Acrylamide–Hemoglobin Adduct Levels in a Japanese Population and Comparison with Acrylamide Exposure Assessed by the Duplicated Method or a Food Frequency Questionnaire
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Data Collection
2.3. Analysis of AA–Hb in Blood
2.4. Assessment of Acrylamide Exposure using DM and FFQ
2.5. Statistical Analysis
2.6. Ethics Statements
3. Results
3.1. Basic Characteristics of the Study Participants
3.2. AA–Hb Levels in Erythrocytes and Estimated Acrylamide Exposure Levels from DM Samples and FFQ
3.3. GM of AA–Hb Levels by Tertiles of Estimated Acrylamide Intake from DM and FFQ
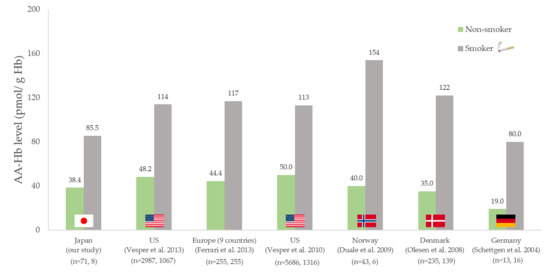
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mottram, D.S.; Wedzicha, B.L.; Dodson, A.T. Acrylamide is formed in the Maillard reaction. Nat. Cell Biol. 2002, 419, 448–449. [Google Scholar] [CrossRef]
- Yaylayan, V.; Wnorowski, A.; Locas, C.P. Why Asparagine Needs Carbohydrates to Generate Acrylamide. J. Agric. Food Chem. 2003, 51, 1753–1757. [Google Scholar] [CrossRef]
- Zyzak, D.V.; Sanders, R.A.; Stojanovic, M.; Tallmadge, D.H.; Eberhart, B.L.; Ewald, D.K.; Gruber, D.C.; Morsch, T.R.; Strothers, M.A.; Rizzi, G.P.; et al. Acrylamide Formation Mechanism in Heated Foods. J. Agric. Food Chem. 2003, 51, 4782–4787. [Google Scholar] [CrossRef]
- Smith, C.J.; Perfetti, T.A.; Rumple, M.A.; Rodgman, A.; Doolittle, D.J. "IARC group 2A Carcinogens" reported in cigarette mainstream smoke. Food Chem. Toxicol. 2000, 38, 371–383. [Google Scholar] [CrossRef]
- Vesper, H.W.; Bernert, J.T.; Ospina, M.; Meyers, T.; Ingham, L.; Smith, A.; Myers, G.L. Assessment of the Relation between Biomarkers for Smoking and Biomarkers for Acrylamide Exposure in Humans. Cancer Epidemiol. Biomark. Prev. 2007, 16, 2471–2478. [Google Scholar] [CrossRef] [Green Version]
- International Agency for Research on Cancer. Some industrial chemicals. In IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans; World Health Organization: Geneva, Switzerland, 1994; Volume 60, pp. 1–560. [Google Scholar]
- European Food Safety Authority. Scientific Opinion on acrylamide in food. EFSA J. 2015, 13, 4104. [Google Scholar]
- European Food Safety Authority. Update on acrylamide levels in food from monitoring years 2007 to 2010. EFSA J. 2012, 10, 2938. [Google Scholar] [CrossRef]
- European Commission. Commission recommendation 2013/647/EU of 8 November 2013 on investigations into the levels of acrylamide in food. Off. J. Eur. Union L 2013, 301, 15–17. [Google Scholar]
- European Commission. Acrylamide Database. Available online: https://ec.europa.eu/food/safety/chemical_safety/contaminants/catalogue/acrylamide_db_en (accessed on 15 December 2020).
- Food Safety Commission of Japan. Evaluation Document of Acrylamide Produced by Heating. Available online: https://www.fsc.go.jp/osirase/acrylamide1.data/acrylamide_hyokasyo1.pdf (accessed on 15 December 2020).
