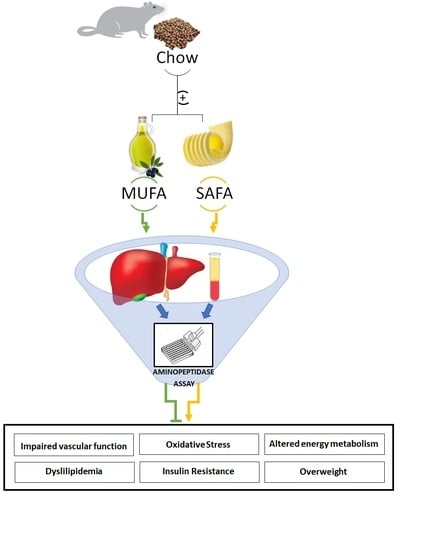
The Role of High Fat Diets and Liver Peptidase Activity in the Development of Obesity and Insulin Resistance in Wistar Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Treatments
2.2. Glucose Tolerance Test
2.3. Determination of Blood Parameters
2.4. Determination of Insulin Resistance
2.5. Sample Preparation for Aminopeptidase Activities Assay
2.6. Peptidase Activities Assay
2.7. Protein Extraction and Western Blot Analysis
2.8. Statistical Analysis
3. Results
3.1. Food and Energy Intakes
3.2. Glucose Tolerance Test (GTT) and Glycemic Control
3.3. Glucagon-Like-Peptide-1 and Dipeptidyl-Peptidase- 4 Activity
3.4. Hepatic TRH-Degrading Pyroglutamate Aminopeptidase Activity and Tyrosine Aminopeptidase Activity
3.5. Hepatic Angiotensinases Activities
3.6. Leucine Aminopeptidase and Gamma Glutamyl Transferase
3.7. Plasma Nitric Oxide (NO) and Hepatic Inducible NO Synthase (iNOS)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guyenet, S.J.; Schwartz, M.W. Regulation of Food Intake, Energy Balance, and Body Fat Mass: Implications for the Pathogenesis and Treatment of Obesity. J. Clin. Endocrinol. Metab. 2012, 97, 745–755. [Google Scholar] [CrossRef] [Green Version]
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef]
- Pi-Sunyer, F.X. The Obesity Epidemic: Pathophysiology and Consequences of Obesity. Obes. Res. 2002, 10, 97S–104S. [Google Scholar] [CrossRef]
- Hariri, N.; Thibault, L. High-fat diet-induced obesity in animal models. Nutr. Res. Rev. 2010, 23, 270–299. [Google Scholar] [CrossRef] [Green Version]
- Villarejo, A.; Ramírez-Sánchez, M.; Segarra, A.B.; Martínez-Cañamero, M.; Prieto, I. Influence of Extra Virgin Olive Oil on Blood Pressure and Kidney Angiotensinase Activities in Spontaneously Hypertensive Rats. Planta Medica 2015, 81, 664–669. [Google Scholar] [CrossRef]
- Gao, M.; Ma, Y.; Liu, D. High-Fat Diet-Induced Adiposity, Adipose Inflammation, Hepatic Steatosis and Hyperinsulinemia in Outbred CD-1 Mice. PLoS ONE 2015, 10, e0119784. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.J.; Wang, B.W.; Zhang, C.; Xia, M.Z.; Chen, Y.H.; Hu, C.Q.; Wang, H.; Chen, X.; Xu, D.X. Vitamin D Deficiency Attenuates High-Fat Diet-Induced Hyperinsulinemia and Hepatic Lipid Accumulation in Male Mice. Endocrinology 2015, 156, 2103–2113. [Google Scholar] [CrossRef]
- Jiang, B.; Le, L.; Wan, W.; Zhai, W.; Hu, K.; Xu, L.; Xiao, P. The Flower Tea Coreopsis tinctoria Increases Insulin Sensitivity and Regulates Hepatic Metabolism in Rats Fed a High-Fat Diet. Endocrinology 2015, 156, 2006–2018. [Google Scholar] [CrossRef] [Green Version]
- Carnevale, R.; Silvestri, R.; Loffredo, L.; Novo, M.; Cammisotto, V.; Castellani, V.; Bartimoccia, S.; Nocella, C.; Violi, F. Oleuropein, a component of extra virgin olive oil, lowers postprandial glycaemia in healthy subjects. BJCP 2018, 84, 1566–1574. [Google Scholar] [CrossRef] [Green Version]
- Domínguez-Vías, G.; Segarra, A.; Martínez-Cañamero, M.; Ramírez-Sánchez, M.; Prieto, I. Influence of a virgin olive oil versus butter plus cholesterol-enriched diet on testicular enzymatic activities in adult male rats. Int. J. Mol. Sci. 2017, 18, 1701. [Google Scholar] [CrossRef] [Green Version]
