The Postprandial Appearance of Features of Cardiometabolic Risk: Acute Induction and Prevention by Nutrients and Other Dietary Substances
Abstract
:1. Introduction
2. The Postprandial Period as a Metabolic Challenge Eliciting Pathophysiological Features Related to Cardiometabolic Risk
3. Fatty Acids, Carbohydrates, and Postprandial Adverse Effects
3.1. Fatty Acids in Challenge Meals
3.2. Carbohydrates in Challenge Meals
4. Relevance to the Effect of the Type of Dietary Protein
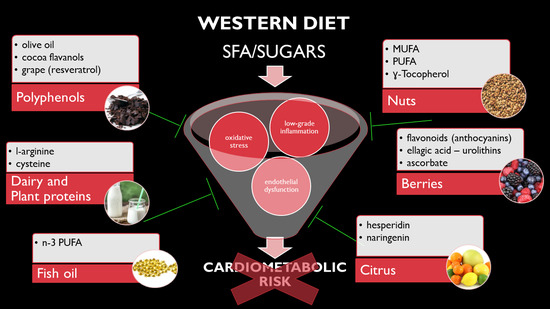
5. Foods, Nutrients, and Other Dietary Substances That May Protect against Adverse Postprandial Effects
5.1. Adding Nuts to a High-Fat/Carbohydrate Meal Prevents Postprandial Endothelial Dysfunction and Oxidative Stress
5.2. Adding Fruit to a High Fat/Carbohydrate Meal Prevents Postprandial Endothelial Dysfunction and Oxidative Stress
6. Key Phytochemicals Identified as Mediating Postprandial Antioxidant and Anti-Inflammatory Effects
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ford, E.S.; Ajani, U.A.; Mokdad, A.H. The Metabolic Syndrome and Concentrations of C-Reactive Protein Among U.S. Youth. Diabetes Care 2005, 28, 878–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grundy, S.M.; Brewer, H.B., Jr.; Cleeman, J.I.; Smith, S.C., Jr.; Lenfant, C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Arterioscler. Thromb. Vasc. Biol. 2004, 24, e13–e18. [Google Scholar] [PubMed]
- Grundy, S.M. Metabolic syndrome pandemic. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.M.; Zimmet, P.; Shaw, J. The metabolic syndrome—A new worldwide definition. Lancet 2005, 366, 1059–1062. [Google Scholar] [CrossRef]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [PubMed]
- Huang, P.L. A comprehensive definition for metabolic syndrome. Dis. Models Mech. 2009, 2, 231–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sattar, N. Inflammation and endothelial dysfunction: Intimate companions in the pathogenesis of vascular disease? Clin. Sci. 2004, 106, 443–445. [Google Scholar] [CrossRef]
- Roberts, C.K.; Hevener, A.L.; Barnard, R.J. Metabolic Syndrome and Insulin Resistance: Underlying Causes and Modification by Exercise Training. Compr. Physiol. 2013, 3, 1–58. [Google Scholar] [Green Version]
- Carey, D.G.; Jenkins, A.B.; Campbell, L.V.; Freund, J.; Chisholm, D.J. Abdominal Fat and Insulin Resistance in Normal and Overweight Women: Direct Measurements Reveal a Strong Relationship in Subjects at Both Low and High Risk of NIDDM. Diabetes 1996, 45, 633–638. [Google Scholar] [CrossRef]
- Godo, S.; Shimokawa, H. Endothelial Functions. Arterioscler. Thromb. Vasc. Biol. 2017, 37, e108–e114. [Google Scholar] [CrossRef] [Green Version]
- Reaven, G.M. The Insulin Resistance Syndrome: Definition and Dietary Approaches to Treatment. Annu. Rev. Nutr. 2005, 25, 391–406. [Google Scholar] [CrossRef]
- Rodriguez-Monforte, M.; Sanchez, E.; Barrio, F.; Costa, B.; Flores-Mateo, G. Metabolic syndrome and dietary patterns: A systematic review and meta-analysis of observational studies. Eur. J. Nutr. 2017, 56, 925–947. [Google Scholar] [CrossRef]
- Keane, D.; Kelly, S.; Healy, N.P.; McArdle, M.A.; Holohan, K.; Roche, H.M. Diet and metabolic syndrome: An overview. Curr. Vasc. Pharmacol. 2013, 11, 842–857. [Google Scholar] [CrossRef]
- Kim, F.; Pham, M.; Maloney, E.; Rizzo, N.O.; Morton, G.J.; Wisse, B.E.; Kirk, E.A.; Chait, A.; Schwartz, M.W. Vascular Inflammation, Insulin Resistance, and Reduced Nitric Oxide Production Precede the Onset of Peripheral Insulin Resistance. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1982–1988. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, W.A.; Lyon, C.J.; Quiñones, M.J. Insulin resistance and the endothelium. Am. J. Med. 2004, 117, 109–117. [Google Scholar] [CrossRef]
- Grandl, G.; Wolfrum, C. Hemostasis, endothelial stress, inflammation, and the metabolic syndrome. Semin. Immunopathol. 2018, 40, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Olver, T.D.; Grunewald, Z.I.; Jurrissen, T.J.; MacPherson, R.E.K.; LeBlanc, P.J.; Schnurbusch, T.R.; Czajkowski, A.M.; Laughlin, M.H.; Rector, R.S.; Bender, S.B.; et al. Microvascular insulin resistance in skeletal muscle and brain occurs early in the development of juvenile obesity in pigs. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 314, R252–R264. [Google Scholar] [CrossRef]
- Muniyappa, R.; Quon, M.J. Insulin action and insulin resistance in vascular endothelium. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 523–530. [Google Scholar] [CrossRef]
- Kim, J.A.; Montagnani, M.; Koh, K.K.; Quon, M.J. Reciprocal relationships between insulin resistance and endothelial dysfunction: Molecular and pathophysiological mechanisms. Circulation 2006, 113, 1888–1904. [Google Scholar] [CrossRef]
- Jonk, A.M.; Houben, A.J.; Schaper, N.C.; de Leeuw, P.W.; Serne, E.H.; Smulders, Y.M.; Stehouwer, C.D. Obesity is associated with impaired endothelial function in the postprandial state. Microvasc. Res. 2011, 82, 423–429. [Google Scholar] [CrossRef]
- Sorop, O.; Van De Wouw, J.; Heinonen, I.; Merkus, D.; Olver, T.D.; Van Duin, R.W.; Duncker, D.J. The microcirculation: A key player in obesity-associated cardiovascular disease. Cardiovasc. Res. 2017, 113, 1035–1045. [Google Scholar] [CrossRef]
- Tousoulis, D.; Tsarpalis, K.; Cokkinos, D.; Stefanadis, C. Effects of insulin resistance on endothelial function: Possible mechanisms and clinical implications. Diabetes Obes. Metab. 2008, 10, 834–842. [Google Scholar] [CrossRef]
- Kubota, T.; Kubota, N.; Kumagai, H.; Yamaguchi, S.; Kozono, H.; Takahashi, T.; Inoue, M.; Itoh, S.; Takamoto, I.; Sasako, T.; et al. Impaired Insulin Signaling in Endothelial Cells Reduces Insulin-Induced Glucose Uptake by Skeletal Muscle. Cell Metab. 2011, 13, 294–307. [Google Scholar] [CrossRef] [Green Version]
- Loader, J.; Khouri, C.; Taylor, F.; Stewart, S.; Lorenzen, C.; Cracowski, J.; Walther, G.; Roustit, M. The continuums of impairment in vascular reactivity across the spectrum of cardiometabolic health: A systematic review and network meta-analysis. Obes. Rev. 2019, 20, 906–920. [Google Scholar] [CrossRef]
- Rizzo, N.O.; Maloney, E.; Pham, M.; Luttrell, I.; Wessell, H.; Tateya, S.; Daum, G.; Handa, P.; Schwartz, M.W.; Kim, F. Reduced NO-cGMP Signaling Contributes to Vascular Inflammation and Insulin Resistance Induced by High-Fat Feeding. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 758–765. [Google Scholar] [CrossRef]
- Sweazea, K.L.; Lekic, M.; Walker, B.R. Comparison of mechanisms involved in impaired vascular reactivity between high sucrose and high fat diets in rats. Nutr. Metab. 2010, 7, 48. [Google Scholar] [CrossRef]
- Vogel, R.A.; Corretti, M.C.; Plotnick, G.D. Effect of a Single High-Fat Meal on Endothelial Function in Healthy Subjects. Am. J. Cardiol. 1997, 79, 350–354. [Google Scholar] [CrossRef]
- Ceriello, A. The post-prandial state and cardiovascular disease: Relevance to diabetes mellitus. Diabetes Metab. Res. Rev. 2000, 16, 125–132. [Google Scholar] [CrossRef]
- De Koning, E.J.; Rabelink, T.J. Endothelial function in the post-prandial state. Atheroscler. Suppl. 2002, 3, 11–16. [Google Scholar] [CrossRef]