- Ministry of Agriculture, Forestry and Fisheries. Guidelines for Reducing Acrylamide in Food. Available online: https://www.maff.go.jp/j/syouan/seisaku/acryl_amide/a_gl/pdf/131127_acrylamide_full.pdf (accessed on 15 December 2020).
- Yoshida, M.; Ono, H.; Chuda, Y.; Yada, H.; Ohnishi-Kameyama, M.; Kobayashi, H.; Ohara-Takada, A.; Matsuura-Endo, C.; Mori, M.; Hayashi, N.; et al. Acrylamide in Japanese Processed Foods and Factors Affecting Acrylamide Level in Potato Chips and Tea. Results Probl. Cell Differ. 2006, 561, 405–413. [Google Scholar] [CrossRef]
- Kotemori, A.; Ishihara, J.; Nakadate, M.; Sawada, N.; Iwasaki, M.; Sobue, T.; Tsugane, S. Validity of a Self-administered Food Frequency Questionnaire for the Estimation of Acrylamide Intake in the Japanese Population: The JPHC FFQ Validation Study. J. Epidemiol. 2018, 28, 482–487. [Google Scholar] [CrossRef] [Green Version]
- Bergmark, E. Hemoglobin Adducts of Acrylamide and Acrylonitrile in Laboratory Workers, Smokers and Nonsmokers. Chem. Res. Toxicol. 1997, 10, 78–84. [Google Scholar] [CrossRef]
- Fennell, T.R.; Sumner, S.C.J.; Snyder, R.W.; Burgess, J.; Spicer, R.; Bridson, W.E.; Friedman, M.A. Metabolism and Hemoglobin Adduct Formation of Acrylamide in Humans. Toxicol. Sci. 2004, 85, 447–459. [Google Scholar] [CrossRef]
- Urban, M.; Kavvadias, D.; Riedel, K.; Scherer, G.; Tricker, A.R. Urinary Mercapturic Acids and a Hemoglobin Adduct for the Dosimetry of Acrylamide Exposure in Smokers and Nonsmokers. Inhal. Toxicol. 2006, 18, 831–839. [Google Scholar] [CrossRef]
- Vesper, H.W.; Caudill, S.P.; Osterloh, J.D.; Meyers, T.; Scott, D.; Myers, G.L. Exposure of the U.S. Population to Acrylamide in the National Health and Nutrition Examination Survey 2003–2004. Environ. Health Perspect. 2010, 118, 278–283. [Google Scholar] [CrossRef] [Green Version]
- Vesper, H.W.; Slimani, N.; Hallmans, G.; Tjønneland, A.; Agudo, A.; Benetou, V.; Bingham, S.; Boeing, H.; Boutron-Ruault, M.-C.; Bueno-De-Mesquita, H.B.; et al. Cross-Sectional Study on Acrylamide Hemoglobin Adducts in Subpopulations from the European Prospective Investigation into Cancer and Nutrition (EPIC) Study. J. Agric. Food Chem. 2008, 56, 6046–6053. [Google Scholar] [CrossRef]
- Fennell, T.R.; Sumner, S.J.; Walker, V.E. A model for the formation and removal of hemoglobin adducts. Cancer Epidemiol. Biomark. Prev. 1992, 1, 213–219. [Google Scholar]
- Friedman, M. Chemistry, Biochemistry, and Safety of Acrylamide. A Review. J. Agric. Food Chem. 2003, 51, 4504–4526. [Google Scholar] [CrossRef]
- Kotemori, A.; Ishihara, J.; Zha, L.; Liu, R.; Sawada, N.; Iwasaki, M.; Sobue, T.; Tsugane, S. The JPHC Study Group Dietary acrylamide intake and risk of breast cancer: The Japan Public Health Center-based Prospective Study. Cancer Sci. 2018, 109, 843–853. [Google Scholar] [CrossRef]
