Natural Products and Acute Myeloid Leukemia: A Review Highlighting Mechanisms of Action
Abstract
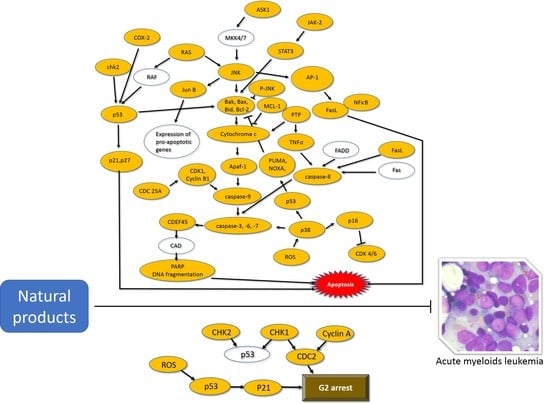
:1. Introduction
2. Methods
3. Natural Compounds and Acute Myeloid Leukemia
3.1. Alkaloids
3.2. Carotenoids
3.3. Nitrogen-Containing Compounds
3.4. Organosulfur Compounds
3.5. Phenolics
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dohner, H.; Weisdorf, D.J.; Bloomfield, C.D. Acute myeloid leukemia. N. Engl. J. Med. 2015, 373, 1136–1152. [Google Scholar] [CrossRef]
- Dombret, H.; Gardin, C. An update of current treatments for adult acute myeloid leukemia. Blood 2016, 127, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Kadia, T.M.; Ravandi, F.; Cortes, J.; Kantarjian, H. New drugs in acute myeloid leukemia. Ann. Oncol. 2016, 27, 770–778. [Google Scholar] [CrossRef]
- Stone, R.M.; Mandrekar, S.J.; Sanford, B.L.; Laumann, K.; Geyer, S.; Bloomfield, C.D.; Thiede, C.; Prior, T.W.; Döhner, K.; Marcucci, G.; et al. Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation. N. Engl. J. Med. 2017, 377, 454–464. [Google Scholar] [CrossRef]
- Jarfelt, M.; Andersen, N.H.; Hasle, H. Is it possible to cure childhood acute myeloid leukaemia without significant cardiotoxicity? Br. J. Haematol. 2016, 175, 577–587. [Google Scholar] [CrossRef]
- Watts, J.; Nimer, S. Recent advances in the understanding and treatment of acute myeloid leukemia. F1000Research 2018, 7, 1196. [Google Scholar] [CrossRef]
- Liu, R.H. Potential synergy of phytochemicals in cancer prevention: Mechanism of action. J. Nutr. 2004, 134, 3479S–3485S. [Google Scholar] [CrossRef]
- Katz, L.; Baltz, R.H. Natural product discovery: Past, present, and future. J. Ind. Microbiol. Biotechnol. 2016, 43, 155–176. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Premoli, M.; Aria, F.; Bonini, S.A.; Maccarinelli, G.; Gianoncelli, A.; Memo, M.; Mastinu, A. Cannabimimetic plants: Are they new cannabinoidergic modulators? Planta 2019, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Drahl, C.; Cravatt, B.F.; Sorensen, E.J. Protein-reactive natural products. Angew. Chem. Int. Ed. Engl. 2005, 44, 5788–5809. [Google Scholar] [CrossRef]
- Bonini, S.A.; Premoli, M.; Tambaro, S.; Kumar, A.; Maccarinelli, G.; Memo, M.; Mastinu, A. Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history. J. Ethnopharmacol. 2018, 227, 300–315. [Google Scholar] [CrossRef]
- Siu, F.-M.; Ma, D.-L.; Cheung, Y.-W.; Lok, C.-N.; Yan, K.; Yang, Z.; Yang, M.; Xu, S.; Ko, B.C.-B.; He, Q.-Y.; et al. Proteomic and transcriptomic study on the action of a cytotoxic saponin (Polyphyllin D): Induction of endoplasmic reticulum stress and mitochondria-mediated apoptotic pathways. Proteomics 2008, 8, 3105–3117. [Google Scholar] [CrossRef]
- Park, J.-W.; Woo, K.J.; Lee, J.-T.; Lim, J.H.; Lee, T.-J.; Kim, S.H.; Choi, Y.H.; Kwon, T.K. Resveratrol induces pro-apoptotic endoplasmic reticulum stress in human colon cancer cells. Oncol. Rep. 2007, 18, 1269–1273. [Google Scholar] [CrossRef] [PubMed]
- Carter, L.G.; D’Orazio, J.A.; Pearson, K.J. Resveratrol and cancer: Focus on in vivo evidence. Endocr. Relat. Cancer 2014, 21, R209–R225. [Google Scholar] [CrossRef] [PubMed]
- Grossman, E.A.; Ward, C.C.; Spradlin, J.N.; Bateman, L.A.; Huffman, T.R.; Miyamoto, D.K.; Kleinman, J.I.; Nomura, D.K. Covalent Ligand Discovery Against Druggable Hotspots Targeted by Anti-Cancer Natural Products. Cell Chem. Biol. 2017, 24, 1368–1376.e4. [Google Scholar] [CrossRef]
- Roomi, M.W.; Kalinovsky, T.; Roomi, N.W.; Niedzwiecki, A.; Rath, M. In vitro and in vivo inhibition of human Fanconi anemia head and neck squamous carcinoma by a phytonutrient combination. Int. J. Oncol. 2015, 46, 2261–2266. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Kim, B. Anti-Cancer Natural Products and Their Bioactive Compounds Inducing ER Stress-Mediated Apoptosis: A Review. Nutrients 2018, 10, 1021. [Google Scholar] [CrossRef]
- Premoli, M.; Aria, F.; Bonini, S.A.; Maccarinelli, G.; Gianoncelli, A.; Della Pina, S.; Tambaro, S.; Memo, M.; Mastinu, A. Cannabidiol: Recent advances and new insights for neuropsychiatric disorders treatment. Life Sci. 2019, 224, 120–127. [Google Scholar] [CrossRef]
- Ames, B.N.; Gold, L.S. Endogenous mutagens and the causes of aging and cancer. Mutat. Res. 1991, 250, 3–16. [Google Scholar] [CrossRef]