- Lee, I.K.; Kim, H.S.; Bae, J.H. Endothelial dysfunction: Its relationship with acute hyperglycaemia and hyperlipidemia. Int. J. Clin. Pract. Suppl. 2002, 129, 59–64. [Google Scholar]
- Nappo, F.; Esposito, K.; Cioffi, M.; Giugliano, G.; Molinari, A.M.; Paolisso, G.; Marfella, R.; Giugliano, D. Postprandial endothelial activation in healthy subjects and in type 2 diabetic patients: Role of fat and carbohydrate meals. J. Am. Coll. Cardiol. 2002, 39, 1145–1150. [Google Scholar] [CrossRef] [Green Version]
- Bae, J.H.; Schwemmer, M.; Lee, I.K.; Lee, H.J.; Park, K.R.; Kim, K.Y.; Bassenge, E. Postprandial hypertriglyceridemia-induced endothelial dysfunction in healthy subjects is independent of lipid oxidation. Int. J. Cardiol. 2003, 87, 259–267. [Google Scholar] [CrossRef]
- Alipour, A.; Elte, J.; Van Zaanen, H.; Rietveld, A.; Cabezas, M.C. Postprandial inflammation and endothelial dysfuction: Figure. Biochem. Soc. Trans. 2007, 35, 466–469. [Google Scholar] [CrossRef]
- Wautier, J.L.; Boulanger, E.; Wautier, M.P. Postprandial hyperglycemia alters inflammatory and hemostatic parameters. Diabetes Metab. 2006, 32, 34–36. [Google Scholar] [CrossRef]
- Burdge, G.C.; Calder, P.C. Plasma cytokine response during the postprandial period: A potential causal process in vascular disease? Br. J. Nutr. 2005, 93, 3–9. [Google Scholar] [CrossRef]
- van Oostrom, A.J.; Sijmonsma, T.P.; Verseyden, C.; Jansen, E.H.; de Koning, E.J.; Rabelink, T.J.; Castro Cabezas, M. Postprandial recruitment of neutrophils may contribute to endothelial dysfunction. J. Lipid Res. 2003, 44, 576–583. [Google Scholar] [CrossRef] [Green Version]
- Blackburn, P.; Després, J.P.; Lamarche, B.; Tremblay, A.; Bergeron, J.; Lemieux, I.; Couillard, C. Postprandial Variations of Plasma Inflammatory Markers in Abdominally Obese Men. Obesity 2006, 14, 1747–1754. [Google Scholar] [CrossRef]
- Poppitt, S.D. Postprandial Lipaemia, Haemostasis, Inflammatory Response and other Emerging Risk Factors for Cardiovascular Disease: The Influence of Fatty Meals. Curr. Nutr. Food Sci. 2005, 1, 23–34. [Google Scholar] [CrossRef] [Green Version]
- Esposito, K.; Ciotola, M.; Sasso, F.C.; Cozzolino, D.; Saccomanno, F.; Assaloni, R.; Ceriello, A.; Giugliano, D. Effect of a single high-fat meal on endothelial function in patients with the metabolic syndrome: Role of tumor necrosis factor-alpha. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 274–279. [Google Scholar] [CrossRef]
- Ceriello, A. Impaired glucose tolerance and cardiovascular disease: The possible role of post-prandial hyperglycemia. Am. Heart J. 2004, 147, 803–807. [Google Scholar] [CrossRef]
- Van Oostrom, A.; Rabelink, T.; Verseyden, C.; Sijmonsma, T.; Plokker, H.; De Jaegere, P.; Cabezas, M.C. Activation of leukocytes by postprandial lipemia in healthy volunteers. Atherosclerosis 2004, 177, 175–182. [Google Scholar] [CrossRef]
- Båvenholm, P.N.; Efendic, S. Postprandial hyperglycaemia and vascular damage—The benefits of acarbose. Diabetes Vasc. Dis. Res. 2006, 3, 72–79. [Google Scholar] [CrossRef]
- Alipour, A.; Elte, J.; Van Zaanen, H.; Rietveld, A.; Cabezas, M.C. Novel aspects of postprandial lipemia in relation to atherosclerosis. Atheroscler. Suppl. 2008, 9, 39–44. [Google Scholar] [CrossRef]
- Ceriello, A.; Quagliaro, L.; Piconi, L.; Assaloni, R.; Da Ros, R.; Maier, A.; Esposito, K.; Giugliano, D. Effect of Postprandial Hypertriglyceridemia and Hyperglycemia on Circulating Adhesion Molecules and Oxidative Stress Generation and the Possible Role of Simvastatin Treatment. Diabetes 2004, 53, 701–710. [Google Scholar] [CrossRef] [Green Version]
- Aljada, A.; Mohanty, P.; Ghanim, H.; Abdo, T.; Tripathy, D.; Chaudhuri, A.; Dandona, P. Increase in intranuclear nuclear factor kappaB and decrease in inhibitor kappaB in mononuclear cells after a mixed meal: Evidence for a proinflammatory effect. Am. J. Clin. Nutr. 2004, 79, 682–690. [Google Scholar] [CrossRef]
- Herieka, M.; Erridge, C. High-fat meal induced postprandial inflammation. Mol. Nutr. Food Res. 2014, 58, 136–146. [Google Scholar] [CrossRef]
- Magné, J.; Mariotti, F.; Fischer, R.; Mathé, V.; Tomé, D.; Huneau, J.F. Early postprandial low-grade inflammation after high-fat meal in healthy rats: Possible involvement of visceral adipose tissue. J. Nutr. Biochem. 2010, 21, 550–555. [Google Scholar] [CrossRef]
- Meneses, M.E.; Camargo, A.; Jimenez-Gomez, Y.; Paniagua, J.A.; Tinahones, F.J.; Roche, H.M.; Perez-Jimenez, F.; Malagón, M.M.; Perez-Martinez, P.; Delgado-Lista, J.; et al. Postprandial inflammatory response in adipose tissue of patients with metabolic syndrome after the intake of different dietary models. Mol. Nutr. Food Res. 2011, 55, 1759–1770. [Google Scholar] [CrossRef]
- Rubin, D.; Claas, S.; Pfeuffer, M.; Nothnagel, M.; Foelsch, U.R.; Schrezenmeir, J. s-ICAM-1 and s-VCAM-1 in healthy men are strongly associated with traits of the metabolic syndrome, becoming evident in the postprandial response to a lipid-rich meal. Lipids Health Dis. 2008, 7, 32. [Google Scholar] [CrossRef]
- Emerson, S.R.; Sciarrillo, C.M.; Kurti, S.P.; Emerson, E.M.; Rosenkranz, S.K. High-Fat Meal-Induced Changes in Markers of Inflammation and Angiogenesis in Healthy Adults Who Differ by Age and Physical Activity Level. Curr. Dev. Nutr. 2019, 3. [Google Scholar] [CrossRef]
- Korkmaz, H.; Onalan, O. Evaluation of Endothelial Dysfunction: Flow-Mediated Dilation. Endothelium 2008, 15, 157–163. [Google Scholar] [CrossRef]
- Thijssen, D.H.J.; Black, M.A.; Pyke, K.E.; Padilla, J.; Atkinson, G.; Harris, R.A.; Parker, B.; Widlansky, M.E.; Tschakovsky, M.E.; Green, D.J. Assessment of flow-mediated dilation in humans: A methodological and physiological guideline. American journal of physiology. Heart Circ. Physiol. 2011, 300, H2–H12. [Google Scholar] [CrossRef]
- Alipour, A.; Van Oostrom, A.J.H.; Izraeljan, A.; Verseyden, C.; Collins, J.M.; Frayn, K.N.; Plokker, T.W.; Elte, J.W.F.; Cabezas, M.C. Leukocyte Activation by Triglyceride-Rich Lipoproteins. Arter. Thromb. Vasc. Biol. 2008, 28, 792–797. [Google Scholar] [CrossRef]
- Norata, G.D.; Grigore, L.; Raselli, S.; Redaelli, L.; Hamsten, A.; Maggi, F.; Eriksson, P.; Catapano, A.L. Post-prandial endothelial dysfunction in hypertriglyceridemic subjects: Molecular mechanisms and gene expression studies. Atherosclerosis 2007, 193, 321–327. [Google Scholar] [CrossRef]
- Dickinson, S.; Hancock, D.P.; Petocz, P.; Ceriello, A.; Brand-Miller, J. High-glycemic index carbohydrate increases nuclear factor-kappaB activation in mononuclear cells of young, lean healthy subjects. Am. J. Clin. Nutr. 2008, 87, 1188–1193. [Google Scholar]
- Bowen, P.E.; Borthakur, G. Postprandial lipid oxidation and cardiovascular disease risk. Curr. Atheroscler. Rep. 2004, 6, 477–484. [Google Scholar] [CrossRef]
- Sies, H.; Stahl, W.; Sevanian, A. Nutritional, Dietary and Postprandial Oxidative Stress. J. Nutr. 2005, 135, 969–972. [Google Scholar] [CrossRef]
- Plotnick, G.D. Effect of antioxidant vitamins on the transient impairment of endothelium-dependent brachial artery vasoactivity following a single high-fat meal. JAMA 1997, 278, 1682–1686. [Google Scholar] [CrossRef]
- Schwander, F.; Kopf-Bolanz, K.A.; Buri, C.; Portmann, R.; Egger, L.; Chollet, M.; McTernan, P.G.; Piya, M.K.; Gijs, M.A.M.; Vionnet, N.; et al. A Dose-Response Strategy Reveals Differences between Normal-Weight and Obese Men in Their Metabolic and Inflammatory Responses to a High-Fat Meal. J. Nutr. 2014, 144, 1517–1523. [Google Scholar] [CrossRef]