- Martínez, N.; Prieto, I.; Hidalgo, M.; Segarra, A.B.; Martínez-Rodríguez, A.; Cobo, A.; Ramírez, M.; Gálvez, A.; Martínez-Cañamero, M. Refined versus Extra Virgin Olive Oil High-Fat Diet Impact on Intestinal Microbiota of Mice and Its Relation to Different Physiological Variables. Microorganisms 2019, 7, 61. [Google Scholar] [CrossRef] [Green Version]
- Nocella, C.; Cammisotto, V.; Fianchini, L.; D’Amico, A.; Novo, M.; Castellani, V.; Stefanini, L.; Violi, F.; Carnevale, R. Extra Virgin Olive Oil and Cardiovascular Diseases: Benefits for Human Health. Endocr. Metab. Immune 2017, 18, 4–13. [Google Scholar] [CrossRef]
- Prieto, I.; Hidalgo, M.; Segarra, A.B.; Martínez-Rodríguez, A.M.; Cobo, A.; Ramírez, M.; Abriouel, H.; Gálvez, A.; Martínez-Cañamero, M. Influence of a diet enriched with virgin olive oil or butter on mouse gut microbiota and its correlation to physiological and biochemical parameters related to metabolic syndrome. PLoS ONE 2018, 13, e0190368. [Google Scholar] [CrossRef] [Green Version]
- Romani, A.; Ieri, F.; Urciuoli, S.; Noce, A.; Marrone, G.; Nediani, C.; Bernini, R. Health Effects of Phenolic Compounds Found in Extra-Virgin Olive Oil, By-Products, and Leaf of Olea europaea L. Nutrients 2019, 11, 1776. [Google Scholar] [CrossRef] [Green Version]
- Yubero-Serrano, E.M.; Lopez-Moreno, J.; Gomez-Delgado, F.; Lopez-Miranda, J. Extra virgin olive oil: More than a healthy fat. Eur. J. Clin. Nutr. 2019, 72, 8–17. [Google Scholar] [CrossRef] [Green Version]
- Elias, S.; Wisam, S.; Luai, A.; Massad, B.; Nimer, A. Lipotoxicity in Obesity: Benefit of Olive Oil. Adv. Exp. Med. Biol. 2017, 960, 607–617. [Google Scholar] [CrossRef]
- Prieto, P.; Cancelas, J.; Villanueva-Peñacarrillo, M.; Valverde, I.; Malaisse, W. Effects of an olive oil-enriched diet on plasma GLP-1 concentration and intestinal content, plasma insulin concentration, and glucose tolerance in normal rats. Endocrine 2005, 26, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.; Homberger, E.; Siegel, E.G.; Allen, R.C.; Eaton, R.P.; Ebert, R.; Creutzfeldt, W. Incretin Effects of Increasing Glucose Loads in Man Calculated from Venous Insulin and C-Peptide Responses*. J. Clin. Endocrinol. Metab. 1986, 63, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.; Stöckmann, F.; Ebert, R.; Creutzfeldt, W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia 1986, 29, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Lovshin, J.A.; Drucker, D.J. Incretin-based therapies for type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2009, 5, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Hare, K.J.; Vilsbøll, T.; Asmar, M.; Deacon, C.F.; Knop, F.K.; Holst, J.J. The glucagonostatic and insulinotropic effects of glucagon-like peptide 1 contribute equally to its glucose-lowering action. Diabetes 2010, 59, 1765–1770. [Google Scholar] [CrossRef] [Green Version]
- Mentlein, R.; Gallwitz, B.; Schmidt, W.E. Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1(7-36)amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur. J. Biochem. 1993, 214, 829–835. [Google Scholar] [CrossRef]