- Yang, C.; Ma, X.; Wang, Z.; Zeng, X.; Hu, Z.; Ye, Z.; Shen, G. Curcumin induces apoptosis and protective autophagy in castration-resistant prostate cancer cells through iron chelation. Drug Des. Dev. Ther. 2017, 11, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, R.; Coppo, E.; Marchese, A.; Daglia, M.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Nabavi, S.M. Phytochemicals for human disease: An update on plant-derived compounds antibacterial activity. Microbiol. Res. 2017, 196, 44–68. [Google Scholar] [CrossRef] [PubMed]
- Lien le, Q.; Linh, T.M.; Giang, V.H.; Mai, N.C.; Nhiem, N.X.; Tai, B.H.; Cuc, N.T.; Anh Hle, T.; Ban, N.K.; Minh, C.V.; et al. New naphthalene derivatives and isoquinoline alkaloids from ancistrocladus cochinchinensis with their anti-proliferative activity on human cancer cells. Bioorg. Med. Chem. Lett. 2016, 26, 3913–3917. [Google Scholar] [CrossRef]
- Vieira Torquato, H.F.; Ribeiro-Filho, A.C.; Buri, M.V.; Araujo Junior, R.T.; Pimenta, R.; de Oliveira, J.S.; Filho, V.C.; Macho, A.; Paredes-Gamero, E.J.; de Oliveira Martins, D.T. Canthin-6-one induces cell death, cell cycle arrest and differentiation in human myeloid leukemia cells. Biochim. Biophys. Acta 2017, 1861, 958–967. [Google Scholar] [CrossRef]
- Dantas, B.B.; Faheina-Martins, G.V.; Coulidiati, T.H.; Bomfim, C.C.B.; Dias, C.D.S.; Barbosa-Filho, J.M.; Araújo, D.A.M. Effects of curine in HL-60 leukemic cells: Cell cycle arrest and apoptosis induction. J. Nat. Med. 2015, 69, 218–223. [Google Scholar] [CrossRef]
- Tesoriere, L.; Attanzio, A.; Allegra, M.; Gentile, C.; Livrea, M.A. Phytochemical indicaxanthin suppresses 7-ketocholesterol-induced thp-1 cell apoptosis by preventing cytosolic ca(2+) increase and oxidative stress. Br. J. Nutr. 2013, 110, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.G.; Su, P.J.; Tsai, P.W.; Yang, L.L.; Wang, C.C. Intermedin a, a new labdane diterpene isolated from alpinia intermedia, prolonged the survival time of p-388d1 tumor-bearing cdf1 mice. Planta Med. 2017, 83, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Grüllich, C.; Ziegler, C.; Finke, J. Rabbit Anti T-Lymphocyte Globulin Induces Apoptosis in Peripheral Blood Mononuclear Cell Compartments and Leukemia Cells, While Hematopoetic Stem Cells Are Apoptosis Resistant. Biol. Blood Marrow Transplant. 2009, 15, 173–182. [Google Scholar] [CrossRef]
- Li, D.-H.; Li, J.-Y.; Xue, C.-M.; Han, T.; Sai, C.-M.; Wang, K.-B.; Lu, J.-C.; Jing, Y.-K.; Hua, H.-M.; Li, Z.-L. Antiproliferative Dimeric Aporphinoid Alkaloids from the Roots of Thalictrum cultratum. J. Nat. Prod. 2017, 80, 2893–2904. [Google Scholar] [CrossRef]
- Lee, Y.-K. Activation of apoptotic protein in U937 cells by a component of turmeric oil. BMB Rep. 2009, 42, 96–100. [Google Scholar] [CrossRef]
- Kim, K.N.; Heo, S.J.; Kang, S.M.; Ahn, G.; Jeon, Y.J. Fucoxanthin induces apoptosis in human leukemia hl-60 cells through a ros-mediated bcl-xl pathway. Toxicol. In Vitro 2010, 24, 1648–1654. [Google Scholar] [CrossRef]
- Saikia, M.; Retnakumari, A.P.; Anwar, S.; Anto, N.P.; Mittal, R.; Shah, S.; Pillai, K.S.; Balachandran, V.S.; Peter, V.; Thomas, R.; et al. Heteronemin, a marine natural product, sensitizes acute myeloid leukemia cells towards cytarabine chemotherapy by regulating farnesylation of Ras. Oncotarget 2018, 9, 18115–18127. [Google Scholar] [CrossRef]
- Choi, J.J.; Kwon, O.-K.; Oh, S.-R.; Lee, H.-K.; Ahn, K.-S. The effect of isolancifolide on the apoptosis in HL-60 cells through caspase-8-dependent and -independent pathways. Arch. Pharmacal Res. 2012, 35, 137–143. [Google Scholar] [CrossRef]
- Fedorov, S.N.; Makarieva, T.N.; Guzii, A.G.; Shubina, L.K.; Kwak, J.Y.; Stonik, V.A. Marine two-headed sphingolipid-like compound rhizochalin inhibits egf-induced transformation of jb6 p+ cl41 cells. Lipids 2009, 44, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Thao, N.P.; Luyen, B.T.T.; Kim, E.-J.; Kang, H.-K.; Kim, S.; Cuong, N.X.; Nam, N.H.; Van Kiem, P.; Van Minh, C.; Kim, Y.H. Asterosaponins from the Starfish Astropecten monacanthus Suppress Growth and Induce Apoptosis in HL-60, PC-3, and SNU-C5 Human Cancer Cell Lines. Boil. Pharm. Bull. 2014, 37, 315–321. [Google Scholar] [CrossRef]