- Patel, C.; Ghanim, H.; Ravishankar, S.; Sia, C.L.; Viswanathan, P.; Mohanty, P.; Dandona, P. Prolonged reactive oxygen species generation and nuclear factor-kappaB activation after a high-fat, high-carbohydrate meal in the obese. J. Clin. Endocrinol. Metab. 2007, 92, 4476–4479. [Google Scholar] [CrossRef]
- Tushuizen, M.E.; Nieuwland, R.; Scheffer, P.G.; Sturk, A.; Heine, R.J.; Diamant, M. Two consecutive high-fat meals affect endothelial-dependent vasodilation, oxidative stress and cellular microparticles in healthy men. J. Thromb. Haemost. 2006, 4, 1003–1010. [Google Scholar] [CrossRef]
- Vincent, M.A.; Clerk, L.H.; Lindner, J.R.; Klibanov, A.L.; Clark, M.G.; Rattigan, S.; Barrett, E.J. Microvascular Recruitment Is an Early Insulin Effect That Regulates Skeletal Muscle Glucose Uptake In Vivo. Diabetes 2004, 53, 1418–1423. [Google Scholar] [CrossRef] [Green Version]
- Vincent, M.A.; Montagnani, M.; Quon, M.J. Molecular and physiologic actions of insulin related to production of nitric oxide in vascular endothelium. Curr. Diabetes Rep. 2003, 3, 279–288. [Google Scholar] [CrossRef]
- Vincent, M.A.; Barrett, E.J.; Lindner, J.R.; Clark, M.G.; Rattigan, S. Inhibiting NOS blocks microvascular recruitment and blunts muscle glucose uptake in response to insulin. Am. J. Physiol. Metab. 2003, 285, 123–129. [Google Scholar] [CrossRef]
- Tessari, P.; Coracina, A.; Puricelli, L.; Vettore, M.; Cosma, A.; Millioni, R.; Cecchet, D.; Avogaro, A.; Tiengo, A.; Kiwanuka, E. Acute effect of insulin on nitric oxide synthesis in humans: A precursor-product isotopic study. Am. J. Physiol. Metab. 2007, 293, 776–782. [Google Scholar] [CrossRef]
- Delgado-Lista, J.; Garcia-Rios, A.; Perez-Martinez, P.; Fuentes, F.; Jimenez-Gomez, Y.; Gomez-Luna, M.J.; Parnell, L.D.; Marin, C.; Lai, C.Q.; Perez-Jimenez, F.; et al. Gene variations of nitric oxide synthase regulate the effects of a saturated fat rich meal on endothelial function. Clin. Nutr. 2011, 30, 234–238. [Google Scholar] [CrossRef]
- Barrett, E.J.; Eggleston, E.M.; Inyard, A.C.; Wang, H.; Li, G.; Chai, W.; Liu, Z. The vascular actions of insulin control its delivery to muscle and regulate the rate-limiting step in skeletal muscle insulin action. Diabetologia 2009, 52, 752–764. [Google Scholar] [CrossRef] [Green Version]
- Giugliano, D.; Marfella, R.; Coppola, L.; Verrazzo, G.; Acampora, R.; Giunta, R.; Nappo, F.; Lucarelli, C.; D’Onofrio, F. Vascular effects of acute hyperglycemia in humans are reversed by L-arginine. Evidence for reduced availability of nitric oxide during hyperglycemia. Circulation 1997, 95, 1783–1790. [Google Scholar] [CrossRef]
- Magné, J.; Huneau, J.F.; Delemasure, S.; Rochette, L.; Tomé, D.; Mariotti, F. Whole-body basal nitric oxide production is impaired in postprandial endothelial dysfunction in healthy rats. Nitric Oxide 2009, 21, 37–43. [Google Scholar] [CrossRef]
- Anfossi, G.; Russo, I.; Doronzo, G.; Trovati, M. Contribution of insulin resistance to vascular dysfunction. Arch. Physiol. Biochem. 2009, 115, 199–217. [Google Scholar] [CrossRef]
- Cohn, J.S. Are we ready for a prospective study to investigate the role of chylomicrons in cardiovascular disease? Atheroscler. Suppl. 2008, 9, 15–18. [Google Scholar] [CrossRef]
- O’Keefe, J.H.; Bell, D.S. Postprandial Hyperglycemia/Hyperlipidemia (Postprandial Dysmetabolism) Is a Cardiovascular Risk Factor. Am. J. Cardiol. 2007, 100, 899–904. [Google Scholar] [CrossRef]
- Lairon, D.; Lopez-Miranda, J.; Williams, C. Methodology for studying postprandial lipid metabolism. Eur. J. Clin. Nutr. 2007, 61, 1145–1161. [Google Scholar] [CrossRef]
- Deopurkar, R.; Ghanim, H.; Friedman, J.; Abuaysheh, S.; Sia, C.L.; Mohanty, P.; Viswanathan, P.; Chaudhuri, A.; Dandona, P. Differential effects of cream, glucose, and orange juice on inflammation, endotoxin, and the expression of Toll-like receptor-4 and suppressor of cytokine signaling-3. Diabetes Care 2010, 33, 991–997. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Lundman, P.; Harmer, J.A.; Cutri, B.; Griffiths, K.A.; Rye, K.A.; Barter, P.J.; Celermajer, D.S. Consumption of Saturated Fat Impairs the Anti-Inflammatory Properties of High-Density Lipoproteins and Endothelial Function. J. Am. Coll. Cardiol. 2006, 48, 715–720. [Google Scholar] [CrossRef]
- Spallarossa, P.; Garibaldi, S.; Barisione, C.; Ghigliotti, G.; Altieri, P.; Tracchi, I.; Fabbi, P.; Barsotti, A.; Brunelli, C. Postprandial serum induces apoptosis in endothelial cells: Role of polymorphonuclear-derived myeloperoxidase and metalloproteinase-9 activity. Atherosclerosis 2008, 198, 458–467. [Google Scholar] [CrossRef]
- Borucki, K.; Aronica, S.; Starke, I.; Luley, C.; Westphal, S. Addition of 2.5 g l-arginine in a fatty meal prevents the lipemia-induced endothelial dysfunction in healthy volunteers. Atherosclerosis 2009, 205, 251–254. [Google Scholar] [CrossRef]
- Rathnayake, K.M.; Weech, M.; Jackson, K.G.; Lovegrove, J.A. Impact of meal fatty acid composition on postprandial lipaemia, vascular function and blood pressure in postmenopausal women. Nutr. Res. Rev. 2018, 31, 193–203. [Google Scholar] [CrossRef]
- Jiménez-Gómez, Y.; López-Miranda, J.; Blanco-Colio, L.M.; Marín, C.; Perez-Martinez, P.; Ruano, J.; Paniagua, J.A.; Rodríguez, F.; Egido, J.; Pérez-Jiménez, F. Olive oil and walnut breakfasts reduce the postprandial inflammatory response in mononuclear cells compared with a butter breakfast in healthy men. Atherosclerosis 2009, 204, e70–e76. [Google Scholar] [CrossRef]
- Pacheco, Y.M.; López, S.; Bermudez, B.; Abia, R.; Villar, J.; Muriana, F.J. A meal rich in oleic acid beneficially modulates postprandial sICAM-1 and sVCAM-1 in normotensive and hypertensive hypertriglyceridemic subjects. J. Nutr. Biochem. 2008, 19, 200–205. [Google Scholar] [CrossRef]
- Tentolouris, N.; Arapostathi, C.; Perrea, D.; Kyriaki, D.; Revenas, C.; Katsilambros, N. Differential Effects of Two Isoenergetic Meals Rich in Saturated or Monounsaturated Fat on Endothelial Function in Subjects With Type 2 Diabetes. Diabetes Care 2008, 31, 2276–2278. [Google Scholar] [CrossRef] [Green Version]
- Newens, K.J.; Thompson, A.K.; Jackson, K.G.; Wright, J.; Williams, C.M. DHA-rich fish oil reverses the detrimental effects of saturated fatty acids on postprandial vascular reactivity. Am. J. Clin. Nutr. 2011, 94, 742–748. [Google Scholar] [CrossRef] [Green Version]
- Armah, C.K.; Jackson, K.G.; Doman, I.; James, L.; Cheghani, F.; Minihane, A.M. Fish oil fatty acids improve postprandial vascular reactivity in healthy men. Clin. Sci. 2008, 114, 679–686. [Google Scholar] [CrossRef] [Green Version]
- Monfort-Pires, M.; Crisma, A.R.; Bordin, S.; Ferreira, S.R.G. Greater expression of postprandial inflammatory genes in humans after intervention with saturated when compared to unsaturated fatty acids. Eur. J. Nutr. 2018, 57, 2887–2895. [Google Scholar] [CrossRef]
- Perez-Martinez, P.; Garcia-Quintana, J.M.; Yubero-Serrano, E.M.; Tasset-Cuevas, I.; Tunez, I.; Garcia-Rios, A.; Delgado-Lista, J.; Marín, C.; Perez-Jimenez, F.; Roche, H.M.; et al. Postprandial oxidative stress is modified by dietary fat: Evidence from a human intervention study. Clin. Sci. 2010, 119, 251–261. [Google Scholar] [CrossRef]