- Kotemori, A.; Ishihara, J.; Zha, L.; Liu, R.; Sawada, N.; Iwasaki, M.; Sobue, T.; Tsugane, S. The JPHC Study Group Dietary acrylamide intake and the risk of endometrial or ovarian cancers in Japanese women. Cancer Sci. 2018, 109, 3316–3325. [Google Scholar] [CrossRef]
- Liu, R.; Sobue, T.; Kitamura, T.; Kitamura, Y.; Ishihara, J.; Kotemori, A.; Zha, L.; Ikeda, S.; Sawada, N.; Iwasaki, M.; et al. Dietary Acrylamide Intake and Risk of Esophageal, Gastric, and Colorectal Cancer: The Japan Public Health Center-based Prospective Study. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1461–1468. [Google Scholar] [CrossRef]
- Wilson, K.M.; Bälter, K.; Adami, H.-O.; Grönberg, H.; Vikström, A.C.; Paulsson, B.; Törnqvist, M.; Mucci, L.A. Acrylamide exposure measured by food frequency questionnaire and hemoglobin adduct levels and prostate cancer risk in the Cancer of the Prostate in Sweden Study. Int. J. Cancer 2009, 124, 2384–2390. [Google Scholar] [CrossRef] [Green Version]
- Obón-Santacana, M.; Freisling, H.; Peeters, P.H.; Lujan-Barroso, L.; Ferrari, P.; Boutron-Ruault, M.-C.; Mesrine, S.; Baglietto, L.; Turzanski-Fortner, R.; Katzke, V.A.; et al. Acrylamide and glycidamide hemoglobin adduct levels and endometrial cancer risk: A nested case-control study in nonsmoking postmenopausal women from the EPIC cohort. Int. J. Cancer 2016, 138, 1129–1138. [Google Scholar] [CrossRef] [Green Version]
- Obón-Santacana, M.; Lujan-Barroso, L.; Travis, R.C.; Freisling, H.; Ferrari, P.; Severi, G.; Baglietto, L.; Boutron-Ruault, M.-C.; Fortner, R.T.; Ose, J.; et al. Acrylamide and Glycidamide Hemoglobin Adducts and Epithelial Ovarian Cancer: A Nested Case-Control Study in Nonsmoking Postmenopausal Women from the EPIC Cohort. Cancer Epidemiol. Biomark. Prev. 2015, 25, 127–134. [Google Scholar] [CrossRef] [Green Version]
- Olesen, P.T.; Olsen, A.; Frandsen, H.L.; Frederiksen, K.; Overvad, K.; Tjønneland, A. Acrylamide exposure and incidence of breast cancer among postmenopausal women in the Danish Diet, Cancer and Health Study. Int. J. Cancer 2008, 122, 2094–2100. [Google Scholar] [CrossRef]
- Xie, J.; Terry, K.L.; Poole, E.M.; Wilson, K.M.; Rosner, B.A.; Willett, W.C.; Vesper, H.W.; Tworoger, S.S. Acrylamide Hemoglobin Adduct Levels and Ovarian Cancer Risk: A nested case-control study. Cancer Epidemiol. Biomark. Prev. 2013, 22, 653–660. [Google Scholar] [CrossRef] [Green Version]
- Kawahara, J.; Zheng, Y.; Terui, M.; Shinohara, A.; Uyama, K.; Yoneyama, M.; Nakajima, D.; Shibata, Y.; Adachi, S. Dietary exposure to acrylamide in a group of Japanese adults based on 24-hour duplicate diet samples. Food Addit. Contam. Part A 2019, 36, 15–25. [Google Scholar] [CrossRef]
- Tsubono, Y.; Takamori, S.; Kobayashi, M.; Takahashi, T.; Iwase, Y.; Iitoi, Y.; Akabane, M.; Yamaguchi, M.; Tsugane, S. A Data-Based Approach for Designing a Semiquantitative Food Frequency Questionnaire for a Population-based Prospective Study in Japan. J. Epidemiol. 1996, 6, 45–53. [Google Scholar] [CrossRef] [Green Version]
