Docosahexaenoic Acid (DHA) Bioavailability in Humans after Oral Intake of DHA-Containing Triacylglycerol or the Structured Phospholipid AceDoPC®
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Volunteers
2.2. Experimental Procedure
2.3. Synthesis of Labeled DHA Sources
2.3.1. 13C-AceDoPC Synthesis
2.3.2. Production of 13C-DHA-Containing Triacylglycerol
2.4. Blood Red Cells and Plasma Preparation
2.5. Extraction and Separation of Lipids from Red Cells and Plasma
2.6. Quantification of 13C by Gas Chromatography Combustion-Isotope Ratio Mass Spectrometry (GC-C-IRMS)
2.7. Measures of Quality Control
2.8. Statistical Analysis
3. Results
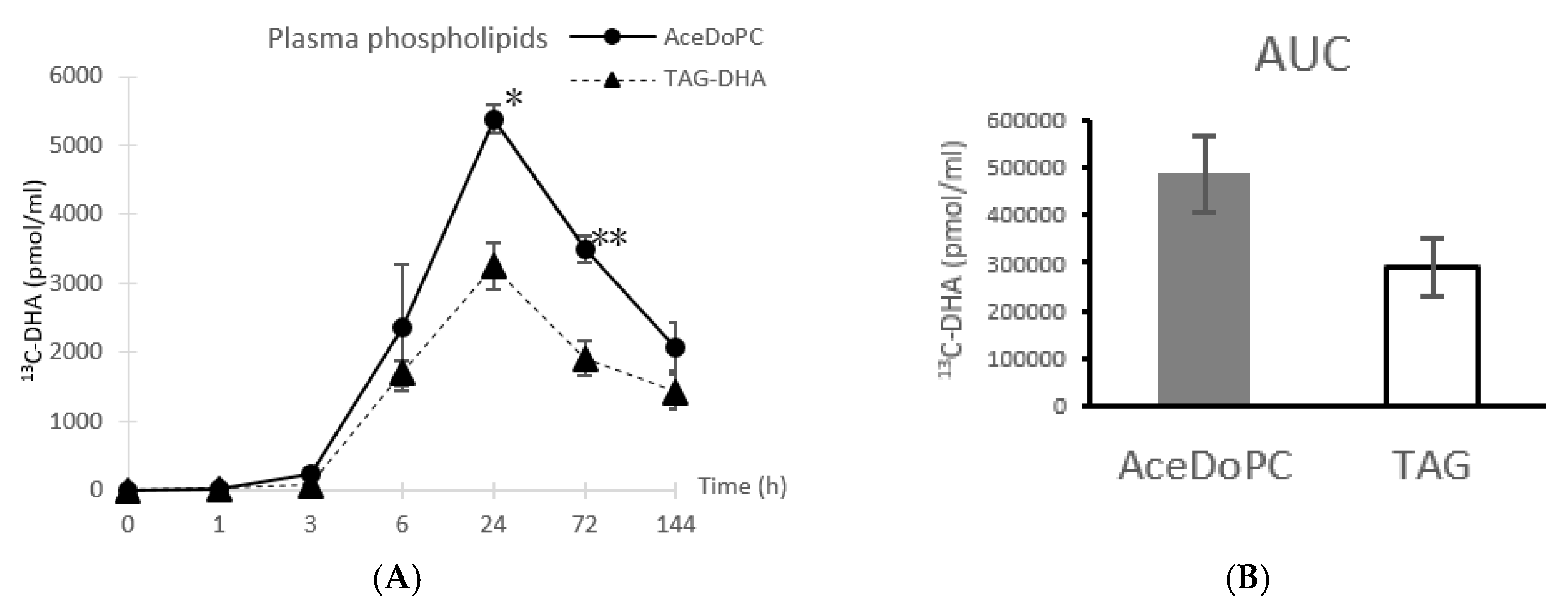
3.1. 13C-DHA Incorporation into Plasma Phospholipids
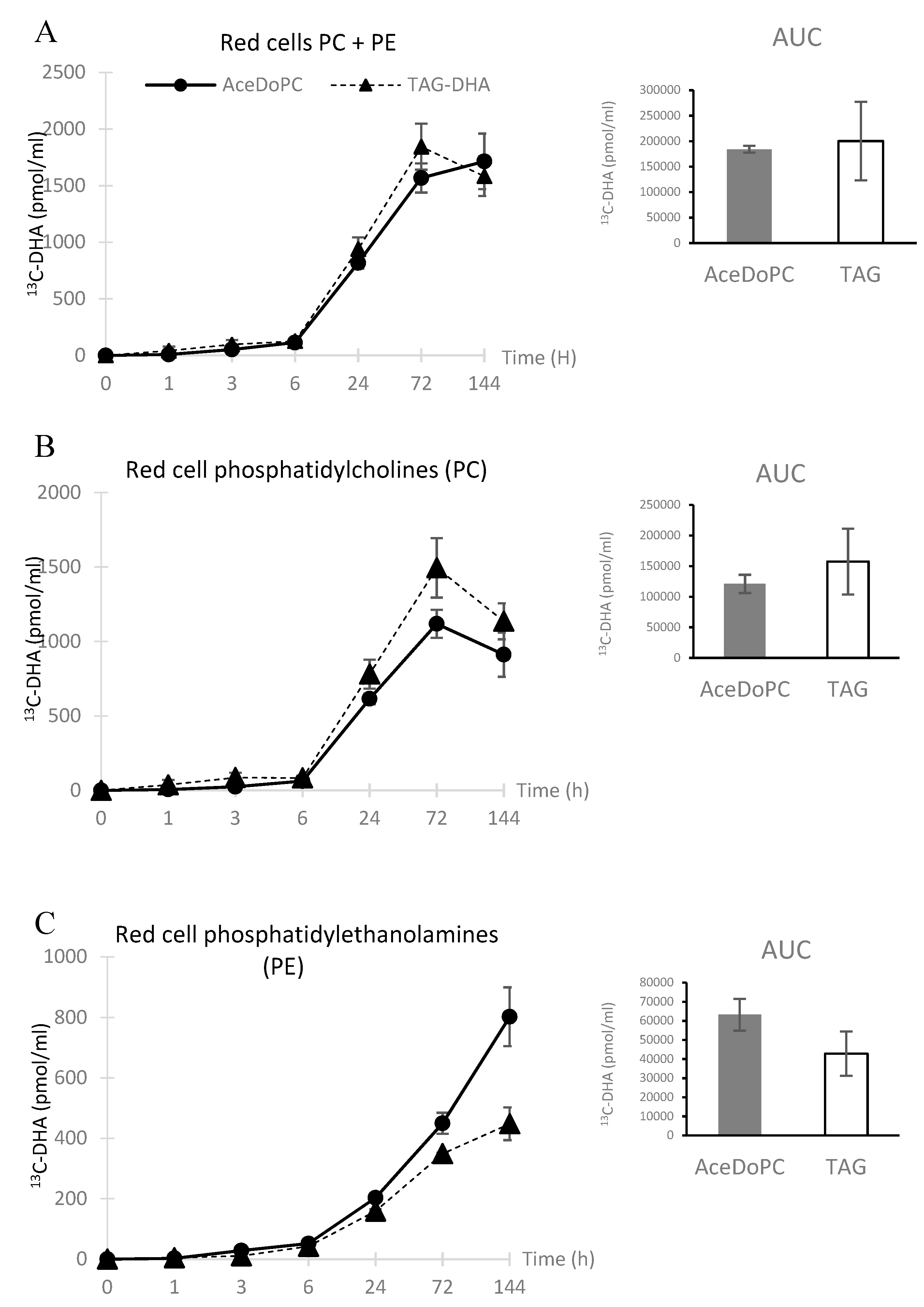
3.2. 13C-DHA Incorporation into Red Cells PC and PE
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Crawford, M.A.; Doyle, W.; Leaf, A.; Leighfield, M.; Ghebremeskel, K.; Phylactos, A. Nutrition and neurodevelopmental disorders. Nutr. Health 1993, 9, 81–97. [Google Scholar] [CrossRef]
- Uauy, R.; Hoffman, D.R. Essential fat requirements of preterm infants. Am. J. Clin. Nutr. 2000, 71, 245S–250S. [Google Scholar] [CrossRef]
- Gharami, K.; Das, M.; Das, S. Essential role of docosahexaenoic acid towards development of a smarter brain. Neurochem. Int. 2015, 89, 51–62. [Google Scholar] [CrossRef]
- Pinot, M.; Vanni, S.; Pagnotta, S.; Lacas-Gervais, S.; Payet, L.A.; Ferreira, T.; Gautier, R.; Goud, B.; Antonny, B.; Barelli, H. Lipid cell biology. Polyunsaturated phospholipids facilitate membrane deformation and fission by endocytic proteins. Science 2014, 345, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Cunnane, S.C.; Schneider, J.A.; Tangney, C.; Tremblay Mercier, J.; Fortier, M.; Bennett, D.A.; Morris, M.C. Plasma and brain fatty acid profiles in mild cognitive impairment and Alzheimer’s disease. J. Alzheimers Dis. 2012, 29, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Belkouch, M.; Hachem, M.; Elgot, A.; Lo Van, A.; Picq, M.; Guichardant, M.; Lagarde, M.; Bernoud-Hubac, N. The pleiotropic effects of omega-3 docosahexaenoic acid on the hallmarks of Alzheimer’s disease. J. Nutr. Biochem. 2016, 38, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Burdge, G. α-Linolenic acid metabolism in men and women: Nutritional and biological implications. Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 137–144. [Google Scholar] [CrossRef]
