Biological and Computational Studies for Dual Cholinesterases Inhibitory Effect of Zerumbone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagent
2.2. Enzyme Kinetics and Substrate Selectivity
2.3. ADMET Predictions and Molecular Docking Study
2.4. Statistics
3. Results and Discussion
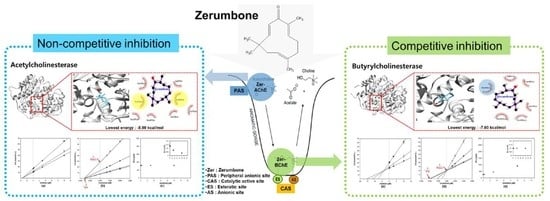
3.1. In Vitro Cholinesterase Inhibitory Property of Zerumbone
3.2. Enzyme Inhibition Kinetics of Zerumbone
3.3. Molecular Docking Studies for Zerumbone
3.4. In Silico ADMET Predictions for the Bioavailability of Zerumbone
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Selkoe, D.J. Alzheimer’s disease: Genes, proteins, and therapy. Physiol. Rev. 2001, 81, 741–766. [Google Scholar] [CrossRef]
- Reid, G.A.; Chilukuri, N.; Darvesh, S. Butyrylcholinesterase and the cholinergic system. Neuroscience 2013, 234, 53–68. [Google Scholar] [CrossRef] [Green Version]
- Ballard, C.G.; Greig, N.H.; Guillozet-Bongaarts, A.L.; Enz, A.; Darvesh, S. Cholinesterases: Role in the brain during health and disease. Curr. Alzheimer Res. 2005, 2, 307–318. [Google Scholar] [CrossRef]
- Inestrosa, N.; Ninamarca, M.; Alvarez, A. Amyloid-cholinesterase interactions. FEBS J. 2008, 275, 25–32. [Google Scholar] [CrossRef]
- Dinamarca, M.; Sagal, J.; Quintanilla, R.; Godoy, J.; Arrázola, M.; Inestrosa, N. Amyloid-beta-acetylcholinesterase complexes potentiate neurodegenerative changes induced by the abeta peptide. Implications for the pathogenesis of Alzheimer’s disease. Mol. Neurodegener 2010, 5, 4–19. [Google Scholar] [CrossRef] [Green Version]
- Pardridge, W.M. The Blood-Brain Barrier: Bottleneck in Brain Drug Development. NeuroRx 2005, 2, 3–14. [Google Scholar] [CrossRef]
- Geula, C.; Mesulam, M.M. Cholinesterases and the pathology of Alzheimer disease. Alzheimer Dis. Assoc. Disord. 1995, 9, 23–28. [Google Scholar] [CrossRef]
- Hemn, H.O.; Hazilawati, H.; Noordin, M.M.; Heshu, S.R.; Zuki, A. Influence of Zerumbone Supplementation a Natural Dietary Product from Zingiber zerumbet smith on Early-Developed Atherosclerotic Lesions in Cholesterol-Fed Rabbits. Open Conf. Proc. J. 2013, 4, 61–64. [Google Scholar] [CrossRef] [Green Version]
- Rahman, H.S.; Rasedee, A.; Othman, H.H.; Chartrand, M.S.; Namvar, F.; Yeap, S.K.; Samad, N.A.; Andas, R.J.; Nadzri, N.M.; Anasamy, T.; et al. Acute toxicity study of zerumbone-loaded nanostructured lipid carrier on BALB/c mice model. Biomed. Res. Int. 2014, 2014, 563930. [Google Scholar] [CrossRef]
- Singh, Y.P.; Girisa, S.; Banik, K.; Ghosh, S.; Swathi, P.; Deka, M.; Padmavathi, G.; Kotoky, J.; Sethi, G.; Fan, L.; et al. Potential application of zerumbone in the prevention and therapy of chronic human diseases. J. Funct. Foods 2019, 53, 248–258. [Google Scholar] [CrossRef]
- Aumeeruddy-Elalfi, Z.; Lall, N.; Fibrich, B.; Van Staden, A.B.; Hosenally, M.; Mahomoodally, M.F. Selected essential oils inhibit key physiological enzymes and possess intracellular and extracellular antimelanogenic properties in vitro. J. Food Drug Anal. 2018, 26, 232–243. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Ji, Y.; Youn, K.; Lim, G.; Lee, J.; Kim, D.H.; Jun, M. Baicalein as a potential inhibitor against BACE1 and AChE: Mechanistic comprehension through in vitro and computational approaches. Nutrients 2019, 11, 2694. [Google Scholar] [CrossRef] [Green Version]
