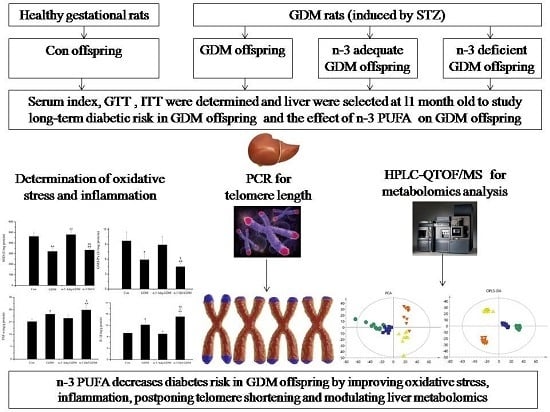
n-3 Polyunsaturated Fatty Acids Decrease Long-Term Diabetic Risk of Offspring of Gestational Diabetes Rats by Postponing Shortening of Hepatic Telomeres and Modulating Liver Metabolism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Model and Offspring Diet Intervention
2.2. Determination of Serum Factors
2.3. Glucose and Insulin Tolerance Tests
2.4. Measurement of Oxidative Stress
2.5. Enzyme-Linked Immunosorbent Assay for Inflammatory Factors
2.6. Telomere Length Measurement
2.7. Metabolomics Analysis
2.7.1. Sample Preparation
2.7.2. High Performance Lipid Chromatography Coupled with Quadrupole-Time of Flight Mass Spectrometry (HPLC-QTOF-MS) Analysis
2.7.3. Data Processing and Metabolites Identification
2.8. Statistical Analysis
3. Results
3.1. Serum Biochemical Index of GDM Offspring and Effect of n-3 PUFA
3.2. Glucose and Insulin Tolerance Test during Growth of GDM Offspring and Effect of n-3 PUFA
3.3. Effect of n-3 PUFA on Oxidative Stress of the Liver of GDM Offspring
3.4. Effect of n-3 PUFA on Inflammatory Factors in the Liver of GDM Offspring
3.5. Influence of GDM on Telomere Length of the Liver of Offspring and the Effect of n-3 PUFA and n-6 PUFA on Telomere Length
3.6. Metabolomics Analysis of the Liver of GDM Offspring at Old Age and the Modulating Effect of n-3 PUFA on the Liver of GDM Offspring
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Pereira, T.J.; Moyce, B.L.; Kereliuk, S.M.; Dolinsky, V.W. Influence of maternal overnutrition and gestational diabetes on the programming of metabolic health outcomes in the offspring: Experimental evidence. Biochem. Cell Biol. 2015, 93, 438–451. [Google Scholar] [CrossRef] [PubMed]
- Riediger, N.D.; Othman, R.A.; Suh, M.; Moghadasian, M.H. A Systemic review of the roles of n-3 Fatty Acids in Health and Disease. J. Am. Diet. Assoc. 2009, 109, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Nettleton, J.; Katz, R. N-3 long-chain polyunsaturated fatty acids in type 2 diabetes: A review. J. Am. Diet. Assoc. 2005, 105, 428–440. [Google Scholar] [CrossRef] [PubMed]
- Poreba, M.; Mostowik, M.; Siniarski, A.; Golebiowska-Wiatrak, R.; Malinowski, K.P.; Haberka, M.; Konduracka, E.; Nessler, J.; Undas, A.; Gajos, G. Treatment with high-dose n-3 PUFAs has no effect on platelet function, coagulation, metabolic status or inflammation in patients with atherosclerosis and type 2 diabetes. Cardiovasc. Diabetol. 2017, 16, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, R.S.; Westgate, J.A.; Beattie, J.; Pattison, N.S.; Gamble, G.; Mildenhall, L.F.J.; Breier, B.H.; Johnstone, F.D. Inverse changes in fetal insulin-like growth factor (IGF)-1 and IGF binding protein-1 in association with higher birth weight in maternal diabetes. Clin. Endocrinol. 2007, 66, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Yessoufou, A.; Soulaimann, N.; Merzouk, S.; Moutairou, K.; Ahissou, H.; Prost, H.; Simmoni, A.M.; Merzouk, A.; Khan, N.A. N-3 Fatty acids modulate antioxidant status in diabetic rats and their macrosomic offspring. Int. J. Obes. 2006, 30, 739–750. [Google Scholar] [CrossRef] [Green Version]