- Tsugane, S.; Sawada, N. The JPHC Study: Design and Some Findings on the Typical Japanese Diet. Jpn. J. Clin. Oncol. 2014, 44, 777–782. [Google Scholar] [CrossRef]
- Tsugane, S.; Sobue, T. Baseline survey of JPHC study-design and participation rate. Japan Public Health Center-based Prospective Study on Cancer and Cardiovascular Diseases. J. Epidemiol. 2001, 11, S24–S29. [Google Scholar] [CrossRef]
- Wirfält, E.; Paulsson, B.; Törnqvist, M.; Axmon, A.; Hagmar, L.; Wirf, E. Associations between estimated acrylamide intakes, and hemoglobin AA adducts in a sample from the Malmö Diet and Cancer cohort. Eur. J. Clin. Nutr. 2007, 62, 314–323. [Google Scholar] [CrossRef]
- Vikström, A.C.; Abramsson-Zetterberg, L.; Naruszewicz, M.; Athanassiadis, I.; Granath, F.N.; Törnqvist, M. In Vivo Doses of Acrylamide and Glycidamide in Humans after Intake of Acrylamide-Rich Food. Toxicol. Sci. 2011, 119, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Ministry of Health, Labor and Welfare. National Health and Nutrition Survey; Ministry of Health, Labor and Welfare: Tokyo, Japan, 2015. [Google Scholar]
- Vesper, H.W.; Sternberg, M.R.; Frame, T.; Pfeiffer, C.M. Among 10 Sociodemographic and Lifestyle Variables, Smoking Is Strongly Associated with Biomarkers of Acrylamide Exposure in a Representative Sample of the U.S. Population. J. Nutr. 2013, 143, 995S–1000S. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, P.; Freisling, H.; Duell, E.J.; Kaaks, R.; Lujan-Barroso, L.; Clavel-Chapelon, F.; Boutron-Ruault, M.-C.; Nailler, L.; Polidoro, S.; Mattiello, A.; et al. Challenges in estimating the validity of dietary acrylamide measurements. Eur. J. Nutr. 2012, 52, 1503–1512. [Google Scholar] [CrossRef]
- Von Stedingk, H.; Vikström, A.C.; Rydberg, P.; Pedersen, M.; Nielsen, J.K.S.; Segerbäck, D.; Knudsen, L.E.; Törnqvist, M. Analysis of Hemoglobin Adducts from Acrylamide, Glycidamide, and Ethylene Oxide in Paired Mother/Cord Blood Samples from Denmark. Chem. Res. Toxicol. 2011, 24, 1957–1965. [Google Scholar] [CrossRef]
- Duale, N.; Bjellaas, T.; Alexander, J.; Becher, G.; Haugen, M.; Paulsen, J.E.; Frandsen, H.; Olesen, P.T.; Brunborg, G. Biomarkers of Human Exposure to Acrylamide and Relation to Polymorphisms in Metabolizing Genes. Toxicol. Sci. 2009, 108, 90–99. [Google Scholar] [CrossRef] [Green Version]
- Bjellaas, T.; Olesen, P.T.; Frandsen, H.; Haugen, M.; Stølen, L.H.; Paulsen, J.E.; Alexander, J.; Lundanes, E.; Becher, G. Comparison of Estimated Dietary Intake of Acrylamide with Hemoglobin Adducts of Acrylamide and Glycidamide. Toxicol. Sci. 2007, 98, 110–117. [Google Scholar] [CrossRef]