- Perez-Martinez, P.; Moreno-Conde, M.; Cruz-Teno, C.; Ruano, J.; Fuentes, F.; Delgado-Lista, J.; Garcia-Rios, A.; Marín, C.; Gómez-Luna, M.J.; Pérez-Jiménez, F.; et al. Dietary fat differentially influences regulatory endothelial function during the postprandial state in patients with metabolic syndrome: From the LIPGENE study. Atherosclerosis 2010, 209, 533–538. [Google Scholar] [CrossRef]
- López-Moreno, J.; García-Carpintero, S.; Jimenez-Lucena, R.; Haro, C.; Rangel-Zúñiga, O.A.; Blanco-Rojo, R.; Yubero-Serrano, E.M.; Tinahones, F.J.; Delgado-Lista, J.; Pérez-Martínez, P.; et al. Effect of Dietary Lipids on Endotoxemia Influences Postprandial Inflammatory Response. J. Agric. Food Chem. 2017, 65, 7756–7763. [Google Scholar] [CrossRef]
- Michalski, M.C.; Vors, C.; LeComte, M.; Laugerette, F. Dietary lipid emulsions and endotoxemia. OCL Oilseeds Fats Crops Lipids 2016, 23. [Google Scholar] [CrossRef]
- Munford, R.S. Endotoxemia—Menace, marker, or mistake? J. Leukoc. Biol. 2016, 100, 687–698. [Google Scholar] [CrossRef]
- West, S.G.; Hecker, K.D.; Mustad, V.A.; Nicholson, S.; Schoemer, S.L.; Wagner, P.; Hinderliter, A.L.; Ulbrecht, J.; Ruey, P.; Kris-Etherton, P.M. Acute effects of monounsaturated fatty acids with and without omega-3 fatty acids on vascular reactivity in individuals with type 2 diabetes. Diabetologia 2005, 48, 113–122. [Google Scholar] [CrossRef]
- Tulk, H.M.; Robinson, L.E. Modifying the n-6/n-3 polyunsaturated fatty acid ratio of a high–saturated fat challenge does not acutely attenuate postprandial changes in inflammatory markers in men with metabolic syndrome. Metabolism 2009, 58, 1709–1716. [Google Scholar] [CrossRef]
- Egert, S.; Stehle, P. Impact of n − 3 fatty acids on endothelial function: Results from human interventions studies. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 121–131. [Google Scholar] [CrossRef]
- Jackson, K.; Armah, C.; Minihane, A. Meal fatty acids and postprandial vascular reactivity: Table. Biochem. Soc. Trans. 2007, 35, 451–453. [Google Scholar] [CrossRef]
- Moreira, A.P.B.; Texeira, T.F.S.; Ferreira, A.B.; Peluzio, M.D.C.G.; Alfenas, R.D.C.G. Influence of a high-fat diet on gut microbiota, intestinal permeability and metabolic endotoxaemia. Br. J. Nutr. 2012, 108, 801–809. [Google Scholar] [CrossRef]
- Thazhath, S.S.; Wu, T.; Bound, M.J.; Checklin, H.L.; Jones, K.L.; Willoughby, S.R.; Horowitz, M.; Rayner, C.K. Changes in meal composition and duration affect postprandial endothelial function in healthy humans. Am. J. Physiol. Liver Physiol. 2014, 307, 1191–1197. [Google Scholar] [CrossRef]
- Motton, D.D.; Keim, N.L.; Tenorio, F.A.; Horn, W.F.; Rutledge, J.C. Postprandial monocyte activation in response to meals with high and low glycemic loads in overweight women. Am. J. Clin. Nutr. 2007, 85, 60–65. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.J.; Kim, J.Y.; Kim, D.R.; Kim, K.S.; Kim, M.K.; Kwon, O. Glycemic index of dietary formula may not be predictive of postprandial endothelial inflammation: A double-blinded, randomized, crossover study in non-diabetic subjects. Nutr. Res. Pract. 2013, 7, 302–308. [Google Scholar] [CrossRef]
- Kendall, C.W.C.; Josse, A.R.; Esfahani, A.; Jenkins, D.J.A. The impact of pistachio intake alone or in combination with high-carbohydrate foods on post-prandial glycemia. Eur. J. Clin. Nutr. 2011, 65, 696–702. [Google Scholar] [CrossRef] [Green Version]
- Zhu, R.; Fan, Z.; Dong, Y.; Liu, M.; Wang, L.; Pan, H. Postprandial Glycaemic Responses of Dried Fruit-Containing Meals in Healthy Adults: Results from a Randomised Trial. Nutrients 2018, 10, 694. [Google Scholar] [CrossRef]
- Ballard, K.D.; Mah, E.; Guo, Y.; Pei, R.; Volek, J.S.; Bruno, R.S. Low-Fat Milk Ingestion Prevents Postprandial Hyperglycemia-Mediated Impairments in Vascular Endothelial Function in Obese Individuals with Metabolic Syndrome. J. Nutr. 2013, 143, 1602–1610. [Google Scholar] [CrossRef]
- Carroll, M.F.; Schade, D.S. Timing of Antioxidant Vitamin Ingestion Alters Postprandial Proatherogenic Serum Markers. Circulation 2003, 108, 24–31. [Google Scholar] [CrossRef]
- Devaraj, S.; Wang-Polagruto, J.; Polagruto, J.; Keen, C.L.; Jialal, I. High-fat, energy-dense, fast-food–style breakfast results in an increase in oxidative stress in metabolic syndrome. Metabolism 2008, 57, 867–870. [Google Scholar] [CrossRef]
- Ghanim, H.; Sia, C.L.; Upadhyay, M.; Korzeniewski, K.; Viswanathan, P.; Abuaysheh, S.; Mohanty, P.; Dandona, P. Orange juice neutralizes the proinflammatory effect of a high-fat, high-carbohydrate meal and prevents endotoxin increase and Toll-like receptor expression. Am. J. Clin. Nutr. 2010, 91, 940–949. [Google Scholar] [CrossRef] [Green Version]
- Sawyer, B.J.; Jarrett, C.L.; Bhammar, D.M.; Ryder, J.R.; Angadi, S.S.; Gaesser, G.A.; Tucker, W.J. High-intensity interval exercise attenuates but does not eliminate endothelial dysfunction after a fast food meal. Am. J. Physiol. Circ. Physiol. 2018, 314, H188–H194. [Google Scholar]
- Ghanim, H.; Abuaysheh, S.; Sia, C.L.; Korzeniewski, K.; Chaudhuri, A.; Fernandez-Real, J.M.; Dandona, P. Increase in plasma endotoxin concentrations and the expression of Toll-like receptors and suppressor of cytokine signaling-3 in mononuclear cells after a high-fat, high-carbohydrate meal: Implications for insulin resistance. Diabetes Care 2009, 32, 2281–2287. [Google Scholar] [CrossRef]
- Järvisalo, M.J.; Jartti, L.; Marniemi, J.; Rönnemaa, T.; Viikari, J.S.A.; Lehtimäki, T.; Raitakari, O.T. Determinants of short-term variation in arterial flow-mediated dilatation in healthy young men. Clin. Sci. 2006, 110, 475–482. [Google Scholar] [CrossRef] [Green Version]
- Dandona, P.; Ghanim, H.; Chaudhuri, A.; Dhindsa, S.; Kim, S.S. Macronutrient intake induces oxidative and inflammatory stress: Potential relevance to atherosclerosis and insulin resistance. Exp. Mol. Med. 2010, 42, 245–253. [Google Scholar] [CrossRef]
- George, T.W.; Waroonphan, S.; Niwat, C.; Gordon, M.H.; Lovegrove, J.A. Effects of acute consumption of a fruit and vegetable puree-based drink on vasodilation and oxidative status. Br. J. Nutr. 2013, 109, 1442–1452. [Google Scholar] [CrossRef]
- Lacroix, S.; Des Rosiers, C.; Gayda, M.; Nozza, A.; Thorin, E.; Tardif, J.C.; Nigam, A. A single Mediterranean meal does not impair postprandial flow-mediated dilatation in healthy men with subclinical metabolic dysregulations. Appl. Physiol. Nutr. Metab. 2016, 41, 888–894. [Google Scholar] [CrossRef] [Green Version]
- Mariotti, F.; Huneau, J.F.; Szezepanski, I.; Petzke, K.J.; Aggoun, Y.; Tomé, D.; Bonnet, D. Meal amino acids with varied levels of arginine do not affect postprandial vascular endothelial function in healthy young men. J. Nutr. 2007, 137, 1383–1389. [Google Scholar] [CrossRef]
- Westphal, S.; Taneva, E.; Kästner, S.; Martens-Lobenhoffer, J.; Bode-Böger, S.; Kropf, S.; Dierkes, J.; Luley, C. Endothelial dysfunction induced by postprandial lipemia is neutralized by addition of proteins to the fatty meal. Atherosclerosis 2006, 185, 313–319. [Google Scholar] [CrossRef]
