoto, M.M.; Markoski, M.M.; Pinto, G.H.; Schaan, B.D. GLUT4 content decreases along with insulin resistance and high levels of inflammatory markers in rats with metabolic syndrome. Cardiovasc. Diabetol. 2012, 11, 100. [Google Scholar] [CrossRef]
- Rogero, M.M.; Calder, P.C. Obesity, Inflammation, Toll-Like Receptor 4 and Fatty Acids. Nutrients 2018, 10, 432. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef]
- Serralde-Zuniga, A.E.; Guevara-Cruz, M.; Tovar, A.R.; Herrera-Hernandez, M.F.; Noriega, L.G.; Granados, O.; Torres, N. Omental adipose tissue gene expression, gene variants, branched-chain amino acids, and their relationship with metabolic syndrome and insulin resistance in humans. Genes Nutr. 2014, 9, 431. [Google Scholar] [CrossRef]
- Buratti, S.; Benedetti, S.; Cosio, M.S. Evaluation of the antioxidant power of honey, propolis and royal jelly by amperometric flow injection analysis. Talanta 2007, 71, 1387–1392. [Google Scholar] [CrossRef]
- Nguyen, H.T.L.; Panyoyai, N.; Kasapis, S.; Pang, E.; Mantri, N. Honey and Its Role in Relieving Multiple Facets of Atherosclerosis. Nutrients 2019, 11, 167. [Google Scholar] [CrossRef]






| Group | Body Weight (g) | Glucose (mg/dL) | Insulin (ng/mL) | Leptin (ng/mL) |
|---|---|---|---|---|
| C + W | 546.4 ± 12.6 c | 79.19 ± 3.3 c | 0.24 ± 0.05 h | 3.92 ± 0.08 g |
| HF + W | 628.2 ± 16 a | 93.55 ± 3.7 b, c | 2.40 ± 0.17 g | 17.58 ± 0.24 d |
| C + 10%S | 680 ± 16.33 a | 143.5 ± 3.5 a,b | 5.00 ± 0.21 e | 9.48 ± 0.43 e |
| HF + 10%S | 720.2 ± 3.5 a | 174.8 ± 7.1 a | 6.78 ± 0.24 b | 35.62 ± 1.17 b |
| C + 10%F | 558.5 ± 1 b | 115.4 ± 6.2 b | 2.83 ± 5.4 g | 6.92 ± 0.25 f |
| HF + 10%F | 587.2 ± 18.7 b | 132.8 ± 2.4 a, b | 5.39 ± 0.16 d | 15.81 ± 0.3 d |
| C + 10%G | 589.7 ± 22.3 b | 133 ± 5.7 a, b | 1.72 ± 0.08 g | 4.94 ± 0.15 g |
| HF + 10%G | 606.8 ± 27.3 a, b | 137.9 ± 4.5 a, b | 3.97 ± 0.05 f | 22.78 ± 0.62 c |
| C + 2.5%SG | 562.2 ± 17.5 c | 89.18 ± 2.6 b, c | 0.28 ± 0.04 h | 5.64 ± 0.26 e |
| HF + 2.5%SG | 540 ± 12.05 c | 138.6 ± 6.9 a, b | 1.72 ± 0.15 g | 9.48 ± 0.37 e |
| C + 10%BS | 593.2 ± 6.5 b | 108.4 ± 3.3 b | 0.71 ± 0.09 h | 3.63 ± 0.27 f |
| HF + 10%BS | 599.2 ± 10.73 a, b | 122.2 ± 3.3 b | 1.76 ± 0.12 g | 7.75 ± 0.26 e |
| C + 10%H | 574.3 ± 18.11 b | 85.7 ± 4.7 b, c | 0.31 ± 0.03 h | 3.62 ± 0.19 g |
| HF + 10%H | 591 ± 11.79 b | 84.8 ± 4.7 b, c | 0.61 ± 0.08 h | 9.57 ± 0.29 e |
| C + 10%SV | 609 ± 19.26 a, b | 138.7 ± 8.1 a, b | 3.7 ± 0.11 f | 8.41 ± 0.35 e |
| HF + 10%SV | 634.2 ± 19.29 a | 141.4 ± 3.4 a, b | 5.05 ± 0.20 e | 27.21 ± 0.52 c |
| C + 1.5%SU | 603.3 ± 21.74 a, b | 151.5 ± 2.7 a | 6.44 ± 0.35 c | 25.54 ± 1.32 c |
| HF + 1.5%SU | 540.8 ± 10.4 c | 177 ± 6.5 a | 10.6 ± 0.31 a | 39.80 ± 0.74 a |
| Sweetener | <0.001 | <0.001 | <0.001 | <0.001 |
| Fat | 0.0349 | <0.001 | <0.001 | <0.001 |
| p | 0.0036 | <0.001 | <0.001 | <0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Tapia, M.; Martínez-Medina, J.; Tovar, A.R.; Torres, N. Natural and Artificial Sweeteners and High Fat Diet Modify Differential Taste Receptors, Insulin, and TLR4-Mediated Inflammatory Pathways in Adipose Tissues of Rats. Nutrients 2019, 11, 880. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11040880
Sánchez-Tapia M, Martínez-Medina J, Tovar AR, Torres N. Natural and Artificial Sweeteners and High Fat Diet Modify Differential Taste Receptors, Insulin, and TLR4-Mediated Inflammatory Pathways in Adipose Tissues of Rats. Nutrients. 2019; 11(4):880. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11040880
Chicago/Turabian StyleSánchez-Tapia, Mónica, Jonathan Martínez-Medina, Armando R Tovar, and Nimbe Torres. 2019. "Natural and Artificial Sweeteners and High Fat Diet Modify Differential Taste Receptors, Insulin, and TLR4-Mediated Inflammatory Pathways in Adipose Tissues of Rats" Nutrients 11, no. 4: 880. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11040880