- Schettgen, T.; Hornig, M.; Beckmann, M.W.; Weiss, T.; Drexler, H.; Angerer, J. Trans-placental exposure of neonates to acrylamide? a pilot study. Int. Arch. Occup. Environ. Health 2004, 77, 213–216. [Google Scholar] [CrossRef]
- Schettgen, T.; Rossbach, B.; Kütting, B.; Letzel, S.; Drexler, H.; Angerer, J. Determination of haemoglobin adducts of acrylamide and glycidamide in smoking and non-smoking persons of the general population. Int. J. Hyg. Environ. Health 2004, 207, 531–539. [Google Scholar] [CrossRef]
- Hogervorst, J.G.F.; Schouten, L.J.; Konings, E.J.M.; Goldbohm, R.A.; Brandt, P.A.V.D. Dietary Acrylamide Intake Is Not Associated with Gastrointestinal Cancer Risk. J. Nutr. 2008, 138, 2229–2236. [Google Scholar] [CrossRef] [Green Version]
- Duarte-Salles, T.; Von Stedingk, H.; Granum, B.; Gützkow, K.B.; Rydberg, P.; Törnqvist, M.; Mendez, M.A.; Brunborg, G.; Brantsæter, A.L.; Meltzer, H.M.; et al. Dietary Acrylamide Intake during Pregnancy and Fetal Growth—Results from the Norwegian Mother and Child Cohort Study (MoBa). Environ. Health Perspect. 2013, 121, 374–379. [Google Scholar] [CrossRef]
- Mucci, L.A.; Adami, H.-O.; Wolk, A. Prospective study of dietary acrylamide and risk of colorectal cancer among women. Int. J. Cancer 2005, 118, 169–173. [Google Scholar] [CrossRef]
- Kawahara, J.; Imaizumi, Y.; Kuroda, K.; Aoki, Y.; Suzuki, N. Estimation of long-term dietary exposure to acrylamide of the Japanese people. Food Addit. Contam. Part A 2018, 35, 1689–1702. [Google Scholar] [CrossRef]
- Konings, E.J.M.; Hogervorst, J.G.F.; Van Rooij, L.; Schouten, L.J.; Sizoo, E.A.; Van Egmond, H.P.; Goldbohm, R.A.; Brandt, P.V.D. Validation of a database on acrylamide for use in epidemiological studies. Eur. J. Clin. Nutr. 2010, 64, 534–540. [Google Scholar] [CrossRef] [Green Version]
- Virk-Baker, M.K.; Nagy, T.R.; Barnes, S.; Groopman, J. Dietary acrylamide and human cancer: A systematic review of literature. Nutr. Cancer 2014, 66, 774–790. [Google Scholar] [CrossRef] [Green Version]
- Ministry of Health, Labor and Welfare. Drinking Water Quality Standards; Ministry of Health, Labor and Welfare: Tokyo, Japan, 2016. [Google Scholar]
| Characteristics | Value |
|---|---|
| Participants, n Age, years (mean, SD) | 89 42.3 (14.4) |
| 20–29, n | 22 |
| 30–39, n | 15 |
| 40–49, n | 22 |
| 50–59, n | 18 |
| 60≥, n | 12 |
| Sex | |
| Men (%) | 40.4 |
| Women (%) | 59.6 |
| Smoking | |
| Current Smoker (%) | 9.0 |
| Past Smoker (%) | 11.2 |
| Never Smoker (%) | 79.8 |
| BMI, kg/m2 (mean, SD) | 22.1 (3.6) |
| 20<, n | 24 |
| 20–25, n | 48 |
| 25>, n | 17 |
| Parameters | All (n = 89) | Never Smoker (n = 71) | Past Smokers (n = 10) | Current Smokers (n = 8) |
|---|---|---|---|---|
| AA-Hb (pmol/g Hb) | ||||
| Mean (SD) | 45.7 (35.4) | 38.4 (30.7) | 65.3 (31.2) | 85.5 (46.6) |
| Median | 35.3 | 31.5 | 67.6 | 80.4 |
| (5th %-ile, 95th %-ile) | (15.7, 104.3) | (15.7, 74.5) | (17.3, 118.9) | (13.8, 150.2) |