- Teng, Y.; Iuchi, K.; Iwasa, E.; Fujishiro, S.; Hamashima, Y.; Dodo, K.; Sodeoka, M. Unnatural enantiomer of chaetocin shows strong apoptosis-inducing activity through caspase-8/caspase-3 activation. Bioorgan. Med. Chem. Lett. 2010, 20, 5085–5088. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, P.; Sengupta, S.B. Role of diallyl disulfide-mediated cleavage of c-Myc and Sp-1 in the regulation of telomerase activity in human lymphoma cell line U937. Nutrition 2015, 31, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Okada, N.; Tanabe, H.; Tazoe, H.; Ishigami, Y.; Fukutomi, R.; Yasui, K.; Isemura, M. Differentiation-associated alteration in sensitivity to apoptosis induced by (−)-epigallocatechin-3-O-gallate in HL-60 cells. Biomed. Res. 2009, 30, 201–206. [Google Scholar] [CrossRef]
- Wu, S.S.; Chen, L.G.; Lin, R.J.; Lin, S.Y.; Lo, Y.E.; Liang, Y.C. Cytotoxicity of (−)-vitisin b in human leukemia cells. Drug Chem. Toxicol. 2013, 36, 313–319. [Google Scholar] [CrossRef]
- Pathania, A.S.; Guru, S.K.; Ashraf, N.U.; Riyaz-Ul-Hassan, S.; Ali, A.; Tasduq, S.A.; Malik, F.; Bhushan, S. A novel stereo bioactive metabolite isolated from an endophytic fungus induces caspase dependent apoptosis and STAT-3 inhibition in human leukemia cells. Eur. J. Pharmacol. 2015, 765, 75–85. [Google Scholar] [CrossRef]
- McMahon, C.M.; Luger, S.M. Maintenance therapy in acute myeloid leukemia: What is the future? Semin. Hematol. 2019, 56, 102–109. [Google Scholar] [CrossRef]
- Christman, L.M.; Dean, L.L.; Allen, J.C.; Godinez, S.F.; Toomer, O.T. Peanut skin phenolic extract attenuates hyperglycemic responses in vivo and in vitro. PLoS ONE 2019, 14, e0214591. [Google Scholar] [CrossRef] [PubMed]
- Shieh, P.-C.; Chen, Y.-O.; Kuo, D.-H.; Chen, F.-A.; Tsai, M.-L.; Chang, I.-S.; Wu, H.; Sang, S.; Ho, C.-T.; Pan, M.-H. Induction of Apoptosis by [8]-shogaol via Reactive Oxygen Species Generation, Glutathione Depletion and Caspase Activation in Human Leukemia Cells. J. Agric. Food Chem. 2010, 58, 3847–3854. [Google Scholar] [CrossRef]
- Sawadogo, W.R.; Cerella, C.; Al-Mourabit, A.; Moriou, C.; Teiten, M.-H.; Guissou, I.P.; Dicato, M.; Diederich, M. Cytotoxic, Antiproliferative and Pro-Apoptotic Effects of 5-Hydroxyl-6,7,3′,4′,5′-Pentamethoxyflavone Isolated from Lantana ukambensis. Nutrients 2015, 7, 10388–10397. [Google Scholar] [CrossRef]
- Devari, S.; Jaglan, S.; Kumar, M.; Deshidi, R.; Guru, S.; Bhushan, S.; Kushwaha, M.; Gupta, A.P.; Gandhi, S.G.; Sharma, J.P.; et al. Capsaicin production by Alternaria alternata, an endophytic fungus from Capsicum annum; LC–ESI–MS/MS analysis. Phytochemistry 2014, 98, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.J.; Yang, J.S.; Lu, C.C.; Chiang, S.Y.; Lin, J.G.; Chung, J.G. Ethanol extract of hedyotis diffusa willd upregulates g0/g1 phase arrest and induces apoptosis in human leukemia cells by modulating caspase cascade signaling and altering associated genes expression was assayed by cdna microarray. Environ. Toxicol. 2015, 30, 1162–1177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Nishimoto, Y.; Tokuda, H.; Suzuki, N.; Yasukawa, K.; Kitdamrongtham, W.; Akazawa, H.; Manosroi, A.; Manosroi, J.; Akihisa, T. Cancer Chemopreventive Effect of Bergenin from Peltophorum pterocarpum Wood. Chem. Biodivers. 2013, 10, 1866–1875. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.Z.; Tan, N.H.; Ji, C.J.; Fan, J.T.; Huang, H.Q.; Han, H.J.; Zhou, G.B. Apoptosis inducement of bigelovin from inula helianthus-aquatica on human leukemia u937 cells. Phytother. Res. 2009, 23, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Mulyaningsih, S.; Youns, M.; El-Readi, M.Z.; Ashour, M.L.; Nibret, E.; Sporer, F.; Herrmann, F.; Reichling, J.; Wink, M.; El-Readi, M.Z.; et al. Biological activity of the essential oil of Kadsura longipedunculata (Schisandraceae) and its major components. J. Pharm. Pharmacol. 2010, 62, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-C.; Chow, J.-M.; Chien, M.-H.; Lin, C.-W.; Chen, H.-Y.; Hsiao, P.-C.; Yang, S.-F. Cantharidic acid induces apoptosis of human leukemic HL-60 cells via c-Jun N-terminal kinase-regulated caspase-8/-9/-3 activation pathway. Environ. Toxicol. 2018, 33, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Masuda, Y.; Asada, K.; Satoh, R.; Takada, K.; Kitajima, J. Capillin, a major constituent of Artemisia capillaris Thunb. flower essential oil, induces apoptosis through the mitochondrial pathway in human leukemia HL-60 cells. Phytomedicine 2015, 22, 545–552. [Google Scholar] [CrossRef]