- Pereira, T.J.; Fonseca, M.A.; Campbell, K.E.; Moyce, B.L.; Cole, L.K.; Hatch, G.M.; Doucette, C.A.; Klein, J.; Aliani, M.; Dolinsky, V.W. Maternal obesity characterized by gestational diabetes increases the susceptibility of rat offspring to hepatic steatosis via a disrupted liver metabolome. J. Physiol. 2015, 593, 3181–3197. [Google Scholar] [CrossRef] [Green Version]
- Ruchat, S.; Hivert, M.; Bouchard, L. Epigenetic programming of obesity and diabetes by in utero exposure to gestational diabetes mellitus. Nutr. Rev. 2013, 71, S88–S94. [Google Scholar] [CrossRef]
- Aubert, G.; Lansdorp, P.M. Telomeres and Aging. Physiol. Rev. 2008, 88, 557–579. [Google Scholar] [CrossRef]
- Epel, E.S.; Blackburn, E.H.; Lin, J.; Dhabhar, F.S.; Adler, N.E.; Morrow, J.D.; Cawthon, R.M. Accelerated telomere shortening in response to life stress. Proc. Natl. Acad. Sci. USA 2004, 101, 17312–17315. [Google Scholar] [CrossRef] [Green Version]
- Blasco, M.A. Telomeres and human disease: Ageing, cancer and beyond. Nat. Rev. Genet. 2005, 6, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, H.; Chung, A.C.K.; Xiang, L.; Li, X.; Zheng, Y.; Luan, H.; Zhu, L.; Liu, W.; Peng, Y.; et al. Large-scale longitudinal metabolomics study reveals different trimester-specific alterations of metabolites in relation to gestational diabetes mellitus. J. Proteome Res. 2018, 18, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huo, X.; Cao, Y.; Li, S.; Du, Z.; Shao, P.; Leng, J.; Zhang, C.; Sun, X.; Ma, R.C.W.; et al. Bile acid metabolites in early pregnancy and risk of gestational diabetes in Chinese women: A nested case-control study. EBioMedicine 2018, 35, 317–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gelaye, B.; Clish, C.B.; Denis, M.; Larrabure, G.; Tadesse, M.G.; Deik, A.; Pierce, K.; Bullock, K.; Dennis, C.; Enquobahrie, D.A.; et al. Metabolomics signatures associated with an oral glucose challenge in pregnant women. Diabetes Metab. 2018, 45, 39–46. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, K.; Alexander, J.; Azuma, R.; Xiao, R.; Snyder, N.; Mesaros, C.; Blair, I.; Pinney, S. Gestational diabetes alters the metabolomic profile in 2nd trimester amniotic fluid in a sex-specific manner. Int. J. Mol. Sci. 2018, 19, 2696. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Reichetzeder, C.; Prehn, C.; von Websky, K.; Slowinski, T.; Chen, Y.; Yin, L.; Kleuser, B.; Yang, X.; Adamski, J.; et al. Fetal serum metabolites are independently associated with gestational diabetes mellitus. Cell. Physiol. Biochem. 2018, 45, 625–638. [Google Scholar] [CrossRef]
- Han, H.; Kang, G.; Kim, J.S.; Choi, B.H.; Koo, S. Regulation of glucose metabolism from a liver-centric perspective. Exp. Mol. Med. 2016, 48, e218. [Google Scholar] [CrossRef]
- Barker, D.J.P. The origins of the developmental origins theory. J. Intern. Med. 2007, 261, 412–417. [Google Scholar] [CrossRef]
- Hocher, B. More than genes: The advanced fetal programming hypothesis. J. Reprod. Immunol. 2014, 104, 8–11. [Google Scholar] [CrossRef]
- Gauster, M.; Desoye, G.; Tötsch, M.; Hiden, U. The placenta and gestational diabetes mellitus. Curr. Diabetes Rep. 2012, 12, 16–23. [Google Scholar] [CrossRef]