- Westphal, S.; Kastner, S.; Taneva, E.; Leodolter, A.; Dierkes, J.; Luley, C. Postprandial lipid and carbohydrate responses after the ingestion of a casein-enriched mixed meal. Am. J. Clin. Nutr. 2004, 80, 284–290. [Google Scholar] [CrossRef]
- Dandona, P.; Chaudhuri, A.; Ghanim, H.; Mohanty, P. Insulin as an Anti-Inflammatory and Antiatherogenic Modulator. J. Am. Coll. Cardiol. 2009, 53, S14–S20. [Google Scholar] [CrossRef] [Green Version]
- Mariotti, F. Postprandial low-grade inflammation and the recent data suggesting a protective impact of dietary protein quantity and sources [L’inflammation postprandiale: Les données récentes suggèrent un rôle préventif des protéines alimentaires et de leur nature]. OCL Ol. Corps Gras Lipides 2011, 18, 14–20. [Google Scholar] [CrossRef]
- Lin, C.C.; Tsai, W.C.; Chen, J.Y.; Li, Y.H.; Lin, L.J.; Chen, J.H. Supplements of l-arginine attenuate the effects of high-fat meal on endothelial function and oxidative stress. Int. J. Cardiol. 2008, 127, 337–341. [Google Scholar] [CrossRef]
- Deveaux, A.; Pham, I.; West, S.G.; Andre, E.; Lantoine-Adam, F.; Bunouf, P.; Sadi, S.; Hermier, D.; Mathé, V.; Fouillet, H.; et al. L-Arginine Supplementation Alleviates Postprandial Endothelial Dysfunction When Baseline Fasting Plasma Arginine Concentration Is Low: A Randomized Controlled Trial in Healthy Overweight Adults with Cardiometabolic Risk Factors. J. Nutr. 2016, 146, 1330–1340. [Google Scholar] [CrossRef]
- Magné, J.; Huneau, J.F.; Tsikas, D.; Delemasure, S.; Rochette, L.; Tomé, D.; Mariotti, F. Rapeseed Protein in a High-Fat Mixed Meal Alleviates Postprandial Systemic and Vascular Oxidative Stress and Prevents Vascular Endothelial Dysfunction in Healthy Rats. J. Nutr. 2009, 139, 1660–1666. [Google Scholar] [CrossRef] [Green Version]
- Blouet, C.; Mariotti, F.; Azzout-Marniche, D.; Mathé, V.; Mikogami, T.; Tomé, D.; Huneau, J.F. Dietary cysteine alleviates sucrose-induced oxidative stress and insulin resistance. Free Radic. Biol. Med. 2007, 42, 1089–1097. [Google Scholar] [CrossRef]
- Luiking, Y.C.; Engelen, M.P.; Deutz, N.E. Regulation of nitric oxide production in health and disease. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 97–104. [Google Scholar] [CrossRef] [Green Version]
- Stonehouse, W.; Brinkworth, G.D.; Noakes, M. Palmolein and olive oil consumed within a high protein test meal have similar effects on postprandial endothelial function in overweight and obese men: A randomized controlled trial. Atherosclerosis 2015, 239, 178–185. [Google Scholar] [CrossRef]
- Teunissen-Beekman, K.F.M.; Dopheide, J.; Geleijnse, J.M.; Bakker, S.J.L.; Brink, E.J.; De Leeuw, P.W.; Schalkwijk, C.G.; Van Baak, M.A. Dietary proteins improve endothelial function under fasting conditions but not in the postprandial state, with no effects on markers of low-grade inflammation. Br. J. Nutr. 2015, 114, 1819–1828. [Google Scholar] [CrossRef] [Green Version]
- Fekete, Á.A.; Givens, D.I.; Lovegrove, J.A. Can milk proteins be a useful tool in the management of cardiometabolic health? An updated review of human intervention trials. Proc. Nutr. Soc. 2016, 75, 328–341. [Google Scholar] [CrossRef] [Green Version]
- Fekete, Á.A.; Giromini, C.; Chatzidiakou, Y.; Givens, D.I.; Lovegrove, J.A. Whey protein lowers systolic blood pressure and Ca-caseinate reduces serum TAG after a high-fat meal in mildly hypertensive adults. Sci. Rep. 2018, 8, 5026. [Google Scholar] [CrossRef]
- Lovegrove, J.A.; Givens, D.I. Dairy food products: Good or bad for cardiometabolic disease? Nutr. Res. Rev. 2016, 29, 249–267. [Google Scholar] [CrossRef]
- Stanhewicz, A.E.; Alba, B.K.; Kenney, W.L.; Alexander, L.M. Dairy cheese consumption ameliorates single-meal sodium-induced cutaneous microvascular dysfunction by reducing ascorbate-sensitive oxidants in healthy older adults. Br. J. Nutr. 2016, 116, 658–665. [Google Scholar] [CrossRef] [Green Version]
- Reis, C.E.; Bordalo, L.A.; Rocha, A.L.; Freitas, D.M.; da Silva, M.V.; de Faria, V.C.; Martino, H.S.; Costa, N.M.; Alfenas, R.C. Ground roasted peanuts leads to a lower post-prandial glycemic response than raw peanuts. Nutr. Hosp. 2011, 26, 745–751. [Google Scholar] [CrossRef]
- Ellis, P.R.; Kendall, C.W.; Ren, Y.; Parker, C.; Pacy, J.F.; Waldron, K.W.; Jenkins, D.J. Role of cell walls in the bioaccessibility of lipids in almond seeds. Am. J. Clin. Nutr. 2004, 80, 604–613. [Google Scholar] [CrossRef] [Green Version]
- Grundy, M.M.; Grassby, T.; Mandalari, G.; Waldron, K.W.; Butterworth, P.J.; Berry, S.E.; Ellis, P.R. Effect of mastication on lipid bioaccessibility of almonds in a randomized human study and its implications for digestion kinetics, metabolizable energy, and postprandial lipemia. Am. J. Clin. Nutr. 2015, 101, 25–33. [Google Scholar] [CrossRef]
- Mariotti, F.; Valette, M.; Lopez, C.; Fouillet, H.; Famelart, M.H.; Mathé, V.; Airinei, G.; Benamouzig, R.; Gaudichon, C.; Tome, D.; et al. Casein Compared with Whey Proteins Affects the Organization of Dietary Fat during Digestion and Attenuates the Postprandial Triglyceride Response to a Mixed High-Fat Meal in Healthy, Overweight Men. J. Nutr. 2015, 145, 2657–2664. [Google Scholar] [CrossRef] [Green Version]
- Pujos-Guillot, E.; Brandolini-Bunlon, M.; Fouillet, H.; Joly, C.; Martin, J.F.; Huneau, J.F.; Dardevet, D.; Mariotti, F. Metabolomics Reveals that the Type of Protein in a High-Fat Meal Modulates Postprandial Mitochondrial Overload and Incomplete Substrate Oxidation in Healthy Overweight Men. J. Nutr. 2018, 148, 876–884. [Google Scholar] [CrossRef]
- Desmarchelier, C.; Borel, P.; Lairon, D.; Maraninchi, M.; Valéro, R. Effect of Nutrient and Micronutrient Intake on Chylomicron Production and Postprandial Lipemia. Nutrients 2019, 11, 1299. [Google Scholar] [CrossRef]
- Abubakar, S.M.; Ukeyima, M.T.; Spencer, J.P.E.; Lovegrove, J.A. Acute Effects of Hibiscus sabdariffa Calyces on Postprandial Blood Pressure, Vascular Function, Blood Lipids, Biomarkers of Insulin Resistance and Inflammation in Humans. Nutrients 2019, 11, 341. [Google Scholar] [CrossRef]
- Skulas-Ray, A.C.; Kris-Etherton, P.M.; Teeter, D.L.; Chen, C.Y.; Vanden Heuvel, J.P.; West, S.G. A high antioxidant spice blend attenuates postprandial insulin and triglyceride responses and increases some plasma measures of antioxidant activity in healthy, overweight men. J. Nutr. 2011, 141, 1451–1457. [Google Scholar] [CrossRef]
- Cortés, B.; Núñez, I.; Cofán, M.; Gilabert, R.; Pérez-Heras, A.; Casals, E.; Deulofeu, R.; Ros, E. Acute Effects of High-Fat Meals Enriched With Walnuts or Olive Oil on Postprandial Endothelial Function. J. Am. Coll. Cardiol. 2006, 48, 1666–1671. [Google Scholar] [CrossRef] [Green Version]
- Berryman, C.E.; Grieger, J.A.; West, S.G.; Chen, C.Y.O.; Blumberg, J.B.; Rothblat, G.H.; Sankaranarayanan, S.; Kris-Etherton, P.M. Acute Consumption of Walnuts and Walnut Components Differentially Affect Postprandial Lipemia, Endothelial Function, Oxidative Stress, and Cholesterol Efflux in Humans with Mild Hypercholesterolemia. J. Nutr. 2013, 143, 788–794. [Google Scholar] [CrossRef] [Green Version]
- Kendall, C.W.; Esfahani, A.; Josse, A.R.; Augustin, L.S.; Vidgen, E.; Jenkins, D.J. The glycemic effect of nut-enriched meals in healthy and diabetic subjects. Nutr. Metab. Cardiovasc. Dis. 2011, 21, S34–S39. [Google Scholar] [CrossRef]