- Wang, R.; Cong, W.-H.; Guo, G.; Li, X.-X.; Chen, X.-L.; Yu, X.-N.; Li, H. Synergism between carnosic acid and arsenic trioxide on induction of acute myeloid leukemia cell apoptosis is associated with modulation of PTEN/Akt signaling pathway. Chin. J. Integr. Med. 2012, 18, 934–941. [Google Scholar] [CrossRef]
- Kikuchi, H.; Yuan, B.; Yuhara, E.; Imai, M.; Furutani, R.; Fukushima, S.; Hazama, S.; Hirobe, C.; Ohyama, K.; Takagi, N.; et al. Involvement of histone H3 phosphorylation via the activation of p38 MAPK pathway and intracellular redox status in cytotoxicity of HL-60 cells induced by Vitex agnus-castus fruit extract. Int. J. Oncol. 2014, 45, 843–852. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, J.; Chen, M.; Li, L.; Huan, F.; Li, A.; Liu, Y.; Xia, Y.; Duan, J.-A.; Ma, S. Metabolomics profiles delineate uridine deficiency contributes to mitochondria-mediated apoptosis induced by celastrol in human acute promyelocytic leukemia cells. Oncotarget 2016, 7, 46557–46572. [Google Scholar] [CrossRef]
- Mallick, S.; Ghosh, P.; Samanta, S.K.; Kinra, S.; Pal, B.C.; Gomes, A.; Vedasiromoni, J.R. Corchorusin-d, a saikosaponin-like compound isolated from corchorus acutangulus lam., targets mitochondrial apoptotic pathways in leukemic cell lines (hl-60 and u937). Cancer Chemother. Pharmacol. 2010, 66, 709–719. [Google Scholar] [CrossRef]
- Choi, J.-H.; Lee, K.-T. Costunolide-Induced Apoptosis in Human Leukemia Cells: Involvement of c-Jun N-Terminal Kinase Activation. Biol. Pharm. Bull. 2009, 32, 1803–1808. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Kang, H.; Xu, Q.; Chen, M.J.; Liao, Y.; Thiyagarajan, M.; O’Donnell, J.; Christensen, D.J.; Nicholson, C.; Iliff, J.J.; et al. Sleep Drives Metabolite Clearance from the Adult Brain. Science 2013, 342, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, R.; Ma, E.; Deng, Y.; Wang, X.; Xiao, J.; Jing, Y. The induction of g2/m cell-cycle arrest and apoptosis by cucurbitacin e is associated with increased phosphorylation of eif2alpha in leukemia cells. Anti-Cancer Drugs 2010, 21, 389–400. [Google Scholar] [CrossRef]
- Yang, C.-W.; Chang, C.-L.; Lee, H.-C.; Chi, C.-W.; Pan, J.-P.; Yang, W.-C. Curcumin induces the apoptosis of human monocytic leukemia THP-1 cells via the activation of JNK/ERK Pathways. BMC Complement. Altern. Med. 2012, 12, 22. [Google Scholar] [CrossRef]
- Hikita, K.; Hattori, N.; Takeda, A.; Yamakage, Y.; Shibata, R.; Yamada, S.; Kato, K.; Murata, T.; Tanaka, H.; Kaneda, N. Potent apoptosis-inducing activity of erypoegin k, an isoflavone isolated from erythrina poeppigiana, against human leukemia hl-60 cells. J. Nat. Med. 2018, 72, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Ma, E.; Wang, X.; Li, Y.; Sun, X.; Tai, W.; Li, T.; Guo, T. Induction of apoptosis by furanodiene in HL60 leukemia cells through activation of TNFR1 signaling pathway. Cancer Lett. 2008, 271, 158–166. [Google Scholar] [CrossRef]
- Kim, N.-S.; Jeong, S.-I.; Hwang, B.-S.; Lee, Y.-E.; Kang, S.-H.; Lee, H.-C.; Oh, C.-H. Gallic Acid Inhibits Cell Viability and Induces Apoptosis in Human Monocytic Cell Line U937. J. Med. Food 2011, 14, 240–246. [Google Scholar] [CrossRef] [PubMed]
- You, Z.; Chen, D.; Wei, Q.; Zhao, L.; Xia, J.; Li, D.; Li, J. Ginsenoside Rh2 inhibits proliferation and promotes apoptosis of leukemia KG1-α cells. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi = Chin. J. Cell. Mol. Immunol. 2014, 30, 565–568. [Google Scholar]
- Chung, K.S.; Cho, S.H.; Shin, J.S.; Kim, D.H.; Choi, J.H.; Choi, S.Y.; Rhee, Y.K.; Hong, H.D.; Lee, K.T. Ginsenoside rh2 induces cell cycle arrest and differentiation in human leukemia cells by upregulating tgf-beta expression. Carcinogenesis 2013, 34, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Saxena, A.K.; Singh, J.; Bhushan, S. Natural antioxidants synergistically enhance the anticancer potential of AP9-cd, a novel lignan composition from Cedrus deodara in human leukemia HL-60 cells. Chem.-Biol. Interact. 2010, 188, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Hien, N.T.; Nhiem, N.X.; Yen, D.T.; Hang, D.T.; Tai, B.H.; Quang, T.H.; Tuan Anh, H.L.; Kiem, P.V.; Minh, C.V.; Kim, E.J.; et al. Chemical constituents of the annona glabra fruit and their cytotoxic activity. Pharm. Biol. 2015, 53, 1602–1607. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-H.; Jeong, S.-J.; Kim, S.-H.; Kim, J.-H.; Jung, J.H.; Koh, W.; Kim, J.H.; Kim, D.K.; Chen, C.-Y.; Kim, S.-H. Icariside II Induces Apoptosis in U937 Acute Myeloid Leukemia Cells: Role of Inactivation of STAT3-Related Signaling. PLoS ONE 2012, 7, e28706. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-D.; Shan, L.; Li, W.; Li, H.-L.; Zhang, W.-D. Isochamaejasmin induces apoptosis in leukemia cells through inhibiting Bcl-2 family proteins. Chin. J. Nat. Med. 2015, 13, 660–666. [Google Scholar] [CrossRef]