- Wang, K.; Jin, X.; Li, Q.; Sawaya, A.C.H.F.; Le Leu, R.K.; Conlon, M.A.; Wu, L.; Hu, F. Propolis from Different Geographic Origins Decreases Intestinal Inflammation and Bacteroides spp. Populations in a Model of DSS-Induced Colitis. Mol. Nutr. Food Res. 2018, 62, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Liu, Y.; Ren, G.; Yin, L.; Liang, X.; Geng, T.; Dang, H.; An, R. Resveratrol limits diabetes-associated cognitive decline in rats by preventing oxidative stress and inflammation and modulating hippocampal structural synaptic plasticity. Brain Res. 2016, 1650, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.B.; Backlund, L.; Wegener, G.; Mathe, A.A.; Lavebratt, C. Telomerase dysregulation in the hippocampus of a rat model of depression: Normalization by lithium. Int. J. Neuropsychopharmacol. 2015, 18, 1–8. [Google Scholar] [CrossRef]
- Kaijser, M.; EdstedtBonamy, A.K.; Akre, O.; Cnattingius, S.; Granath, F.; Norman, M.; Ekbom, A. Perinatal Risk Factors for Diabetes in Later Life. Diabetes 2009, 58, 523–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gastaldelli, A.; Cusi, K.; Pettiti, M.; Hardies, J.; Miyazaki, Y.; Berria, R.; Buzzigoli, E.; Sironi, A.M.; Cersosimo, E.; Ferrannini, E.; et al. Relationship Between Hepatic/Visceral Fat and Hepatic Insulin Resistance in Nondiabetic and Type 2 Diabetic Subjects. Gastroenterology 2007, 133, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Aref, A.M.; Ahmed, O.M.; Ali, L.A.; Semmler, M. Maternal rat diabetes mellitus deleteriously affects insulin sensitivity and beta-cell function in the offspring. J. Diabetes Res. 2013, 2013, 429154. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Wang, F.; Shu, J.; Tian, S.; Jiang, Y.; Zhang, D.; Wang, N.; Luo, Q.; Zhang, Y.; Jin, F.; et al. Transgenerational glucose intolerance with Igf2/H19 epigenetic alterations in mouse islet induced by intrauterine hyperglycemia. Diabetes 2012, 61, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Kamel, M.A.; Helmy, M.H.; Hanafi, M.Y.; Mahmoud, S.A.; Abo Elfetooh, H. Impaired peripheral glucose sensing in F1 offspring of diabetic pregnancy. J. Physiol. Biochem. 2014, 70, 685–699. [Google Scholar] [CrossRef]
- Malcolm, J.C.; Lawson, M.L.; Gaboury, I.; Lough, G.; Keely, E. Glucose tolerance of offspring of mother with gestational diabetes mellitus in a low-risk population. Diabet. Med. 2006, 23, 565–570. [Google Scholar] [CrossRef]
- Franks, P.W.; Looker, H.C.; Kobes, S.; Touger, L.; Tataranni, P.A.; Hanson, R.L.; Knowler, W.C. Gestational glucose tolerance and risk of type 2 diabetes in young Pima Indian offspring. Diabetes 2006, 55, 460–465. [Google Scholar] [CrossRef]
- Dabelea, D.; Knowler, W.C.; Pettitt, D.J. Effect of diabetes in pregnancy on offspring: Follow-up research in the Pima Indians. J. Matern.-Fetal Med. 2000, 9, 83–88. [Google Scholar]
- Moussa, S.A. Oxidative stress in diabetes mellitus. Romanian. J. Biophys. 2008, 18, 225–236. [Google Scholar]
- Ouassila, A.; Saad, S.; Meriem, K.; Amira, M.; Imen, M. The investigation of the oxidative stress-related parameters in type 2 diabetes mellitue. Can. J. Diabetes 2015, 39, 44–49. [Google Scholar]
- Weitao, C.; Dandan, R.; Yuanhu, X.; Chao, N.; Youli, T.; Yang, W.; Kungao, Z.; Lu, C.; Litai, J.; Yi, T. Cardiac-specific overexpression of catalase prevents diabetes-induced pathological changes by inhibiting NF-κB signaling activation in the heart. J. Mol. Cell. Cardiol. 2015, 89, 314–325. [Google Scholar]