- Lugari, R.; Cas, A.D.; Ugolotti, D.; Barilli, A.L.; Camellini, C.; Ganzerla, G.C.; Luciani, A.; Salerni, B.; Mitternperger, F.; Nodari, S.; et al. Glucagon-like Peptide 1 (GLP-1) Secretion and Plasma Dipeptidyl Peptidase IV (DPP-IV) Activity in Morbidly Obese Patients Undergoing Biliopancreatic Diversion. Horm. Metab. Res. 2004, 36, 111–115. [Google Scholar] [CrossRef]
- Barrett, A.J.; Rawlings, N.D.; Woessner, J.F. Handbook of Proteolytic Enzymes; Elsevier Academic Press: Amsterdam, The Netherlands, 2004. [Google Scholar]
- de Gasparo, M.; Whitebread, S.; Bottari, S.P.; Levens, N.R. Heterogeneity of Angiotensin Receptor Subtypes. In Medicinal Chemistry of the Renin-Angiotensin System; Pbmwm, T., Wexler, R.R., Eds.; Elsevier: Amsterdam, The Netherlands, 1994; pp. 269–294. [Google Scholar]
- García-Sáinz, J.A.; Martínez-Alfaro, M.; Romero-Avila, M.T.; González-Espinosa, C. Characterization of the AT1 angiotensin II receptor expressed in guinea pig liver. J. Endocrinol. 1997, 154, 133–138. [Google Scholar] [CrossRef]
- Chai, S.Y.; Fernando, R.; Peck, G.; Ye, S.Y.; Mendelsohn, F.A.O.; Jenkins, T.A.; Albiston, A.L. What’s new in the renin-angiotensin system? The angiotensin IV/AT4 receptor. Cell. Mol. Life Sci. 2004, 61, 2728–2737. [Google Scholar] [CrossRef]
- Coleman, J.K.; Krebs, L.T.; Hamilton, T.A.; Ong, B.; Lawrence, K.A.; Sardinia, M.F.; Harding, J.W.; Wright, J.W. Autoradiographic identification of kidney angiotensin IV binding sites and angiotensin IV-induced renal cortical blood flow changes in rats. Peptides 1998, 19, 269–277. [Google Scholar] [CrossRef]
- Segarra, A.B.; Ramirez, M.; Banegas, I.; Alba, F.; Vives, F.; de Gasparo, M.; Ortega, E.; Ruiz, E.; Prieto, I. Dietary Fat Influences Testosterone, Cholesterol, Aminopeptidase A, and Blood Pressure in Male Rats. Horm. Metab. Res. 2008, 40, 289–291. [Google Scholar] [CrossRef]
- Segarra, A.B.; Ruiz-Sanz, J.I.; Ruiz-Larrea, M.B.; Ramírez-Sánchez, M.; de Gasparo, M.; Banegas, I.; Martínez-Cañamero, M.; Vives, F.; Prieto, I. The Profile of Fatty Acids in Frontal Cortex of Rats Depends on the Type of Fat Used in the Diet and Correlates with Neuropeptidase Activities. Horm. Metab. Res. 2011, 43, 86–91. [Google Scholar] [CrossRef]
- de Macêdo, S.M.; Antunes-Guimarães, T.; Feltenberger, J.D.; Santos, S.H.S. The role of renin-angiotensin system modulation on treatment and prevention of liver diseases. Peptides 2014, 62, 189–196. [Google Scholar] [CrossRef]
- Afroze, S.H.; Munshi, M.K.; Martínez, A.K.; Uddin, M.; Gergely, M.; Szynkarski, C.; Guerrier, M.; Nizamutdinov, D.; Dostal, D.; Glaser, S. Activation of the renin-angiotensin system stimulates biliary hyperplasia during cholestasis induced by extrahepatic bile duct ligation. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G691–G701. [Google Scholar] [CrossRef] [Green Version]
- Hayden, M.R.; Sowers, K.M.; Pulakat, L.; Joginpally, T.; Krueger, B.; Whaley-Connell, A.; Sowers, J.R. Possible Mechanisms of Local Tissue Renin-Angiotensin System Activation in the Cardiorenal Metabolic Syndrome and Type 2 Diabetes Mellitus. Cardiorenal Med. 2011, 1, 193–210. [Google Scholar] [CrossRef] [Green Version]