- Guo, J.-R.; Chen, Q.-Q.; Lam, C.W.-K.; Zhang, W. Effects of karanjin on cell cycle arrest and apoptosis in human A549, HepG2 and HL-60 cancer cells. Biol. Res. 2015, 48, 818. [Google Scholar] [CrossRef]
- Chen, Y.J.; Chou, C.J.; Chang, T.T. Compound mmh01 possesses toxicity against human leukemia and pancreatic cancer cells. Toxicol. In Vitro 2009, 23, 418–424. [Google Scholar] [CrossRef]
- Tran, M.H.; Nguyen, M.T.; Nguyen, H.D.; Nguyen, T.D.; Phuong, T.T. Cytotoxic constituents from the seeds of Vietnamese Caesalpinia sappan. Pharm. Biol. 2015, 53, 1549–1554. [Google Scholar] [CrossRef]
- Blaschke, M.; McKinnon, R.; Nguyen, C.H.; Holzner, S.; Zehl, M.; Atanasov, A.G.; Schelch, K.; Krieger, S.; Diaz, R.; Frisch, R.; et al. A eudesmane-type sesquiterpene isolated from Pluchea odorata (L.) Cass. combats three hallmarks of cancer cells: Unrestricted proliferation, escape from apoptosis and early metastatic outgrowth in vitro. Mutat. Res. 2015, 777, 79–90. [Google Scholar] [CrossRef]
- Minker, C.; Duban, L.; Karas, D.; Järvinen, P.; Lobstein, A.; Muller, C.D. Impact of Procyanidins from Different Berries on Caspase 8 Activation in Colon Cancer. Oxidative Med. Cell. Longev. 2015, 2015, 1–13. [Google Scholar] [CrossRef]
- Duan, D.; Zhang, B.; Yao, J.; Liu, Y.; Fang, J. Shikonin targets cytosolic thioredoxin reductase to induce ROS-mediated apoptosis in human promyelocytic leukemia HL-60 cells. Free Radic. Biol. Med. 2014, 70, 182–193. [Google Scholar] [CrossRef]
- Trivedi, R.; Müller, G.A.; Rathore, M.S.; Mishra, D.P.; Dihazi, H. Anti-Leukemic Activity of Shikonin: Role of ERP57 in Shikonin Induced Apoptosis in Acute Myeloid Leukemia. Cell. Physiol. Biochem. 2016, 39, 604–616. [Google Scholar] [CrossRef]
- Alachkar, H.; Santhanam, R.; Harb, J.G.; Lucas, D.M.; Oaks, J.J.; Hickey, C.J.; Pan, L.; Kinghorn, A.D.; Caligiuri, M.A.; Perrotti, D.; et al. Silvestrol exhibits significant in vivo and in vitro antileukemic activities and inhibits FLT3 and miR-155 expressions in acute myeloid leukemia. J. Hematol. Oncol. 2013, 6, 21. [Google Scholar] [CrossRef]
- Liu, J.-J.; Liu, W.-D.; Yang, H.-Z.; Zhang, Y.; Fang, Z.-G.; Liu, P.-Q.; Lin, D.-J.; Xiao, R.-Z.; Hu, Y.; Wang, C.-Z.; et al. Inactivation of PI3k/Akt signaling pathway and activation of caspase-3 are involved in tanshinone I-induced apoptosis in myeloid leukemia cells in vitro. Ann. Hematol. 2010, 89, 1089–1097. [Google Scholar] [CrossRef]
- Liu, C.; Li, J.; Wang, L.; Wu, F.; Huang, L.; Xu, Y.; Ye, J.; Xiao, B.; Meng, F.; Chen, S.; et al. Analysis of tanshinone IIA induced cellular apoptosis in leukemia cells by genome-wide expression profiling. BMC Complement. Altern. Med. 2012, 12, 5. [Google Scholar] [CrossRef]
- Enomoto, R.; Koshiba, C.; Suzuki, C.; Lee, E. Wogonin potentiates the antitumor action of etoposide and ameliorates its adverse effects. Cancer Chemother. Pharmacol. 2011, 67, 1063–1072. [Google Scholar] [CrossRef]
- Huang, S.-T.; Wang, C.-Y.; Yang, R.-C.; Chu, C.-J.; Wu, H.-T.; Pang, J.-H.S. Wogonin, an active compound in Scutellaria baicalensis, induces apoptosis and reduces telomerase activity in the HL-60 leukemia cells. Phytomedicine 2010, 17, 47–54. [Google Scholar] [CrossRef]
- Nibret, E.; Youns, M.; Krauth-Siegel, R.L.; Wink, M. Biological Activities of Xanthatin from Xanthium strumarium Leaves. Phytother. Res. 2011, 25, 1883–1890. [Google Scholar] [CrossRef]
- Omer, F.A.A.; Hashim, N.B.M.; Ibrahim, M.Y.; Dehghan, F.; Yahayu, M.; Karimian, H.; Salim, L.Z.A.; Mohan, S. Beta-mangostin from Cratoxylum arborescens activates the intrinsic apoptosis pathway through reactive oxygen species with downregulation of the HSP70 gene in the HL60 cells associated with a G0/G1 cell-cycle arrest. Tumor Biol. 2017, 39. [Google Scholar] [CrossRef]
- Baron, J.; Wang, E.S. Gemtuzumab ozogamicin for the treatment of acute myeloid leukemia. Expert Rev. Clin. Pharmacol. 2018, 11, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Click, Z.R.; Seddon, A.N.; Bae, Y.R.; Fisher, J.D.; Ogunniyi, A. New Food and Drug Administration-Approved and Emerging Novel Treatment Options for Acute Myeloid Leukemia. Pharmacotherapy 2018, 38, 1143–1154. [Google Scholar] [CrossRef]