- Pires, D.E.V.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef]
- Lee, S.; Youn, K.; Lim, G.; Lee, J.; Jun, M. In Silico Docking and In Vitro Approaches towards BACE1 and Cholinesterases Inhibitory Effect of Citrus Flavanones. Molecules 2018, 23, 1509. [Google Scholar] [CrossRef] [Green Version]
- Murakami, A.; Takahashi, D.; Kinoshita, T.; Koshimizu, K.; Kim, H.W.; Yoshihiro, A.; Nakamura, Y.; Jiwajinda, S.; Terao, J.; Ohigashi, H. Zerumbone, a Southeast Asian Ginger Sesquiterpene, Markedly Suppresses Free Radical Generation, Proinflammatory Protein Production, and Cancer Cell Proliferation Accompanied by Apoptosis: The Alpha, beta-Unsaturated Carbonyl Group Is a Prerequisite. Carcinogenesis 2002, 23, 795–802. [Google Scholar] [CrossRef] [Green Version]
- Ozougwu, V.E.O.; Akuba, B.O. In vitro inhibition of carbohydrate metabolizing enzymes and in vivo anti-hyperglycemic potential of methanol extract of Desmodium velutinum leaves. J. Med. Plant Res. 2018, 12, 48–56. [Google Scholar]
- Ujan, R.; Saeed, A.; Channar, P.A.; Larik, F.A.; Abbas, Q.; Alajmi, M.F.; El-Seedi, H.R.; Rind, M.A.; Hassan, M.; Raza, H.; et al. Drug-1, 3, 4-Thiadiazole Conjugates as Novel Mixed-Type Inhibitors of Acetylcholinesterase: Synthesis, Molecular Docking, Pharmacokinetics, and ADMET Evaluation. Molecules 2019, 24, 860. [Google Scholar] [CrossRef] [Green Version]
- Mehrotra, N.; Gupta, M.; Kovar, A.; Meibohm, B. The role of pharmacokinetics and pharmacodynamics in phosphodiesterase-5 inhibitor therapy. Int. J. Impot. Res. 2007, 19, 253–264. [Google Scholar] [CrossRef]
- Al-Zubairi, A.S.; Abdul, A.B.; Yousif, M.; Abdelwahab, S.I.; Elhassan, M.M.; Mohan, S. In vivo and in vitro genotoxic effects of zerumbone. Caryologia 2010, 63, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.B.; Seo, W.D.; Lee, Y.J.; Lee, Y.-S.; Lee, H.J. Toxicological evaluation of zerumbone on antitumor effects in mice. Afr. J. Pharm. Pharmacol. 2013, 7, 466–473. [Google Scholar] [CrossRef]
- Li, A.P. Screening for human ADME/Tox drug properties in drug discovery. Drug Discov. Today 2001, 6, 357–366. [Google Scholar] [CrossRef]
- Burk, C.; Sabbagh, M.N. Successes and failures for drugs in late-stage development for Alzheimer’s disease. Drug Aging 2013, 10, 738–792. [Google Scholar] [CrossRef]
- Sugimoto, H.; Yamanishi, Y.; Iimura, Y.; Kawakami, Y. Donepezil hydrochloride (E2020) and other acetylcholinesterase inhibitors. Curr. Med. Chem. 2000, 7, 303–339. [Google Scholar] [CrossRef]
- Liu, B.; Li, S.; Hu, J. Technological advances in high-throughput screening. Am. J. Pharmacogenomics 2004, 4, 263–276. [Google Scholar] [CrossRef]
- Schneider, G. Prediction of Drug-Like Properties. Madame Curie Bioscience Database; Landes Bioscience: Austin, TX, USA, 2013. [Google Scholar]
- Merlot, C. Computational toxicology-a tool for early safety evaluation. Drug Dicov. Today 2010, 15, 16–22. [Google Scholar] [CrossRef]
- Jafarian, S.; Ling, K.-H.; Hassan, Z.; Perimal-Lewis, L.; Sulaiman, M.R.; Perimal, E.K. Effect of zerumbone on scopolamine-induced memory impairment and anxiety-like behaviours in rats. Alzheimer’s Dement. 2019, 5, 637–643. [Google Scholar] [CrossRef]