- Takeshita, Y.; Takamura, T.; Ando, H.; Hamaguchi, E.; Takazakura, A.; Matsuzawa-Nagata, N.; Kaneko, S. Cross talk of tumor necrosis factor-alpha and the renin-angiotensin system in tumor necrosis factor-alpha-induced plasminogen activator inhibitor-1 production from hepatocytes. Eur. J. Pharmacol. 2008, 579, 426–432. [Google Scholar] [CrossRef]
- Orlic, L.; Mikolasevic, I.; Lukenda, V.; Anic, K.; Jelic, I.; Racki, S. Nonalcoholic fatty liver disease and the renin-angiotensin system blockers in the patients with chronic kidney disease. Wien. Klin. Wochenschr. 2015, 127, 355–362. [Google Scholar] [CrossRef]
- Tao, X.R.; Rong, J.B.; Lu, H.S.; Daugherty, A.; Shi, P.; Ke, C.L.; Zhang, Z.C.; Xu, Y.C.; Wang, J.A. Angiotensinogen in hepatocytes contributes to Western diet-induced liver steatosis. J. Lipid Res. 2019, 60, 1983–1995. [Google Scholar] [CrossRef] [Green Version]
- Sansoè, G.; Aragno, M.; Wong, F. Pathways of hepatic and renal damage through non-classical activation of the renin-angiotensin system in chronic liver disease. Liver Int. 2019, 40, 18–31. [Google Scholar] [CrossRef] [Green Version]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Abbas, A.; Sakr, H.F. Simvastatin and vitamin E effects on cardiac and hepatic oxidative stress in rats fed on high fat diet. J. Physiol. Biochem. 2013, 69, 737–750. [Google Scholar] [CrossRef]
- Pan, X.; Wang, P.; Luo, J.; Wang, Z.; Song, Y.; Ye, J.; Hou, X. Adipogenic changes of hepatocytes in a high-fat diet-induced fatty liver mice model and non-alcoholic fatty liver disease patients. Endocrine 2015, 48, 834–847. [Google Scholar] [CrossRef]
- Taylor, B.S.; Alarcon, L.H.; Billiar, T.R. Inducible nitric oxide synthase in the liver: Regulation and function. Biochemistry 1998, 63, 766–781. [Google Scholar]
- Scharfmann, R.; Aratan-Spire, S. Ontogeny of two topologically distinct TRH-degrading pyroglutamate aminopeptidase activities in the rat liver. Regul. Pept. 1991, 32, 75–83. [Google Scholar] [CrossRef]
- Ra, K.S.; Suh, H.J.; Choi, J.W. Hypoglycemic effects of Cyclo (His-Pro) in streptozotocin-induced diabetic rats. Biotechnol. Bioproc. E 2012, 17, 176–184. [Google Scholar] [CrossRef]
- Arechaga, G.; Prieto, I.; Segarra, A.B.; Alba, F.; Ruiz-Larrea, M.B.; Ruiz-Sanz, J.I.; de Gasparo, M.; Ramirez, M. Dietary fatty acid composition affects aminopeptidase activities in the testes of mice. Int. J. Androl. 2002, 25, 113–118. [Google Scholar] [CrossRef]
- Mendez, I.A.; Ostlund, S.B.; Maidment, N.T.; Murphy, N.P. Involvement of Endogenous Enkephalins and β-Endorphin in Feeding and Diet-Induced Obesity. Neuropsychopharmacology 2015, 40, 2103–2112. [Google Scholar] [CrossRef] [Green Version]
- Cieśla, A.; Mach, T.; Pierzchała-Koziec, K.; Skwara, P.; Szczepański, W. Met-enkephalin in the liver as a marker of hepatocellular damage in chronic viral hepatitis type B and C. Adv. Med. Sci. Poland 2006, 51, 261–264. [Google Scholar]
- Owczarek, D.; Garlicka, M.; Pierzchała-Koziec, K.; Skulina, D.; Szulewski, P. Met-enkephalin plasma concentration and content in liver tissue in patients with primary biliary cirrhosis. Przegl. Lek. 2003, 60, 461–466. [Google Scholar]