- Yates, J.W.; Wallace, H.J., Jr.; Ellison, R.R.; Holland, J.F. Cytosine arabinoside (NSC-63878) and daunorubicin (NSC-83142) therapy in acute nonlymphocytic leukemia. Cancer Chemother. Rep. 1973, 57, 485–488. [Google Scholar] [PubMed]
- Kadia, T.M.; Ravandi, F.; O’Brien, S.; Cortes, J.; Kantarjian, H.M. Progress in acute myeloid leukemia. Clin. Lymphoma Myeloma Leuk. 2015, 15, 139–151. [Google Scholar] [CrossRef]
- Dohner, H.; Estey, E.H.; Amadori, S.; Appelbaum, F.R.; Buchner, T.; Burnett, A.K.; Dombret, H.; Fenaux, P.; Grimwade, D.; Larson, R.A.; et al. Diagnosis and management of acute myeloid leukemia in adults: Recommendations from an international expert panel, on behalf of the European leukemianet. Blood 2010, 115, 453–474. [Google Scholar] [CrossRef]
- Burnett, A.K.; Russell, N.H.; Hills, R.K.; Hunter, A.E.; Kjeldsen, L.; Yin, J.; Gibson, B.E.; Wheatley, K.; Milligan, D. Optimization of Chemotherapy for Younger Patients with Acute Myeloid Leukemia: Results of the Medical Research Council AML15 Trial. J. Clin. Oncol. 2013, 31, 3360–3368. [Google Scholar] [CrossRef] [PubMed]
- Borthakur, G.; Kantarjian, H.; Wang, X.; Plunkett, W.K., Jr.; Gandhi, V.V.; Faderl, S.; Garcia-Manero, G.; Ravandi, F.; Pierce, S.; Estey, E.H. Treatment of core-binding-factor in acute myelogenous leukemia with fludarabine, cytarabine, and granulocyte colony-stimulating factor results in improved event-free survival. Cancer 2008, 113, 3181–3185. [Google Scholar] [CrossRef] [PubMed]
- Acheampong, D.O.; Adokoh, C.K.; Asante, D.-B.; Asiamah, E.A.; Barnie, P.A.; Bonsu, D.O.; Kyei, F. Immunotherapy for acute myeloid leukemia (AML): A potent alternative therapy. Biomed. Pharmacother. 2018, 97, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Cooper, S.L.; Brown, P.A. Treatment of pediatric acute lymphoblastic leukemia. Pediatr. Clin. N. Am. 2015, 62, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Siveen, K.S.; Uddin, S.; Mohammad, R.M. Targeting acute myeloid leukemia stem cell signaling by natural products. Mol. Cancer 2017, 16, 13. [Google Scholar] [CrossRef] [PubMed]
- Huber, B.; Eberl, L.; Feucht, W.; Polster, J. Influence of Polyphenols on Bacterial Biofilm Formation and Quorum-sensing. Z. Nat. C 2003, 58, 879–884. [Google Scholar] [CrossRef]
- Karakaya, S.; Koca, M.; Yılmaz, S.V.; Yıldırım, K.; Pınar, N.M.; Demirci, B.; Brestic, M.; Sytar, O. Molecular Docking Studies of Coumarins Isolated from Extracts and Essential Oils of Zosima absinthifolia Link as Potential Inhibitors for Alzheimer’s Disease. Molecules 2019, 24, 722. [Google Scholar] [CrossRef]
- Siddique, A.B.; Ebrahim, H.; Mohyeldin, M.; Qusa, M.; Batarseh, Y.; Fayyad, A.; Tajmim, A.; Nazzal, S.; Kaddoumi, A.; El Sayed, K. Novel liquid-liquid extraction and self-emulsion methods for simplified isolation of extra-virgin olive oil phenolics with emphasis on (−)-oleocanthal and its oral anti-breast cancer activity. PLoS ONE 2019, 14, e0214798. [Google Scholar] [CrossRef] [PubMed]
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, pharmacology and treatment. Br. J. Pharmacol. 2017, 174, 1290–1324. [Google Scholar] [CrossRef]
- Williamson, E.M. Synergy and other interactions in phytomedicines. Phytomedicine 2001, 8, 401–409. [Google Scholar] [CrossRef] [PubMed]


| Classification | Compound | Source | Cell Line/Animal Model | Dose/Duration | Mechanism | Reference |
|---|---|---|---|---|---|---|
| Alkaloids | 4′-methoxy-5-epi-ancistecrorine A1 | Ancistrocladus cochinchinensis | HL-60 | IC50 3.8–6.2 μM | [22] | |
| Alkaloids | Canthin-6-one | Various plant genera and from fungi | Kasumi-1 | 45 μM; 24 h | c-capase-3, -8, -9, ROS, ASK-1, p38, JNK, CHK2, p53, H2A.X, p16, INK4A, p27-Kip1 ↑ | [23] |
| MMP, p-RB ↓ | ||||||
| Alkaloids | Curine | Chondrodendron platyphyllum | HL-60 | 15 μM; 48h | MMP ↓ | [24] |
| Alkaloids | Indicaxanthin | Cactus pear fruit | THP-1 | 13.5, 15, 15.5, 15.9, 16 µM (16 mM-7-KC); 12, 24 h | c-caspase-3, c-PARP-1, free intracellular Ca2+ ↑ | [25] |
| Ik-Bα ↓ | ||||||
| Alkaloids | Intermedin A | Alpinia intermedia | HL-60 | 23.57 ± 2.15 µg/Ml; 12 h | c-caspase-3, c-PARP ↑ | [26] |
| Alkaloids | Polyclonal anti-T-lymphocyte globulins | Rabbit ATG- Fresenius® | HL-60 | KG1: 100, 200, 300, 400, 500 μg/mL; 6 h | GVHD prophylaxis ↑ | [27] |