- Thies, F.; Pillon, C.; Moliere, P.; Lagarde, M.; Lecerf, J. Preferential incorporation of sn-2 lysoPC DHA over unesterified DHA in the young rat brain. Am. J. Physiol. 1994, 267, R1273–R1279. [Google Scholar] [CrossRef]
- Sugasini, D.; Thomas, R.; Yalagala, P.C.R.; Tai, L.M.; Subbaiah, P.V. Dietary docosahexaenoic acid (DHA) as lysophosphatidylcholine, but not as free acid, enriches brain DHA and improves memory in adult mice. Sci. Rep. 2017, 7, 11263. [Google Scholar] [CrossRef]
- Chouinard-Watkins, R.; Chen, C.T.; Metherel, A.H.; Lacombe, R.J.S.; Thies, F.; Masoodi, M.; Bazinet, R.P. Phospholipid class-specific brain enrichment in response to lysophosphatidylcholine docosahexaenoic acid infusion. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 1092–1098. [Google Scholar] [CrossRef]
- Bazinet, R.P.; Bernoud-Hubac, N.; Lagarde, M. How the plasma lysophospholipid and unesterified fatty acid pools supply the brain with docosahexaenoic acid. Prostaglandins Leukot. Essent. Fatty Acids 2019, 142, 1–3. [Google Scholar] [CrossRef]
- Bernoud, N.; Fenart, L.; Molière, P.; Dehouck, M.-P.; Lagarde, M.; Cecchelli, R.; Jecerf, J. Preferential Transfer of 2-Docosahexaenoyl-1-Lysophosphatidylcholine Through an In Vitro Blood-Brain Barrier Over Unesterified Docosahexaenoic Acid. J. Neurochem. 1999, 72, 338–345. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, L.N.; Ma, D.; Shui, G.; Wong, P.; Cazenave-Gassiot, A.; Zhang, X.; Silver, D.L. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 2014, 509, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Thies, F.; Delachambre, M.C.; Bentejac, M.; Lagarde, M.; Lecerf, J. Unsaturated fatty acids esterified in 2-acyl-l-lysophosphatidylcholine bound to albumin are more efficiently taken up by the young rat brain than the unesterified form. J. Neurochem. 1992, 59, 1110–1116. [Google Scholar] [CrossRef] [PubMed]