- Akash, M.S.H.; Rehman, K.; Liaqat, A. Tumor necrosis factor-alpha: Role in development of insulin resistance and pathogenesis of type 2 diabetes mellitus. J. Cell. Biochem. 2018, 119, 105–110. [Google Scholar] [CrossRef]
- Cai, D.; Yuan, M.; Frantz, D.F.; Melendez, P.A.; Hansen, L.; Lee, J.; Shoelson, S.E. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta andNF-kappa B. Nat. Med. 2005, 11, 183–190. [Google Scholar] [CrossRef]
- Vasconcelos-Moreno, M.P.; Fries, G.R.; Gubert, C.; dos Santos, B.T.M.Q.; Fijtman, A.; Sartori, J.; Ferrari, P.; Grun, L.K.; Parisi, M.M.; Guma, F.T.C.R.; et al. Telomere length, oxidative stress, inflammation and BDNF levels in siblings of patients with bipolar disorder: Implications for accelerated cellular aging. Int. J. Neuropsychopharmacol. 2017, 6, 445–454. [Google Scholar] [CrossRef]
- Zhang, J.; Rane, G.; Dai, X.; Shanmugam, M.K.; Arfuso, F.; Samy, R.P.; Lai, M.K.P.; Kappei, D.; Kumar, A.P.; Sethi, G. Aging and the telomere connections: An intimate relationship with inflammation. Ageing Res. Rev. 2016, 25, 55–69. [Google Scholar] [CrossRef]
- Xu, J.; Ye, J.; Wu, Y.; Zhang, H.; Luo, Q.; Han, C.; Ye, X.; Wang, H.; He, J.; Huang, H.; et al. Reduced fetal telomere length in gestational diabetes. PLoS ONE 2014, 9, e86161. [Google Scholar] [CrossRef]
- Cross, J.A.; Brennan, C.; Gray, T.; Temple, R.C.; Dozio, N.; Hughes, J.C.; Levell, N.J.; Murphy, H.; Fowler, D.; Hughes, D.A.; et al. Absence of telomere shortening and oxidative DNA damage in the young adult offspring of women with pre-gestational type 1 diabetes. Diabetologia 2009, 52, 226–234. [Google Scholar] [CrossRef]
- Gilfillan, C.; Naidu, P.; Gunawan, F.; Hassan, F.; Tian, P.; Elwood, N. Leukocyte telomere length in the neonatal offspring of mothers with gestational and pre-gestational diabetes. PLoS ONE 2016, 11, e163824. [Google Scholar] [CrossRef]
- Chavez, J.A.; Summers, S.A. A Ceramide-centric view of insulin resistance. Cell Metab. 2012, 15, 585–594. [Google Scholar] [CrossRef]
- Pardo, V.; González-Rodríguez, Á.; Muntané, J.; Kozma, S.C.; Valverde, Á.M. Role of hepatocyte S6K1 in palmitic acid-induced endoplasmic reticulum stress, lipotoxicity, insulin resistance and in oleic acid-induced protection. Food Chem. Toxicol. 2015, 80, 298–309. [Google Scholar] [CrossRef]
- Boden, G.; Chen, X.; Stein, T.P. Gluconeogenesis in moderately and severely hyperglycemic patients with type 2 diabetes mellitus. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E23–E30. [Google Scholar] [CrossRef]
- De Weerth, C.; Zijl, R.H.; Buitelaar, J.K. Development of cortisol circadian rhythm in infancy. Early Hum. Dev. 2003, 73, 39–52. [Google Scholar] [CrossRef]
- Phillips, D.I.; Barker, D.J.; Fall, C.H.; Seckl, J.R.; Whorwood, C.B.; Wood, P.J.; Walker, B.R. Elevated plasma cortisol concentrations: A link between low birth weight and the insulin resistance syndrome? J. Clin. Endocrinol. Metab. 1998, 83, 757–760. [Google Scholar]
- Wu, J.H.; Micha, R.; Imamura, F.; Pan, A.; Biggs, M.L.; Ajaz, O.; Djousse, L.; Hu, F.B.; Mozaffarian, D. Omega-3 fatty acids and incident type 2 diabetes: A systematic review and meta-analysis. Br. J. Nutr. 2012, 107, S214–S227. [Google Scholar] [CrossRef]