- Kendall, C.W.C.; Josse, A.R.; Esfahani, A.; Jenkins, D.J.A. Nuts, metabolic syndrome and diabetes. Br. J. Nutr. 2010, 104, 465–473. [Google Scholar] [CrossRef]
- Liu, X.; Hill, A.M.; West, S.G.; Gabauer, R.M.; McCrea, C.E.; Fleming, J.A.; Kris-Etherton, P.M. Acute Peanut Consumption Alters Postprandial Lipids and Vascular Responses in Healthy Overweight or Obese Men. J. Nutr. 2017, 147, 835–840. [Google Scholar] [CrossRef] [Green Version]
- Naissides, M.; Mamo, J.C.; James, A.P.; Pal, S. The effect of acute red wine polyphenol consumption on postprandial lipaemia in postmenopausal women. Atherosclerosis 2004, 177, 401–408. [Google Scholar] [CrossRef]
- Testa, R.; Bonfigli, A.R.; Prattichizzo, F.; La Sala, L.; De Nigris, V.; Ceriello, A. The “Metabolic Memory” Theory and the Early Treatment of Hyperglycemia in Prevention of Diabetic Complications. Nutrients 2017, 9, 437. [Google Scholar] [CrossRef]
- Schisano, B.; Tripathi, G.; McGee, K.; McTernan, P.G.; Ceriello, A. Glucose oscillations, more than constant high glucose, induce p53 activation and a metabolic memory in human endothelial cells. Diabetologia 2011, 54, 1219–1226. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, K.; Watanabe, K.; Futami-Suda, S.; Yano, H.; Motoyama, M.; Matsumura, N.; Igari, Y.; Suzuki, T.; Nakano, H.; Oba, K. The effects of postprandial glucose and insulin levels on postprandial endothelial function in subjects with normal glucose tolerance. Cardiovasc. Diabetol. 2012, 11, 98. [Google Scholar] [CrossRef]
- Thom, N.J.; Early, A.R.; Hunt, B.E.; Harris, R.A.; Herring, M.P. Eating and arterial endothelial function: A meta-analysis of the acute effects of meal consumption on flow-mediated dilation. Obes. Rev. 2016, 17, 1080–1090. [Google Scholar] [CrossRef]
- Tuccinardi, D.; Farr, O.M.; Upadhyay, J.; Oussaada, S.M.; Klapa, M.I.; Candela, M.; Rampelli, S.; Lehoux, S.; Lazaro, I.; Sala-Vila, A.; et al. Mechanisms Underlying the Cardiometabolic Protective Effect of Walnut Consumption in Obese Subjects: A Cross-Over, Randomized, Double-Blinded, Controlled Inpatient Physiology Study. Diabetes Obes. Metab. 2019. [Google Scholar] [CrossRef]
- Wu, L.; Piotrowski, K.; Rau, T.; Waldmann, E.; Broedl, U.C.; Demmelmair, H.; Koletzko, B.; Stark, R.G.; Nagel, J.M.; Mantzoros, C.S.; et al. Walnut-enriched diet reduces fasting non-HDL-cholesterol and apolipoprotein B in healthy Caucasian subjects: A randomized controlled cross-over clinical trial. Metabolism 2014, 63, 382–391. [Google Scholar] [CrossRef]
- Ros, E.; Núñez, I.; Pérez-Heras, A.; Serra, M.; Gilabert, R.; Casals, E.; Deulofeu, R. A walnut diet improves endothelial function in hypercholesterolemic subjects: A randomized crossover trial. Circulation 2004, 109, 1609–1614. [Google Scholar] [CrossRef]
- Fitschen, P.J.; Rolfhus, K.R.; Winfrey, M.R.; Allen, B.K.; Manzy, M.; Maher, M.A. Cardiovascular Effects of Consumption of Black Versus English Walnuts. J. Med. Food 2011, 14, 890–898. [Google Scholar] [CrossRef]
- Casas-Agustench, P.; Bulló, M.; Salas-Salvadó, J. Nuts, inflammation and insulin resistance. Asia Pac. J. Clin. Nutr. 2010, 19, 124–130. [Google Scholar]
- Halvorsen, B.L.; Holte, K.; Barikmo, I.; Remberg, S.F.; Wold, A.B.; Haffner, K.; Baugerød, H.; Andersen, L.F.; Moskaug, O.; Jacobs, D.R.; et al. A Systematic Screening of Total Antioxidants in Dietary Plants. J. Nutr. 2002, 132, 461–471. [Google Scholar] [CrossRef] [Green Version]
- Haddad, E.H.; Gaban-Chong, N.; Oda, K.; Sabaté, J. Effect of a walnut meal on postprandial oxidative stress and antioxidants in healthy individuals. Nutr. J. 2014, 13, 4. [Google Scholar] [CrossRef]
- Dreher, M.L. Pistachio nuts: Composition and potential health benefits. Nutr. Rev. 2012, 70, 234–240. [Google Scholar] [CrossRef]
- Jenkins, D.J.A.; Josse, A.R.; Salvatore, S.; Vidgen, E.; Rao, A.V.; Kendall, C.W.C.; Brighenti, F.; Augustin, L.S.A.; Ellis, P.R. Almonds Decrease Postprandial Glycemia, Insulinemia, and Oxidative Damage in Healthy Individuals. J. Nutr. 2006, 136, 2987–2992. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Li, Z.; Liu, Y.; Lv, X.; Yang, W. Effects of pistachios on body weight in Chinese subjects with metabolic syndrome. Nutr. J. 2012, 11, 20. [Google Scholar] [CrossRef]
- Kay, C.D.; Gebauer, S.K.; West, S.G.; Kris-Etherton, P.M. Pistachios Increase Serum Antioxidants and Lower Serum Oxidized-LDL in Hypercholesterolemic Adults. J. Nutr. 2010, 140, 1093–1098. [Google Scholar] [CrossRef] [Green Version]
- Salas-Salvadó, J.; Baldrich-Mora, M.; Juanola-Falgarona, M.; Hernández-Alonso, P.; Bulló, M. Beneficial Effect of Pistachio Consumption on Glucose Metabolism, Insulin Resistance, Inflammation, and Related Metabolic Risk Markers: A Randomized Clinical Trial. Diabetes Care 2014, 37, 3098–3105. [Google Scholar] [Green Version]
- Sari, I.; Baltaci, Y.; Bagci, C.; Davutoglu, V.; Erel, O.; Çelik, H.; Ozer, O.; Aksoy, N.; Aksoy, M. Effect of pistachio diet on lipid parameters, endothelial function, inflammation, and oxidative status: A prospective study. Nutrition 2010, 26, 399–404. [Google Scholar] [CrossRef]
- Kocyigit, A.; Koylu, A.; Keles, H. Effects of pistachio nuts consumption on plasma lipid profile and oxidative status in healthy volunteers. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 202–209. [Google Scholar] [CrossRef]
- Kendall, C.W.C.; West, S.G.; Augustin, L.S.; Esfahani, A.; Vidgen, E.; Bashyam, B.; Sauder, K.A.; Campbell, J.; Chiavaroli, L.; Jenkins, A.L.; et al. Acute effects of pistachio consumption on glucose and insulin, satiety hormones and endothelial function in the metabolic syndrome. Eur. J. Clin. Nutr. 2014, 68, 370–375. [Google Scholar] [CrossRef]
- Rangel-Zuniga, O.A.; Haro, C.; Tormos, C.; Perez-Martinez, P.; Delgado-Lista, J.; Marin, C.; Quintana-Navarro, G.M.; Cerda, C.; Saez, G.T.; Lopez-Segura, F.; et al. Frying oils with high natural or added antioxidants content, which protect against postprandial oxidative stress, also protect against DNA oxidation damage. Eur. J. Nutr. 2017, 56, 1597–1607. [Google Scholar] [CrossRef]
- Lyte, J.M.; Gabler, N.K.; Hollis, J.H. Postprandial serum endotoxin in healthy humans is modulated by dietary fat in a randomized, controlled, cross-over study. Lipids Health Dis. 2016, 15, 186. [Google Scholar] [CrossRef]
- Carnevale, R.; Pignatelli, P.; Nocella, C.; Loffredo, L.; Pastori, D.; Vicario, T.; Petruccioli, A.; Bartimoccia, S.; Violi, F. Extra virgin olive oil blunt post-prandial oxidative stress via NOX2 down-regulation. Atherosclerosis 2014, 235, 649–658. [Google Scholar] [CrossRef]
- Park, E.; Edirisinghe, I.; Burton-Freeman, B. Avocado Fruit on Postprandial Markers of Cardio-Metabolic Risk: A Randomized Controlled Dose Response Trial in Overweight and Obese Men and Women. Nutrients 2018, 10, 1287. [Google Scholar] [CrossRef]
- Burton-Freeman, B. Postprandial metabolic events and fruit-derived phenolics: A review of the science. Br. J. Nutr. 2010, 104 (Suppl. S3), S1–S14. [Google Scholar] [CrossRef]
- AlQurashi, R.M.; Galante, L.A.; Rowland, I.R.; Spencer, J.P.; Commane, D.M. Consumption of a flavonoid-rich açai meal is associated with acute improvements in vascular function and a reduction in total oxidative status in healthy overweight men. Am. J. Clin. Nutr. 2016, 104, 1227–1235. [Google Scholar] [CrossRef]