- Croset, M.; Brossard, N.; Polette, A.; Lagarde, M. Characterization of plasma unsaturated lysophosphatidylcholines in human and rat. Biochem. J. 2000, 345, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Polette, A.; Deshayes, C.; Chantegrel, B.; Croset, M.; Armstrong, J.M.; Lagarde, M. Synthesis of acetyl,docosahexaenoyl-glycerophosphocholine and its characterization using nuclear magnetic resonance. Lipids 1999, 34, 1333–1337. [Google Scholar] [CrossRef] [PubMed]
- Chauveau, F.; Cho, T.H.; Perez, M.; Guichardant, M.; Riou, A.; Aguettaz, P.; Picq, M.; Lagarde, M.; Berthezène, Y.; Nighoghossian, N.; et al. Brain-targeting form of docosahexaenoic acid for experimental stroke treatment: MRI evaluation and anti-oxidant impact. Curr. Neurovasc. Res. 2011, 8, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Hachem, M.; Géloën, A.; Van, A.L.; Foumaux, B.; Fenart, L.; Gosselet, F.; Da Silva, P.; Breton, G.; Lagarde, M.; Picq, M.; et al. Efficient Docosahexaenoic Acid Uptake by the Brain from a Structured Phospholipid. Mol. Neurobiol. 2016, 53, 3205–3215. [Google Scholar] [CrossRef] [PubMed]
- Fourrier, C.; Remus-Borel, J.; Greenhalgh, A.D.; Guichardant, M.; Bernoud-Hubac, N.; Lagarde, M.; Joffre, C.; Layé, S. Docosahexaenoic acid-containing choline phospholipid modulates LPS-induced neuroinflammation in vivo and in microglia In Vitro. J. Neuroinflamm. 2017, 14, 170. [Google Scholar] [CrossRef]
- Lo Van, A.; Sakayori, N.; Hachem, M.; Belkouch, M.; Picq, M.; Fourmaux, B.; Lagarde, M.; Osumi, N.; Bernoud-Hubac, N. Targeting the Brain with a Neuroprotective Omega-3 Fatty Acid to Enhance Neurogenesis in Hypoxic Condition in Culture. Mol. Neurobiol. 2019, 56, 986–999. [Google Scholar] [CrossRef]
- Lagarde, M.; Bryon, P.A.; Guichardant, M.; Dechavanne, M. A simple and efficient method for platelet isolation from their plasma. Thromb. Res. 1980, 17, 581–588. [Google Scholar] [CrossRef]
- Gabert, L.; Vors, C.; Louche-Pélissier, C.; Sauvinet, V.; Lambert-Porcheron, S.; Drai, J.; Michalski, M.C. 13C tracer recovery in human stools after digestion of a fat-rich meal labelled with [1, 1, 1-13C3]tripalmitin and [1, 1, 1-13C3]triolein. Rapid Commun. Mass Spectrom. RCM 2011, 25, 2697–2703. [Google Scholar] [CrossRef] [PubMed]
- Brossard, N.; Croset, M.; Normand, S.; Pousin, J.; Lecerf, J.; Laville, M.; Tayot, J.L.; Lagarde, M. Human plasma albumin transports [13C]docosahexaenoic acid in two lipid forms to blood cells. J. Lipid Res. 1997, 38, 1571–1582. [Google Scholar] [PubMed]