| DM (ng/kg bw/day) | ||||
| Mean (SD) | 225.2 (267.8) | 243.5 (288.0) | 157.3 (140.5) | 147.6 (173.4) |
| T1 | 50.9 | 59.7 | 37.7 | 22.0 |
| T2 | 139.7 | 148.7 | 123.8 | 62.3 |
| T3 | 479.2 | 514.5 | 321.7 | 316.7 |
| FFQ (ng/kg bw/day) | ||||
| Mean (SD) | 130.6 (79.4) | 135.9 (81.2) | 102.7 (60.4) | 120.2 (84.4) |
| T1 | 60.8 | 66.0 | 53.5 | 40.4 |
| T2 | 112.3 | 116.6 | 77.3 | 89.0 |
| T3 | 218.8 | 225.0 | 185.8 | 204.5 |
| Variables | All (n = 89) | Never Smoker (n = 71) | Past Smokers (n = 10) | Current Smoker (n = 8) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | GM (95% CI) a | n | GM (95% CI) a | n | GM (95% CI) a | n | GM (95% CI) a | |||||
| Tertile of each acrylamide intakes | ||||||||||||
| DM | ||||||||||||
| T1 | 29 | 46.0 b | (36.9–57.3) | 20 | 29.2 | (23.0–37.1) | 5 | 48.6 | (22.1–106.9) | 4 | 63.8 | (17.1–238.2) |
| T2 | 30 | 48.3 b | (38.0–61.4) | 25 | 29.3 | (23.8–36.1) | 3 | 61.1 | (23.9–156.2) | 2 | 62.5 | (11.4–342.4) |
| T3 | 30 | 64.5 b | (50.5–82.5) | 26 | 38.0 | (31.1–46.3) | 2 | 86.4 | (28.0–266.2) | 2 | 97.2 | (13.0–726.8) |
| p for trend | 0.02 | 0.08 | 0.34 | 0.68 | ||||||||
| FFQ | ||||||||||||
| T1 | 29 | 44.4 b | (35.4–55.8) | 21 | 27.8 | (22.2–34.7) | 4 | 52.9 | (27.3–102.4) | 4 | 69.2 | (18.9–254.0) |
| T2 | 30 | 52.5 b | (41.2–66.9) | 25 | 33.6 | (27.3–41.2) | 3 | 39.0 | (15.6–97.8) | 2 | 94.0 | (17.6–503.4) |
| T3 | 30 | 59.9 b | (47.0–76.3) | 25 | 36.2 | (29.3–44.7) | 3 | 97.0 | (44.4–211.9) | 2 | 54.9 | (7.5–399.9) |
| p for trend | 0.04 | 0.09 | 0.20 | 0.82 | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamamoto, J.; Ishihara, J.; Matsui, Y.; Matsuda, T.; Kotemori, A.; Zheng, Y.; Nakajima, D.; Terui, M.; Shinohara, A.; Adachi, S.; et al. Acrylamide–Hemoglobin Adduct Levels in a Japanese Population and Comparison with Acrylamide Exposure Assessed by the Duplicated Method or a Food Frequency Questionnaire. Nutrients 2020, 12, 3863. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12123863
Yamamoto J, Ishihara J, Matsui Y, Matsuda T, Kotemori A, Zheng Y, Nakajima D, Terui M, Shinohara A, Adachi S, et al. Acrylamide–Hemoglobin Adduct Levels in a Japanese Population and Comparison with Acrylamide Exposure Assessed by the Duplicated Method or a Food Frequency Questionnaire. Nutrients. 2020; 12(12):3863. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12123863
Chicago/Turabian StyleYamamoto, Junpei, Junko Ishihara, Yasuto Matsui, Tomonari Matsuda, Ayaka Kotemori, Yazhi Zheng, Daisuke Nakajima, Miho Terui, Akiko Shinohara, Shuichi Adachi, and et al. 2020. "Acrylamide–Hemoglobin Adduct Levels in a Japanese Population and Comparison with Acrylamide Exposure Assessed by the Duplicated Method or a Food Frequency Questionnaire" Nutrients 12, no. 12: 3863. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12123863