- Muramatsu, T.; Yatsuya, H.; Toyoshima, H.; Sasaki, S.; Li, Y.; Otsuka, R.; Wada, K.; Hotta, Y.; Mitsuhashi, H.; Matsushita, K. Higher dietary intake of alpha-linolenic acid is associated with lower insulin resistance in middle-aged Japanese. Prev. Med. 2010, 50, 272–276. [Google Scholar] [CrossRef]
- Djoussé, L.; Biggs, M.L.; Lemaitre, R.N.; King, I.B.; Song, X.; Ix, J.H.; Mukamal, K.J.; Siscovick, D.S.; Mozaffarian, D. Plasma omega-3 fatty acids and incident diabetes in older adults. Am. J. Clin. Nutr. 2011, 94, 527–533. [Google Scholar] [CrossRef] [Green Version]
- Behme, M.T. Nicotinamide and diabetes prevention. Nutr. Rev. 1995, 53, 137–139. [Google Scholar] [CrossRef]
- Olmos, P.R.; Hodgson, M.I.; Maiz, A.; Manrique, M.; De Valdés, M.D.; Foncea, R.; Acosta, A.M.; Emmerich, M.V.; Velasco, S.; Muñiz, O.P.; et al. Nicotinamide protected first-phase insulin response (FPIR) and prevented clinical disease in first-degree relatives of type-1 diabetics. Diabetes Res. Clin. Pract. 2006, 71, 320–333. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, B.A.; Harmon, B.V.; Cameron, D.P.; Allan, D.J. Nicotinamide prevents the development of diabetes in the cyclophosphamide-induced NOD mouse model by reducing beta-cell apoptosis. J. Pathol. 2000, 191, 86–92. [Google Scholar] [CrossRef]
- Baliarsingh, S.; Beg, Z.H.; Ahmad, J. The therapeutic impacts of tocotrienols in type 2 diabetic patients with hyperlipidemia. Atherosclerosis 2005, 182, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Kuhad, A.; Bishnoi, M.; Tiwari, V.; Chopra, K. Suppression of NF-κβ signaling pathway by tocotrienol can prevent diabetes associated cognitive deficits. Pharmacol. Biochem. Behav. 2009, 92, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Erdinest, N.; Shmueli, O.; Grossman, Y.; Ovadia, H.; Solomon, A. Anti-inflammatory effects of alpha linolenic acid on human corneal epithelial cells. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4396–4406. [Google Scholar] [CrossRef]
- Boon, J.; Hoy, A.J.; Stark, R.; Brown, R.D.; Meex, R.C.; Henstridge, D.C.; Schenk, S.; Meikle, P.J.; Horowitz, J.F.; Kingwell, B.A.; et al. Ceramides contained in LDL are elevated in type 2 diabetes and promote inflammation and skeletal muscle insulin resistance. Diabetes 2013, 62, 401–410. [Google Scholar] [CrossRef]
- Berglund, T.; Wallström, A.; Nguyen, T.V.; Laurell, C.; Ohlsson, A.B. Nicotinamide: Antioxidative and DNA hypomethylation effects in plant cells. Plant Physiol. Biochem. 2017, 118, 551–560. [Google Scholar] [CrossRef]
- Ying, W.; Min, Z. Nicotinamide improves sevoflurane-induced cognitive impairment through suppression of inflammation and anti-apoptosis in rat. Int. J. Clin. Exp. Med. 2015, 8, 20079–20085. [Google Scholar]
- Abu-Bakar, A.; Hakkola, J.; Juvonen, R.; Rahnasto-Rilla, M.; Raunio, H.; A. Lang, M. Function and regulation of the Cyp2a5/CYP2A6 genes in response to toxic insults in the liver. Curr. Drug Metab. 2013, 14, 137–150. [Google Scholar] [CrossRef]
- Skowrońska, M.; Albrecht, J. Alterations of blood brain barrier function in hyperammonemia: An overview. Neurotox. Res. 2012, 21, 236–244. [Google Scholar] [CrossRef]









| 3 Week (Weaning) | Con | GDM |
|---|---|---|
| Fasting glucose (mmol/L) | 6.17 ± 1.24 | 6.01 ± 1.3 |
| Postprandial glucose (mmol/L) | 7.34 ± 0.61 | 7.57 ± 0.49 |
| Fasting insulin (mU/L) | 12.01 ± 0.59 | 9.7 ± 0.46 * |
| Postprandial insulin (mU/L) | 13.23 ± 0.78 | 20.17 ± 1.45 ** |