- Lemaitre-Delaunay, D.; Pachiaudi, C.; Laville, M.; Pousin, J.; Armstrong, M.; Lagarde, M. Blood compartmental metabolism of docosahexaenoic acid (DHA) in humans after ingestion of a single dose of [13C]DHA in phosphatidylcholine. J. Lipid Res. 1999, 40, 1867–1874. [Google Scholar] [PubMed]
- Connor, W.E.; Neuringer, M.; Lin, D.S. Dietary effects on brain fatty acid composition: The reversibility of n-3 fatty acid deficiency and turnover of docosahexaenoic acid in the brain, erythrocytes, and plasma of rhesus monkeys. J. Lipid Res. 1990, 31, 237–247. [Google Scholar]
- Létondor, A.; Buaud, B.; Vaysse, C.; Fonseca, L.; Herrouin, C.; Servat, B.; Layé, S.; Pallet, V.; Alfos, S. Erythrocyte DHA level as a biomarker of DHA status in specific brain regions of n-3 long-chain PUFA-supplemented aged rats. Br. J. Nutr. 2014, 112, 1805–1818. [Google Scholar] [CrossRef] [Green Version]
- Acetyl,2acyl-Glycerophosphocholines for the Treatment of Inflammation or of Intestinal Cancer. Patent WO 2017/006047 Al, 12 January 2017.
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hachem, M.; Nacir, H.; Picq, M.; Belkouch, M.; Bernoud-Hubac, N.; Windust, A.; Meiller, L.; Sauvinet, V.; Feugier, N.; Lambert-Porcheron, S.; et al. Docosahexaenoic Acid (DHA) Bioavailability in Humans after Oral Intake of DHA-Containing Triacylglycerol or the Structured Phospholipid AceDoPC®. Nutrients 2020, 12, 251. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12010251
Hachem M, Nacir H, Picq M, Belkouch M, Bernoud-Hubac N, Windust A, Meiller L, Sauvinet V, Feugier N, Lambert-Porcheron S, et al. Docosahexaenoic Acid (DHA) Bioavailability in Humans after Oral Intake of DHA-Containing Triacylglycerol or the Structured Phospholipid AceDoPC®. Nutrients. 2020; 12(1):251. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12010251
Chicago/Turabian StyleHachem, Mayssa, Houda Nacir, Madeleine Picq, Mounir Belkouch, Nathalie Bernoud-Hubac, Anthony Windust, Laure Meiller, Valerie Sauvinet, Nathalie Feugier, Stephanie Lambert-Porcheron, and et al. 2020. "Docosahexaenoic Acid (DHA) Bioavailability in Humans after Oral Intake of DHA-Containing Triacylglycerol or the Structured Phospholipid AceDoPC®" Nutrients 12, no. 1: 251. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12010251