- Karatayev, O.; Gaysinskaya, V.; Chang, G.Q.; Leibowitz, S.F. Circulating triglycerides after a high-fat meal: Predictor of increased caloric intake, orexigenic peptide expression, and dietary obesity. Brain. Res. 2009, 1298, 111–122. [Google Scholar] [CrossRef] [Green Version]
- Hill-Pryor, C.; Dunbar, J.C. The Effect of High Fat-Induced Obesity on Cardiovascular and Physical Activity and Opioid Responsiveness in Conscious Rats. Clin. Exp. Hypertens. 2006, 28, 133–145. [Google Scholar] [CrossRef]
- Fernández, D.; Valdivia, A.; Irazusta, J.; Ochoa, C.; Casis, L. Peptidase activities in human semen. Peptides 2002, 23, 461–468. [Google Scholar] [CrossRef]
- HOMA2 Calculator: Overview. The Oxford Centre for diabetes endocrinoly and metabolism. Diabetes Trials Unit, HOMA Calculator. (n.d.). Available online: https://www.dtu.ox.ac.uk/homacalculator/ (accessed on 14 December 2019).
- Alba, F.; Arenas, J.C.; Lopez, M.A. Properties of rat brain dipeptidyl aminopeptidases in the presence of detergents. Peptides 1995, 16, 325–329. [Google Scholar] [CrossRef] [Green Version]
- Ramírez, M.; Prieto, I.; Banegas, I.; Segarra, A.B.; Alba, F. Neuropeptidases. Methods Mol. Biol. 2011, 789, 287–294. [Google Scholar] [CrossRef]
- Segarra, A.B.; Arechaga, G.; Prieto, I.; Ramirez-Exposito, M.J.; Martinez-Martos, J.M.; Ramirez, M.; Alba, F.; Ruiz-Larrea, M.B.; Ruiz-Sanz, J.I. Effects of dietary supplementation with fish oil, lard, or coconut oil on oxytocinase activity in the testis of mice. Arch. Androl. 2002, 48, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Prasad, C.; Imrhan, V.; Juma, S.; Maziarz, M.; Prasad, A.; Tiernan, C.; Vijayagopal, P. Bioactive Plant Metabolites in the Management of Non-Communicable Metabolic Diseases: Looking at Opportunities beyond the Horizon. Metabolites 2015, 5, 733–765. [Google Scholar] [CrossRef] [Green Version]
- Scharfmann, R.; Ebiou, J.; Morgat, J.; Aratan-Spire, S. Thyroid status regulates particulate but not soluble TRH-degrading pyroglutamate aminopeptidase activity in the rat liver. Acta Endocrinol. 1990, 123, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Boozer, C.N.; Schoenbach, G.; Atkinson, R.L. Dietary fat and adiposity: A dose-response relationship in adult male rats fed isocalorically. Am. J. Physiol. Endocrinol. Metab. 1995, 268, E546–E550. [Google Scholar] [CrossRef]
- Ghibaudi, L.; Cook, J.; Farley, C.; van Heek, M.; Hwa, J.J. Fat Intake Affects Adiposity, Comorbidity Factors, and Energy Metabolism of Sprague-Dawley Rats. Obes. Res. 2002, 10, 956–963. [Google Scholar] [CrossRef]
- Blundell, J.E.; Gillett, A. Control of Food Intake in the Obese. Obes. Res. 2001, 9, S263–S270. [Google Scholar] [CrossRef]
- Iossa, S.; Lionetti, L.; Mollica, M.P.; Crescenzo, R.; Botta, M.; Barletta, A.; Liverini, G. Effect of high-fat feeding on metabolic efficiency and mitochondrial oxidative capacity in adult rats. Br. J. Nutr. 2003, 90, 953–960. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Martínez, P.; García-Ríos, A.; Delgado-Lista, J.; Pérez-Jiménez, F.; López-Miranda, J. Mediterranean diet rich in olive oil and obesity, metabolic syndrome and diabetes mellitus. Curr. Pharm. Des. 2011, 17, 769–777. [Google Scholar] [CrossRef] [Green Version]