- Rodriguez-Mateos, A.; Del Pino-García, R.; George, T.W.; Vidal-Diez, A.; Heiss, C.; Spencer, J.P.E.; Rodriguez-Mateos, A.; Del Pino-García, R.; Vidal-Diez, A. Impact of processing on the bioavailability and vascular effects of blueberry (poly)phenols. Mol. Nutr. Food Res. 2014, 58, 1952–1961. [Google Scholar] [CrossRef]
- Istas, G.; Feliciano, R.P.; Weber, T.; García-Villalba, R.; Tomás-Barberán, F.; Heiss, C.; Rodriguez-Mateos, A. Plasma urolithin metabolites correlate with improvements in endothelial function after red raspberry consumption: A double-blind randomized controlled trial. Arch. Biochem. Biophys. 2018, 651, 43–51. [Google Scholar] [CrossRef]
- Schell, J.; Betts, N.M.; Foster, M.; Scofield, R.H.; Basu, A. Cranberries improve postprandial glucose excursions in type 2 diabetes. Food Funct. 2017, 8, 3083–3090. [Google Scholar] [CrossRef]
- Hannum, S.M. Potential Impact of Strawberries on Human Health: A Review of the Science. Crit. Rev. Food Sci. Nutr. 2004, 44, 1–17. [Google Scholar] [CrossRef]
- Joseph, S.V.; Edirisinghe, I.; Burton-Freeman, B.M. Fruit Polyphenols: A Review of Anti-inflammatory Effects in Humans. Crit. Rev. Food Sci. Nutr. 2016, 56, 419–444. [Google Scholar] [CrossRef]
- Burton-Freeman, B.; Linares, A.; Hyson, D.; Kappagoda, T. Strawberry modulates LDL oxidation and postprandial lipemia in response to high-fat meal in overweight hyperlipidemic men and women. J. Am. Coll. Nutr. 2010, 29, 46–54. [Google Scholar] [CrossRef]
- Richter, C.K.; Skulas-Ray, A.C.; Gaugler, T.L.; Lambert, J.D.; Proctor, D.N.; Kris-Etherton, P.M. Incorporating freeze-dried strawberry powder into a high-fat meal does not alter postprandial vascular function or blood markers of cardiovascular disease risk: A randomized controlled trial. Am. J. Clin. Nutr. 2017, 105, 313–322. [Google Scholar] [CrossRef]
- Lee, Y.J.; Ahn, Y.; Kwon, O.; Lee, M.Y.; Lee, C.H.; Lee, S.; Park, T.; Kwon, S.W.; Kim, J.Y. Dietary Wolfberry Extract Modifies Oxidative Stress by Controlling the Expression of Inflammatory mRNAs in Overweight and Hypercholesterolemic Subjects: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Agric. Food Chem. 2017, 65, 309–316. [Google Scholar] [CrossRef]
- Urquiaga, I.; Ávila, F.; Echeverria, G.; Perez, D.; Trejo, S.; Leighton, F. A Chilean Berry Concentrate Protects against Postprandial Oxidative Stress and Increases Plasma Antioxidant Activity in Healthy Humans. Oxidative Med. Cell. Longev. 2017, 2017, 8361493. [Google Scholar] [CrossRef]
- Peluso, I.; Palmery, M. Risks of Misinterpretation in the Evaluation of the Effect of Fruit-Based Drinks in Postprandial Studies. Gastroenterol. Res. Pract. 2014, 2014, 870547. [Google Scholar] [CrossRef]
- Blanco-Colio, L.M.; Valderrama, M.; Alvarez-Sala, L.A.; Bustos, C.; Ortego, M.; Hernández-Presa, M.A.; Cancelas, P.; Gómez-Gerique, J.; Millán, J.; Egido, J. Red wine intake prevents nuclear factor-kappaB activation in peripheral blood mononuclear cells of healthy volunteers during postprandial lipemia. Circulation 2000, 102, 1020–1026. [Google Scholar] [CrossRef]
- Covas, M.I.; Gambert, P.; Fito, M.; de la Torre, R. Wine and oxidative stress: Up-to-date evidence of the effects of moderate wine consumption on oxidative damage in humans. Atherosclerosis 2010, 208, 297–304. [Google Scholar] [CrossRef]
- Schmitt, C.A.; Heiss, E.H.; Dirsch, V.M. Effect of resveratrol on endothelial cell function: Molecular mechanisms. BioFactors 2010, 36, 342–349. [Google Scholar] [CrossRef]
- Ghanim, H.; Sia, C.L.; Korzeniewski, K.; Lohano, T.; Abuaysheh, S.; Marumganti, A.; Chaudhuri, A.; Dandona, P. A Resveratrol and Polyphenol Preparation Suppresses Oxidative and Inflammatory Stress Response to a High-Fat, High-Carbohydrate Meal. J. Clin. Endocrinol. Metab. 2011, 96, 1409–1414. [Google Scholar] [CrossRef] [Green Version]
- Hampton, S.M.; Isherwood, C.; Kirkpatrick, V.J.E.; Lynne-Smith, A.C.; Griffin, B.A. The influence of alcohol consumed with a meal on endothelial function in healthy individuals. J. Hum. Nutr. Diet. 2010, 23, 120–125. [Google Scholar] [CrossRef]
- Karatzi, K.; Papamichael, C.; Karatzis, E.; Papaioannou, T.G.; Voidonikola, P.T.; Vamvakou, G.D.; Lekakis, J.; Zampelas, A. Postprandial improvement of endothelial function by red wine and olive oil antioxidants: A synergistic effect of components of the Mediterranean diet. J. Am. Coll. Nutr. 2008, 27, 448–453. [Google Scholar] [CrossRef]
- Dhindsa, S.; Tripathy, D.; Mohanty, P.; Ghanim, H.; Syed, T.; Aljada, A.; Dandona, P. Differential effects of glucose and alcohol on reactive oxygen species generation and intranuclear nuclear factor-kappaB in mononuclear cells. Metabolism 2004, 53, 330–334. [Google Scholar] [CrossRef]
- Bulut, D.; Jelich, U.; Dacanay-Schwarz, R.; Mügge, A. Red Wine Ingestion Prevents Microparticle Formation After a Single High-Fat Meal—A Crossover Study in Healthy Humans. J. Cardiovasc. Pharmacol. 2013, 61, 489–494. [Google Scholar] [CrossRef]
- Fragopoulou, E.; Choleva, M.; Antonopoulou, S.; Demopoulos, C.A. Wine and its metabolic effects. A comprehensive review of clinical trials. Metabolism 2018, 83, 102–119. [Google Scholar] [CrossRef]
- Argyrou, C.; Vlachogianni, I.; Stamatakis, G.; Demopoulos, C.A.; Antonopoulou, S.; Fragopoulou, E. Postprandial effects of wine consumption on Platelet Activating Factor metabolic enzymes. Prostaglandins Other Lipid Mediat. 2017, 130, 23–29. [Google Scholar] [CrossRef]
- Botham, K.M.; Wheeler-Jones, C.P. Postprandial lipoproteins and the molecular regulation of vascular homeostasis. Prog. Lipid Res. 2013, 52, 446–464. [Google Scholar] [CrossRef]
- Bardagjy, A.S.; Hu, Q.; Giebler, K.A.; Ford, A.; Steinberg, F.M. Effects of grape consumption on biomarkers of inflammation, endothelial function, and PBMC gene expression in obese subjects. Arch. Biochem. Biophys. 2018, 646, 145–152. [Google Scholar] [CrossRef]
- Erridge, C.; Attinà, T.; Spickett, C.M.; Webb, D.J. A high-fat meal induces low-grade endotoxemia: Evidence of a novel mechanism of postprandial inflammation. Am. J. Clin. Nutr. 2007, 86, 1286–1292. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic Endotoxemia Initiates Obesity and Insulin Resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [Green Version]
- Hermier, D.; Mathé, V.; Lan, A.; Santini, C.; Quignard-Boulangé, A.; Huneau, J.F.; Mariotti, F. Postprandial low-grade inflammation does not specifically require TLR4 activation in the rat. Nutr. Metab. 2017, 14, 65. [Google Scholar] [CrossRef]
- Mele, L.; Mena, P.; Piemontese, A.; Marino, V.; López-Gutiérrez, N.; Bernini, F.; Brighenti, F.; Zanotti, I.; Del Rio, D. Antiatherogenic effects of ellagic acid and urolithins in vitro. Arch. Biochem. Biophys. 2016, 599, 42–50. [Google Scholar] [CrossRef]
- Morand, C.; Dubray, C.; Milenkovic, D.; Lioger, D.; Martin, J.F.; Scalbert, A.; Mazur, A. Hesperidin contributes to the vascular protective effects of orange juice: A randomized crossover study in healthy volunteers. Am. J. Clin. Nutr. 2010, 93, 73–80. [Google Scholar] [CrossRef]