| TG (mmol/L) | 0.45 ± 0.1 | 0.46 ± 0.11 |
| TC (mmol/L) | 1.76 ± 0.19 | 1.98 ± 0.16 |
| 11 Months old | Con | GDM | n-3 Adq-GDM | n-3 Def-GDM |
|---|---|---|---|---|
| Glucose (mmol/L) | 6.05 ± 0.37 | 8.39 ± 2.07 | 6.3 ± 0.54 | 8.38 ± 1.66 |
| Insulin (mU/L) | 11.73 ± 0.55 | 8.03 ± 0.47 ** | 10 ± 0.46 *## | 7.8 ± 0.78 ** |
| TG (mmol/L) | 0.79 ± 0.13 | 0.63 ± 0.19 | 0.43 ± 0.19 *# | 0.61 ± 0.19 |
| TC (mmol/L) | 1.87 ± 0.04 | 1.79 ± 0.36 | 0.69 ± 0.16 **## | 1.7 ± 0.17 |
| HDL (mmol/L) | 0.53 ± 0.03 | 0.52 ± 0.18 | 0.27 ± 0.04 **## | 0.47 ± 0.03 |
| TC/HDL | 3.52 ± 0.12 | 3.44 ± 0.66 | 2.55 ± 0.28 **## | 3.62 ± 0.19 |
| Identification | RT (Min) | m/z | Changing Trend | Significance | ||
|---|---|---|---|---|---|---|
| GDM vs. Con | n-3adq vs. GDM | n-3def vs. GDM | ||||
| Ceramide (d18:1/16:0) | 17.78 | 560.5073 | ↑ | ↓* | - | Biomarker for diabetes; impair insulin signaling and cause insulin resistance; increase oxidative stress; promote inflammation; contribute to non-alcohol fatty liver disease |
| Tetrahydro-11-deoxycortisol | 11.56 | 337.2756 | ↑ | ↓** | - | Impact cortisol and further impact insulin production and glucose metabolism; inhibit glycogen synthesis; cause insulin resistance |
| 9’-Carboxy-γ-tocotrienol | 16.50 | 395.2234 | ↓ | - | ↓** | Antioxidant effect; improve glycemic control; prevent hyperlipidemia; suppress inflammation |
| α-Linolenic acid | 11.93 | 570.3029 | ↓ | - | ↓** | Decreases diabetic risk; improve insulin resistance; improve oxidative stress and inflammation; regulate lipid metabolism; improve non-alcohol fatty liver disease |
| Hexadecenoic acid | 15.99 | 271.2649 | ↑ | - | - | Induce endoplasmic reticulum stress and insulin resistance; lipotoxicity; enhance oxidative stress and inflammation; contribute to non-alcohol fatty liver disease |
| Niacinamide | 0.95 | 123.0515 | ↓ | - | - | Prevent diabetes; protect β cell; antioxidative role; anti-inflammatory effect |
| Oxalacetic acid | 18.17 | 154.9962 | ↓ | - | ↓* | Impact citric acid cycle and glucose and lipid metabolism; decrease of it indicate gluconeogenesis |
| Phenylethylamine | 2.52 | 122.0956 | ↑ | - | ↑** | Indicate possibility of hepatic damage and hepatic encephalopathy |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Xiao, H.; Li, J.; Guo, X.; Cai, W.; Li, D. n-3 Polyunsaturated Fatty Acids Decrease Long-Term Diabetic Risk of Offspring of Gestational Diabetes Rats by Postponing Shortening of Hepatic Telomeres and Modulating Liver Metabolism. Nutrients 2019, 11, 1699. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11071699
Gao J, Xiao H, Li J, Guo X, Cai W, Li D. n-3 Polyunsaturated Fatty Acids Decrease Long-Term Diabetic Risk of Offspring of Gestational Diabetes Rats by Postponing Shortening of Hepatic Telomeres and Modulating Liver Metabolism. Nutrients. 2019; 11(7):1699. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11071699
Chicago/Turabian StyleGao, Jinlong, Hailong Xiao, Jiaomei Li, Xiaofei Guo, Wenwen Cai, and Duo Li. 2019. "n-3 Polyunsaturated Fatty Acids Decrease Long-Term Diabetic Risk of Offspring of Gestational Diabetes Rats by Postponing Shortening of Hepatic Telomeres and Modulating Liver Metabolism" Nutrients 11, no. 7: 1699. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11071699