- Soriguer, F.; Almaraz, M.C.; Ruiz-de-Adana, M.S.; Esteva, I.; Linares, F.; García-Almeida, J.M.; Morcillo, S.; García-Escobar, E.; Olveira-Fuster, G.; Rojo-Martínez, G. Incidence of obesity is lower in persons who consume olive oil. Eur. J. Clin. Nutr. 2009, 63, 1371–1374. [Google Scholar] [CrossRef]
- Polley, K.R.; Miller, M.K.; Johnson, M.; Vaughan, R.; Paton, C.M.; Cooper, J.A. Metabolic responses to high-fat diets rich in MUFA v. PUFA. Br. J. Nutr. 2018, 120, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.; Ajuwon, K.M. Effects of Diets Differing in Composition of 18-C Fatty Acids on Adipose Tissue Thermogenic Gene Expression in Mice Fed High-Fat Diets. Nutrients 2018, 10, 256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oi-Kano, Y.; Kawada, T.; Watanabe, T.; Koyama, F.; Watanabe, K.; Senbongi, R.; Iwai, K. Extra virgin olive oil increases uncoupling protein 1 content in brown adipose tissue and enhances noradrenaline and adrenaline secretions in rats. J. Nutr. Biochem. 2007, 18, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Castro-Barquero, S.; Lamuela-Raventós, R.M.; Doménech, M.; Estruch, R. Relationship between Mediterranean Dietary Polyphenol Intake and Obesity. Nutrients 2018, 10, 1523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Durillo, F.; Segarra, A.B.; Villarejo, A.; Ramírez-Sánchez, M.; Prieto, I. Influence of Diet and Gender on Plasma DPP4 Activity and GLP-1 in Patients with Metabolic Syndrome: An Experimental Pilot Study. Molecules 2018, 23, 1564. [Google Scholar] [CrossRef] [Green Version]
- Cancelas, J.; Prieto, P.G.; Villanueva-Peñacarrillo, M.L.; Valverde, I.; Malaisse, W.J. Effects of an olive oil-enriched diet on glucagon-like peptide 1 release and intestinal content, plasma insulin concentration, glucose tolerance and pancreatic insulin content in an animal model of type 2 diabetes. Horm. Metab. Res. 2006, 38, 98–105. [Google Scholar] [CrossRef]
- Rocca, A.S.; LaGreca, J.; Kalitsky, J.; Brubaker, P.L. Monounsaturated fatty acid diets improve glycemic tolerance through increased secretion of glucagon-like peptide-1. Endocrinology 2001, 142, 1148–1155. [Google Scholar] [CrossRef]
- Song, M.K.; Rosenthal, M.J.; Song, A.M.; Yang, H.; Ao, Y.; Yamaguchi, D.T. Raw vegetable food containing high cyclo (his-pro) improved insulin sensitivity and body weight control. Metabolism 2005, 54, 1480–1489. [Google Scholar] [CrossRef]
- Hwang, I.K.; Go, V.L.W.; Harris, D.M.; Yip, I.; Kang, K.W.; Song, M.K. Effects of cyclo (his-pro) plus zinc on glucose metabolism in genetically diabetic obese mice. Diabetes Obes. Metab. 2003, 5, 317–324. [Google Scholar] [CrossRef]
- Araujo, R.L.; Andrade, B.M.; Padrón, A.S.; Gaidhu, M.P.; Perry, R.L.S.; Carvalho, D.P.; Ceddia, R.B. High-Fat Diet Increases Thyrotropin and Oxygen Consumption without Altering Circulating 3,5,3′-Triiodothyronine (T3) and Thyroxine in Rats: The Role of Iodothyronine Deiodinases, Reverse T3 Production, and Whole-Body Fat Oxidation. Endocrinology 2010, 151, 3460–3469. [Google Scholar] [CrossRef]
- Bergasa, N.V.; Boyella, V.D. Liver derived endogenous opioids may interfere with the therapeutic effect of interferon in chronic hepatitis C. Med. Hypotheses 2008, 70, 556–559. [Google Scholar] [CrossRef]