- Rendeiro, C.; Dong, H.; Saunders, C.; Harkness, L.; Blaze, M.; Hou, Y.; Belanger, R.L.; Corona, G.; Lovegrove, J.A.; Spencer, J.P.E. Flavanone-rich citrus beverages counteract the transient decline in postprandial endothelial function in humans: A randomised, controlled, double-masked, cross-over intervention study. Br. J. Nutr. 2016, 116, 1999–2010. [Google Scholar] [CrossRef]
- Salden, B.N.; Troost, F.J.; De Groot, E.; Stevens, Y.R.; Garces-Rimon, M.; Possemiers, S.; Winkens, B.; Masclee, A.A. Randomized clinical trial on the efficacy of hesperidin 2S on validated cardiovascular biomarkers in healthy overweight individuals. Am. J. Clin. Nutr. 2016, 104, 1523–1533. [Google Scholar] [CrossRef] [Green Version]
- Ono-Moore, K.D.; Snodgrass, R.G.; Huang, S.; Singh, S.; Freytag, T.L.; Burnett, D.J.; Bonnel, E.L.; Woodhouse, L.R.; Zunino, S.J.; Peerson, J.M.; et al. Postprandial Inflammatory Responses and Free Fatty Acids in Plasma of Adults Who Consumed a Moderately High-Fat Breakfast with and without Blueberry Powder in a Randomized Placebo-Controlled Trial. J. Nutr. 2016, 146, 1411–1419. [Google Scholar] [CrossRef]
- Huebbe, P.; Giller, K.; de Pascual-Teresa, S.; Arkenau, A.; Adolphi, B.; Portius, S.; Arkenau, C.N.; Rimbach, G. Effects of blackcurrant-based juice on atherosclerosis-related biomarkers in cultured macrophages and in human subjects after consumption of a high-energy meal. Br. J. Nutr. 2012, 108, 234–244. [Google Scholar] [CrossRef]
- Jin, Y.; Alimbetov, D.; George, T.; Gordon, M.H.; Lovegrove, J.A. A randomised trial to investigate the effects of acute consumption of a blackcurrant juice drink on markers of vascular reactivity and bioavailability of anthocyanins in human subjects. Eur. J. Clin. Nutr. 2011, 65, 849–856. [Google Scholar] [CrossRef]
- Castro-Acosta, M.L.; Smith, L.; Miller, R.J.; McCarthy, D.I.; Farrimond, J.A.; Hall, W.L. Drinks containing anthocyanin-rich blackcurrant extract decrease postprandial blood glucose, insulin and incretin concentrations. J. Nutr. Biochem. 2016, 38, 154–161. [Google Scholar] [CrossRef] [Green Version]
- Wiswedel, I.; Hirsch, D.; Kropf, S.; Gruening, M.; Pfister, E.; Schewe, T.; Sies, H. Flavanol-rich cocoa drink lowers plasma F 2 -isoprostane concentrations in humans. Free Radic. Biol. Med. 2004, 37, 411–421. [Google Scholar] [CrossRef]
- Magrone, T.; Russo, M.A.; Jirillo, E. Cocoa and Dark Chocolate Polyphenols: From Biology to Clinical Applications. Front. Immunol. 2017, 8, 677. [Google Scholar] [CrossRef]
- Edirisinghe, I.; Burton-Freeman, B.; Kappagoda, C.T. Mechanism of the endothelium-dependent relaxation evoked by a grape seed extract. Clin. Sci. 2008, 114, 331–337. [Google Scholar] [CrossRef]
- Edirisinghe, I.; Burton-Freeman, B.; Varelis, P.; Kappagoda, T. Strawberry Extract Caused Endothelium-Dependent Relaxation through the Activation of PI3 Kinase/Akt. J. Agric. Food Chem. 2008, 56, 9383–9390. [Google Scholar] [CrossRef]
- Sies, H.; Schewe, T.; Heiss, C.; Kelm, M. Cocoa polyphenols and inflammatory mediators. Am. J. Clin. Nutr. 2005, 81, 304–312. [Google Scholar] [CrossRef]
- Schnorr, O.; Brossette, T.; Momma, T.Y.; Kleinbongard, P.; Keen, C.L.; Schroeter, H.; Sies, H. Cocoa flavanols lower vascular arginase activity in human endothelial cells in vitro and in erythrocytes in vivo. Arch. Biochem. Biophys. 2008, 476, 211–215. [Google Scholar] [CrossRef]
- Selmi, C.; Cocchi, C.A.; Lanfredini, M.; Keen, C.L.; Gershwin, M.E. Chocolate at heart: The anti-inflammatory impact of cocoa flavanols. Mol. Nutr. Food Res. 2008, 52, 1340–1348. [Google Scholar] [CrossRef]
- Grassi, D.; Desideri, G.; Necozione, S.; Ruggieri, F.; Blumberg, J.B.; Stornello, M.; Ferri, C. Protective Effects of Flavanol-Rich Dark Chocolate on Endothelial Function and Wave Reflection During Acute Hyperglycemia. Hypertension 2012, 60, 827–832. [Google Scholar] [CrossRef] [Green Version]
- Westphal, S.; Luley, C. Flavanol-rich cocoa ameliorates lipemia-induced endothelial dysfunction. Heart Vessel. 2011, 26, 511–515. [Google Scholar] [CrossRef]
- Varadhan, K.; Limb, M.C.; Phillips, B.E.; Atherton, P.J.; Williams, J.P.; Smith, K. Acute cocoa flavanol supplementation improves muscle macro- and microvascular but not anabolic responses to amino acids in older men. Appl. Physiol. Nutr. Metab. 2016, 41, 548–556. [Google Scholar]
- García-Conesa, M.T.; Chambers, K.; Combet, E.; Pinto, P.; Garcia-Aloy, M.; Andrés-Lacueva, C.; De Pascual-Teresa, S.; Mena, P.; Ristic, A.K.; Hollands, W.J.; et al. Meta-Analysis of the Effects of Foods and Derived Products Containing Ellagitannins and Anthocyanins on Cardiometabolic Biomarkers: Analysis of Factors Influencing Variability of the Individual Responses. Int. J. Mol. Sci. 2018, 19, 694. [Google Scholar] [CrossRef]
- Ndiaye, M.; Chataigneau, M.; Lobysheva, I.; Schini-Kerth, V.B. Red wine polyphenol-induced, endothelium-dependent NO-mediated relaxation is due to the redox-sensitive PI3-kinase/Akt-dependent phosphorylation of endothelial NO-synthase in the isolated porcine coronary artery. FASEB J. 2005, 19, 455–457. [Google Scholar] [CrossRef]
- Ndiaye, M.; Chataigneau, T.; Chataigneau, M.; Schini-Kerth, V.B. Red wine polyphenols induce EDHF-mediated relaxations in porcine coronary arteries through the redox-sensitive activation of the PI3-kinase/Akt pathway. Br. J. Pharmacol. 2004, 142, 1131–1136. [Google Scholar] [CrossRef] [Green Version]
- Heiss, C.; Kleinbongard, P.; Dejam, A.; Perré, S.; Schroeter, H.; Sies, H.; Kelm, M. Acute Consumption of Flavanol-Rich Cocoa and the Reversal of Endothelial Dysfunction in Smokers. J. Am. Coll. Cardiol. 2005, 46, 1276–1283. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, C.A.; Dirsch, V.M. Modulation of endothelial nitric oxide by plant-derived products. Nitric Oxide 2009, 21, 77–91. [Google Scholar] [CrossRef]
- Bollenbach, A.; Huneau, J.F.; Mariotti, F.; Tsikas, D. Asymmetric and Symmetric Protein Arginine Dimethylation: Concept and Postprandial Effects of High-Fat Protein Meals in Healthy Overweight Men. Nutrients 2019, 11, 1463. [Google Scholar] [CrossRef]
- McDonald, J.D.; Mah, E.; Chitchumroonchokchai, C.; Reverri, E.J.; Li, J.; Volek, J.S.; Villamena, F.A.; Bruno, R.S. Co-ingestion of whole eggs or egg whites with glucose protects against postprandial hyperglycaemia-induced oxidative stress and dysregulated arginine metabolism in association with improved vascular endothelial function in prediabetic men. Br. J. Nutr. 2018, 120, 901–913. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Remesy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230–242. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimina, L.; Mariotti, F. The Postprandial Appearance of Features of Cardiometabolic Risk: Acute Induction and Prevention by Nutrients and Other Dietary Substances. Nutrients 2019, 11, 1963. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11091963
Dimina L, Mariotti F. The Postprandial Appearance of Features of Cardiometabolic Risk: Acute Induction and Prevention by Nutrients and Other Dietary Substances. Nutrients. 2019; 11(9):1963. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11091963
Chicago/Turabian StyleDimina, Laurianne, and François Mariotti. 2019. "The Postprandial Appearance of Features of Cardiometabolic Risk: Acute Induction and Prevention by Nutrients and Other Dietary Substances" Nutrients 11, no. 9: 1963. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11091963