| KG-1 | U937: 100, 200, 300, 400, 500 μg/mL; 6 h | |||||
| U937 | ||||||
| Alkaloids | Thalicultratine C | Thalictrum cultratum | HL-60 | 0.06, 0.3, 1.5 μM; 24, 48, 72 h | MMP ↓ | [28] |
| Classification | Compound | Source | Cell Line/Animal Model | Dose/Duration | Mechanism | Reference |
|---|---|---|---|---|---|---|
| Carotenoids | Aromatic (ar)-tumerone | Turmeric oil | U937 | 40, 80, 120 µg/mL; 48 h | Bax, c-caspase-3, cytochrome c, p53 ↑ | [29] |
| Carotenoids | Fucoxanthin | Ishige okamurae | HL-60 | 7.5, 15, 30 μg; 72 h | c-caspase-3, -7, c-PARP, ROS ↑ | [30] |
| Bcl-xL ↓ | ||||||
| Carotenoids | Heteronemin | Hyrtios sp. | HL-60 | 5 nM; 72 h | AP-1, c-myc, MAPK, NF-κB, Ras ↑ | [31] |
| Carotenoids | Isolancifolide | Actinodaphne lancifolia | HL-60 | HL-60: 25 μM; 2, 6, 12 h | c-caspase-8, -9, c-PARP ↑ | [32] |
| Bid, pro-caspase-3, -8 ↓ |
| Classification | Compound | Source | Cell Line/Animal Model | Dose/Duration | Mechanism | Reference |
|---|---|---|---|---|---|---|
| Nitrogen-containing compounds | Rhizochalin | Rhizochalina incrustata | THP-1 | 10, 20 mM; 6, 24 h | p53 ↑ | [33] |
| Classification | Compound | Source | Cell Line/Animal Model | Dose/Duration | Mechanism | Reference |
|---|---|---|---|---|---|---|
| Organosulfur compounds | Asterosaponin | Astropecten monacanthus | HL-60 | 0.01, 0.1, 1, 10, 50, 100 µM; 72 h | Bax, c-caspase-3, -9, c-PARP ↑ | [34] |
| AKT, Bcl-2, c-myc, ERK 1/2, MAPK, PI3K ↓ | ||||||
| Organosulfur compounds | Chaetocin | Chaetomium species fungi | HL-60 | 0.3 μM; 4, 24 h | c-caspase-3, -8 ↑ | [35] |
| Organosulfur compounds | Diallyl disulfide (DADS) | Allium sativum | U937 | 25, 50, 100, 150 μM; 24 h | c-myc, Mad1, Sp-1 ↑ | [36] |
| hTERT ↓ |
| Classification | Compound | Source | Cell Line/Animal Model | Dose/Duration | Mechanism | Reference |
|---|---|---|---|---|---|---|
| Phenolics | Epigallocatechin-3-O-gallate (EGCG) | Green tea | HL-60 | 50, 100, 150, 200, 250 μg; 24 h | 67LR ↓ | [37] |
| Phenolics | Vitisin B | Vitis thunbergii var. taiwaniana | HL-60 | HL-60: 12.5, 25 μM; 24, 36, 48 h | Bax, c-caspase-3, -8, -9, c-PARP, FasL, JNK ↑ | [38] |
| Phenolics | (R)-5-hydroxy-2-methylchroman-4-one (HMC) | Cryptosporiopsis sp. H2-1, NFCCI-2856 | HL-60 | 10, 20, 30 µg/mL; 48 h | c-caspase-3, -8, -9, c-PARP-1, CDK1, Cyclin A, cytochrome c, p21, p53, PUMA ↑ | [39] |
| Bax, Bcl-2, Bcl-xL, Bid, c-IAP-1, c-myc, cyclin D1, MMP, STAT3, survivin, VEGF ↓ | ||||||
| Phenolics | (8)-shogaol | Ginger | HL-60 | 30 µM | c-caspase-3, -9, c-DFF-45, c-PARP, ROS ↑ | [42] |
| Bid, glutathione, MMP, pro-caspase-8 ↓ | ||||||
| Phenolics | 5-hydroxy-6,7,3′,4′,5′-pentamethoxyflavone | Lantana ukambensis | U937 | 10 μg/mL; 24 h | [43] | |
| Phenolics | Alternariol-10-methyl ether | Alternaria alternata | HL-60 | 100, 200 µM; 72 h | c-caspases ↑ | [44] |
| MMP ↓ | ||||||
| Phenolics | Anthraquinone | Hedyotis diffusa Willd | HL-60 | 25, 50, 100, 200 μg/mL; 48 h | c-caspase-3, -8, -9 ↑ | [45] |
| Phenolics | Bergenin | Peltophorum pterocarpum | HL-60 | HL-60: 1–100 μM; 48 h | cytochrome P450 ↓ | [46] |
| Phenolics | Bigelovin | Inula helianthus-aquatia C. Y. Wu | U937 | 1 µM; 24, 48 h | [47] | |
| Phenolics | Camphene | Kadsura longipedunculata | HL-60 | 167.75 µg/mL; 24 h | c-caspase-3, -7 ↑ | [48] |
| Phenolics | Cantharidic acid | Blister beetles | HL-60 | 10 μM; 24 h | c-caspase-3, -8, -9, c-PARP, JNK, p38 ↑ | [49] |
| Phenolics | Capillin | Artemisia capillaris Thunb. Flower. | HL-60 | 2 μM; 6 h | cytochrome c, ERK1/2, JNK ↑ | [50] |
| IC50 6.5 ± 2.9 µM | ||||||
| AP-1, MAPK, NF ↓ | ||||||
| Phenolics | Carnosic acid | Rosmarinus officinalis L | HL-60 | HL-60: 10, 15, 20 μM/L; 24–48 h | c-caspase-9, p27, PTEN ↑ | [51] |
| p-AKT, p-BAD ↓ | ||||||
| Phenolics | Casticin | Vitex agnus Castus | HL-60 | 30, 40, 50 μg/mL; 24 h, 48 h | p-Histone H3 ↑ | [52] |
| Phenolics | Celastrol | Thunder God Vine | HL-60 | 0.5 μM; 24 h | Bax, c-caspase -3, -9, p53 ↑ | [53] |
| DHODH, uridine ↓ | ||||||
| Phenolics | Corchorusin-D | Corchorus acutangulus | HL-60, U937 | HL-60: 25, 50, 75, 100, 120, 150 μg/mL; 24 h | Bax ↑ | [54] |
| U937: 50, 75, 100, 125, 150 μg/mL; 24 h | Bcl-2, MMP ↓ | |||||
| Phenolics | Costunolide | Magnolia sieboldii | U937 | 10 µM; 0.5, 1,2 h | Bcl-2, p-ERK1/2, p-JNK ↑ | [55] |
| Bcl-2, ROS ↓ | ||||||
| Phenolics | Coumarin | Zanthoxylum schinifolium | HL-60 | HL-60: 5 μM; 24, 48 h | Bax, c-caspase -3, -9, c-PARP ↑ | [56] |