- Li, L.; Wu, X.H.; Zhao, X.J.; Xu, L.; Pan, C.L.; Zhang, Z.Y. Zerumbone ameliorates behavioral impairments and neuropathology in transgenic APP/PS1 mice by suppressing MAPK signaling. J. Neuroinflamm. 2020, 17, 61. [Google Scholar] [CrossRef] [Green Version]



| Sample | AChE | BChE | ||||
|---|---|---|---|---|---|---|
| IC50 1 | Ki Value 2 | Inhibition Type 3 | IC50 | Ki Value | Inhibition Type | |
| Zerumbone | 2.78 ± 0.48 | 3.5 | Non-competitive | 4.12 ± 0.42 | 3.8 | Competitive |
| Galantamine 4 | 1.50 ± 0.05 | - | Competitive | 14.47 ± 0.33 | Competitive 5 | |
| Compounds (µM) | Trypsin | Chymotrypsin | Elastase | BACE1 | |
|---|---|---|---|---|---|
| Zerumbone | 50 | 5.00 ± 1.94 | 3.45 ± 0.30 | 3.46 ± 1.85 | 13.35 ± 1.20 |
| 100 | 3.75 ± 2.03 | 2.71 ± 0.45 | 3.57 ± 0.94 | 16.08 ± 1.36 | |
| Enzymes | Lowest Energy (Kcal/mol) | Van der Waals Residues |
|---|---|---|
| AChE | −8.0 | TYR72, ASP74, LEU76, TYR124, TRP286, PHE297, TYR337, TYR341 |
| BChE | −7.6 | ASP70, TRP82, PHE329, TYR332, TRP430, HIS438 |
| Properties | Predicted Values | |
|---|---|---|
| Absorption | Water solubility | −4.03 (log mol/L) |
| Permeability of Caco-2 | 1.43 (log Papp in 10−6 cm/s) | |
| Human intestinal absorption | 95.78 (% Absorbed) | |
| Skin permeability | −2.06 (log Kp) | |
| Substrate of P-glycoprotein (P-gp) | No | |
| Inhibitor of P-gp I, II | No | |
| Distribution | Volume of distribution (VDss, human) | 0.28 (log L/kg) |
| Fraction unbound (human) | 0.40 (Fu) | |
| Permeability of BBB | 0.52 (log BB) | |
| CNS permeability | −2.65 (log PS) | |
| Metabolism | Substrate of CYP2D6/CYP3A4 | No |
| Inhibitor of CYP1A2/CYP2C19/CYP2C9/CYP2D6/CYP3A4 | No | |
| Excretion | Total clearance | 1.31 (log ml/min/kg) |
| Renal OCT2 substrate 1 | No | |
| Toxicity | Ames toxicity 2 | No |
| Maximum tolerated dose (human) | 0.53 (log mg/kg/day) | |
| Inhibitor of hERG 3 I/II | No | |
| Hepatotoxicity | No | |
| Oral Rat Acute Toxicity (LD50) | 1.08 (mol/kg) | |
| Oral Rat Chronic Toxicity (LOAEL) | 1.18 (log mg/kg_body weigh)t/day) | |
| Skin Sensitization | Yes | |
| T. Pyriformis toxicity (IGC50) 4 | 1.39 (log µg/L) | |
| Minnow toxicity (LC50) 5 | 1.03 (log mM) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, J.; Youn, K.; Ji, Y.; Lee, S.; Lim, G.; Lee, J.; Ho, C.-T.; Leem, S.-H.; Jun, M. Biological and Computational Studies for Dual Cholinesterases Inhibitory Effect of Zerumbone. Nutrients 2020, 12, 1215. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12051215
Hwang J, Youn K, Ji Y, Lee S, Lim G, Lee J, Ho C-T, Leem S-H, Jun M. Biological and Computational Studies for Dual Cholinesterases Inhibitory Effect of Zerumbone. Nutrients. 2020; 12(5):1215. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12051215
Chicago/Turabian StyleHwang, Jayeong, Kumju Youn, Yeongseon Ji, Seonah Lee, Gyutae Lim, Jinhyuk Lee, Chi-Tang Ho, Sun-Hee Leem, and Mira Jun. 2020. "Biological and Computational Studies for Dual Cholinesterases Inhibitory Effect of Zerumbone" Nutrients 12, no. 5: 1215. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12051215