- Suo, J.; Zhao, X.; Guo, X.; Zhao, X. Met-enkephalin improves metabolic syndrome in high fat diet challenged mice through promotion of adipose tissue browning. Toxicol. Appl. Pharm. 2018, 359, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Fukasawa, K.M.; Fukasawa, K.; Kanai, M.; Fujii, S.; Harada, M. Molecular cloning and expression of rat liver aminopeptidase B. J. Biol. Chem. 1996, 271, 30731–30735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagasaka, T.; Nomura, S.; Okamura, M.; Tsujimoto, M.; Nakazato, H.; Oiso, Y.; Nakashima, N.; Mizutani, S. Immunohistochemical localization of placental leucine aminopeptidase/oxytocinase in normal human placental, fetal and adult tissues. Reprod. Fertil. Dev. 1997, 9, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Shim, K.Y.; Eom, Y.W.; Kim, M.Y.; Kang, S.H.; Baik, S.K. Role of the renin-angiotensin system in hepatic fibrosis and portal hypertension. Korean J. Intern. Med. 2018, 33, 453–461. [Google Scholar] [CrossRef] [Green Version]
- van den Hoven, A.F.; Smits, M.L.J.; Rosenbaum, C.E.N.M.; Verkooijen, H.M.; van den Bosch, M.A.A.J.; Lam, M.G.E.H. The effect of intra-arterial angiotensin II on the hepatic tumor to non-tumor blood flow ratio for radioembolization: A systematic review. PLoS ONE 2014, 9, e86394. [Google Scholar] [CrossRef]
- Graus-Nunes, F.; de Santos, F.O.; de Marinho, T.S.; Miranda, C.S.; Barbosa-da-Silva, S.; Souza-Mello, V. Beneficial effects of losartan or telmisartan on the local hepatic renin-angiotensin system to counter obesity in an experimental model. World J. Hepatol. 2019, 11, 359–369. [Google Scholar] [CrossRef]
- Min, L.; Sim, M.K.; Xu, X.G. Effects of des-aspartate-angiotensin I on angiotensin II-induced incorporation of phenylalanine and thymidine in cultured rat cardiomyocytes and aortic smooth muscle cells. Regul. Pept. 2000, 95, 93–97. [Google Scholar] [CrossRef]
- Reaux, A.; Fournie-Zaluski, M.C.; David, C.; Zini, S.; Roques, B.P.; Corvol, P.; Llorens-Cortes, C. Aminopeptidase A inhibitors as potential central antihypertensive agents. Proc. Natl. Acad. Sci. USA 1999, 96, 13415–13420. [Google Scholar] [CrossRef] [Green Version]
- Segarra, A.B.; Prieto, I.; Martínez-Cañamero, M.; de Gasparo, M.; Luna, J.d.D.; Ramírez-Sánchez, M. Thyroid Disorders Change the Pattern of Response of Angiotensinase Activities in the Hypothalamus-Pituitary-Adrenal Axis of Male Rats. Front. Endocrinol. 2018, 9, 731. [Google Scholar] [CrossRef] [Green Version]
- Gajdosechova, L.; Krskova, K.; Segarra, A.B.; Spolcova, A.; Suski, M.; Olszanecki, R.; Zorad, S. Hypooxytocinaemia in obese Zucker rats relates to oxytocin degradation in liver and adipose tissue. J. Endocrinol. 2014, 220, 333–343. [Google Scholar] [CrossRef] [Green Version]
- Prieto, I.; Segarra, A.B.; de Gasparo, M.; Martínez-Cañamero, M.; Ramírez-Sánchez, M. Divergent profile between hypothalamic and plasmatic aminopeptidase activities in WKY and SHR. Influence of beta-adrenergic blockade. Life Sci. 2018, 192, 9–17. [Google Scholar] [CrossRef]