| Bcl-2, c-myc, p-AKT, p-ERK1/2 ↓ | ||||||
| Phenolics | Cucurbitacin E | Cucurbitaceae | HL-60 | 1–10 mol/L | Bax ↑ | [57] |
| IAP, Mcl-1, survivin ↓ | ||||||
| Phenolics | Curcumin | turmeric | THP-1 | 50 μM; 3, 6, 12 h | c-caspase-3, -8, -9, c-PARP-1, JNK, p-ERK ↑ | [58] |
| Phenolics | Erypoegin K | Erythrina poeppigiana | HL-60 | 0.175 ± 0.004 μM; 48h | c-caspase-3 ↑ | [59] |
| 10 μM; 24h | ||||||
| Phenolics | Furanodiene | Curcuma wenyujin | HL-60 | 10, 30, 50, 70 μM; 6 h | Bid, c-caspase-3, -8, -9, c-PARP, TNFα ↑ | [60] |
| Phenolics | Gallic acid | Rhus chinensis | U937 | 5.8, 58, 580 µM; 24 h | p53, NF-κB, ↑ | [61] |
| GAPDH, I-κB ↓ | ||||||
| Phenolics | Ginsenoside Rh2 | KG-1 | 100, 200, 300, 400, 500 μg/mL; 6 h | c-caspase-3, p21, p53 ↑ | [62] | |
| Phenolics | Ginsenoside Rh2 | Panax ginseng | HL-60, U937 | HL-60: 10, 20, 30 μM; 24, 48, 72 h | p21, p27 ↑ | [63] |
| U937: 10, 20, 30 μM; 24, 48, 72 h | CDK4, CDK6, Cyclin D1, Cyclin D2, Cyclin D3, Cyclin E ↓ | |||||
| Phenolics | Glaucocalyxin A | Rabdosia japonica var. glaucocalyx | HL-60 | 10.0 μg/mL; 24 h | Bax, c-caspase-3, -9 ↑ | [64] |
| Bcl-2 ↓ | ||||||
| Phenolics | Icariside D2 | Annona glabra Linn | HL-60 | IC50 9.0 ± 1.0 µM; 72h | Bax, c-caspase -3 ↑ | [65] |
| 9.0 µM; 24, 48 h | Bcl-2, c-myc, c-PARP, p-AKT ↓ | |||||
| Phenolics | Icariside II | Epimedium koreanum | U937 | 25, 50 μM; 24, 48, 72 h | c-caspase-3, c-PARP, PTP, SHP-1 ↑ | [66] |
| Bcl-2, Bcl-X, COX-2, JAK2, Src, STAT3, survivin ↓ | ||||||
| Phenolics | Isochamaejasmin | Stellera chamaejasme L. (Thymelaeaceae) | HL-60 | HL-60: IC50 50.40 ± 1.21 μmol·L−1 25 and 50 μmol·L−1; 48 h | c-caspase-3, -9, c-PARP ↑ | [67] |
| Bcl-2 ↓ | ||||||
| K562: IC50 24.51 ± 1.62 μmol·L−1 | ||||||
| Phenolics | Karanjin | Fordia cauliflora | HL-60 | 2, 4, 6 µM; 72 h | [68] | |
| Phenolics | MMH01 | Antrodia cinnamomea | U937 | 5, 10 µg/mL; 24 h | Bax, Bcl-2, Chk2, Cyclin B1 ↑ | [69] |
| Phenolics | Phanginin D | Caesalpinia sappan Linn. (Leguminosae) | HL-60 | 10, 30 µM; 24, 48 h | c-caspase-3 ↑ | [70] |
| Phenolics | PO-1 | Pluchea odorata | HL-60 | IC50 8.9 μM; 72 h | α-tubulin, Cdc2, FAK, JunB, MYPT, NF-κB ↑ | [71] |
| 25, 50, 100 μM; 24, 48, 72 h | CCID, Cdc25A, Chk2, MCF-7 ↓ | |||||
| Phenolics | Proanthocyanidins | 11 berry species | THP-1 | 50 μg/mL; 24 h | c-caspase 8 ↑ | [72] |
| Phenolics | Shikonin | Lithospermum erythrorhizon | HL-60 | 1, 2, 5 μM; 24, 48 h | ROS, c-caspase-3 ↑ | [73] |
| Phenolics | Shikonin | Lithospermum erythrorhizon | HL-60 | 2.5 µM; 24 h | ATF6, calreticulin, CHOP, ERp57, IRE-1 ↓ | [74] |
| Phenolics | Silvestrol | Aglaia foveolata | THP-1 | 50 nm; 24 h | FLT3, miR-155 ↓ | [75] |
| Phenolics | Tanshinone I | Salvia miltiorrhiza Bunge | HL-60 | 31 ± 7.1 μmol/L; 24 h, | Bax ↑ | [76] |
| 22 ± 7.6 μmol/L; 48 h, | ||||||
| c-caspase-3, survivin ↓ | ||||||
| 15 ± 4.3 μmol/L; 72 h | ||||||
| Phenolics | Tanshinone IIA | Salvia miltiorrhiza | U937 | 2, 3, 5, 10 µg/mL; 12 h, 24, 36, 48 h | c-caspase 3, PXR ↑ | [77] |
| CCL2, NF-κB ↓ | ||||||
| Phenolics | Wogonin | Scutellaria baicalensis Georgi | HL-60 | 10 μM; 24h | [3H]etoposide ↑ | [78] |
| P-gp ↓ | ||||||
| Phenolics | Wogonin | Scutellaria baicalensis | HL-60 | 10 mg/mL; 20, 25, 30, 35, 40 min | c-caspase-3 ↑ | [79] |
| Bcl-2, c-myc, telomerase ↓ | ||||||
| Phenolics | Xanthatin | Xanthium strumarium | HL-60 | HL-60: 100 μg/mL; 48 h | c-caspase-3, -7 ↑ | [80] |
| PGE2 ↓ | ||||||
| Phenolics | β-mangostin | Cratoxylum arborescens | HL-60 | 58 µM; 24 h | Bax, c-caspase -3, -9, cytochrome c, p53 ↑ | [81] |
| Bcl-2, HSP70 ↓ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, D.; Kim, M.; Park, H.; Jeong, M.I.; Jung, W.; Kim, B. Natural Products and Acute Myeloid Leukemia: A Review Highlighting Mechanisms of Action. Nutrients 2019, 11, 1010. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11051010
Hwang D, Kim M, Park H, Jeong MI, Jung W, Kim B. Natural Products and Acute Myeloid Leukemia: A Review Highlighting Mechanisms of Action. Nutrients. 2019; 11(5):1010. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11051010
Chicago/Turabian StyleHwang, Dongwon, Minsun Kim, Hyejin Park, Myung In Jeong, Woojin Jung, and Bonglee Kim. 2019. "Natural Products and Acute Myeloid Leukemia: A Review Highlighting Mechanisms of Action" Nutrients 11, no. 5: 1010. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11051010