- Villarejo, A.B.; Segarra, A.B.; Ramírez, M.; Banegas, I.; Wangensteen, R.; de Gasparo, M.; Cobo, J.; Alba, F.; Vives, F.; Prieto, I. Angiotensinase and vasopressinase activities in hypothalamus, plasma, and kidney after inhibition of angiotensin-converting enzyme: Basis for a new working hypothesis. Horm. Metab. Res. 2012, 44, 152–154. [Google Scholar] [CrossRef] [PubMed]
- Prieto, I.; Villarejo, A.B.; Segarra, A.B.; Wangensteen, R.; Banegas, I.; de Gasparo, M.; Vanderheyden, P.; Zorad, S.; Vives, F.; Ramírez-Sánchez, M. Tissue distribution of CysAP activity and its relationship to blood pressure and water balance. Life Sci 2015, 134, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Segarra, A.B.; Prieto, I.; Martinez-Canamero, M.; Vargas, F.; De Gasparo, M.; Vanderheyden, P.; Zorad, S.; Ramirez-Sanchez, M. Cystinyl and pyroglutamyl-beta-naphthylamide hydrolyzing activities are modified coordinately between hypothalamus, liver and plasma depending on the thyroid status of adult male rats. J. Physiol. Pharmacol. 2018, 69, 197–204. [Google Scholar] [CrossRef]
- Kanno, T.; Maekawa, M.; Kanda, S.; Kohno, H.; Sudo, K. Evaluation of Cytosolic Aminopeptidase in Human Sera: Evaluation in Hepatic Disorders. Am. J. Clin. Pathol. 1984, 82, 700–705. [Google Scholar] [CrossRef] [PubMed]
- Porta, M.; Pumarega, J.; Guarner, L.; Malats, N.; Solà, R.; Real, F.X.; PANKRAS II Study Group. Relationships of hepatic and pancreatic biomarkers with the cholestatic syndrome and tumor stage in pancreatic cancer. Biomarkers 2012, 17, 557–565. [Google Scholar] [CrossRef]
- Bezerra-Duarte, S.M.; Faintuch, J.; Stefano, J.T.; Sobral de Oliveira, M.B.; de Campos Mazo, D.F.; Rabelo, F.; Vanni, D.; Nogueira, M.A.; Carrilho, F.J.; Marques Souza de Oliveira, C.P. Hypocaloric high-protein diet improves clinical and biochemical markers in patients with nonalcoholic fatty liver disease (NAFLD). Nutr. Hosp. 2014, 29, 94–101. [Google Scholar] [CrossRef]
- Li, Q.; Feenstra, M.; Pfaffendorf, M.; Eijsman, L.; van Zwieten, P.A. Comparative Vasoconstrictor Effects of Angiotensin II, III, and IV in Human Isolated Saphenous Vein. J. Cardiovasc. Pharmacol. 1997, 29, 451–456. [Google Scholar] [CrossRef] [Green Version]
- Hassanin, A.; Malek, H.A.; Saleh, D. Heparin modulation on hepatic nitric oxide synthase in experimental steatohepatitis. Exp. Ther. Med. 2014, 8, 1551–1558. [Google Scholar] [CrossRef]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domínguez-Vías, G.; Segarra, A.B.; Ramírez-Sánchez, M.; Prieto, I. The Role of High Fat Diets and Liver Peptidase Activity in the Development of Obesity and Insulin Resistance in Wistar Rats. Nutrients 2020, 12, 636. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12030636
Domínguez-Vías G, Segarra AB, Ramírez-Sánchez M, Prieto I. The Role of High Fat Diets and Liver Peptidase Activity in the Development of Obesity and Insulin Resistance in Wistar Rats. Nutrients. 2020; 12(3):636. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12030636
Chicago/Turabian StyleDomínguez-Vías, Germán, Ana Belén Segarra, Manuel Ramírez-Sánchez, and Isabel Prieto. 2020. "The Role of High Fat Diets and Liver Peptidase Activity in the Development of Obesity and Insulin Resistance in Wistar Rats" Nutrients 12, no. 3: 636. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12030636