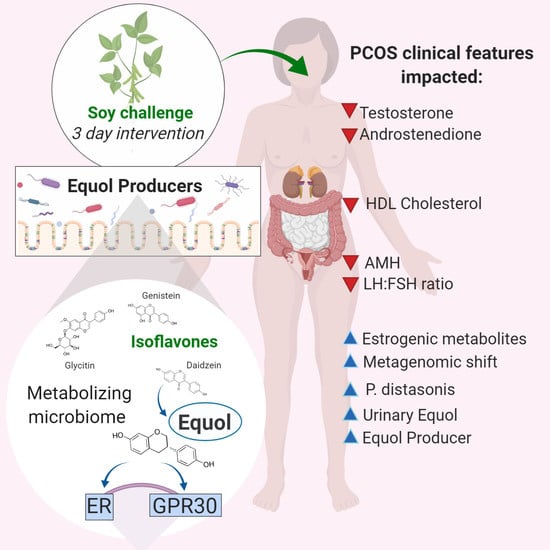
Impact of Short-Term Isoflavone Intervention in Polycystic Ovary Syndrome (PCOS) Patients on Microbiota Composition and Metagenomics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Visits and Sampling
2.3. Biochemical Measurements
2.4. Calculation of Indices
2.5. Next-Generation Sequencing of Stool Samples
2.6. Processing of Sequencing Data
2.7. Predicted Metagenome Analysis
2.8. Statistical Analysis and Sample Calculations
3. Results
3.1. Patient Characteristics
3.2. Isoflavone Metabolism and Characteristics
3.3. Metabolic Changes after Isoflavone Intervention
3.4. Predicted Metagenome
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Meier, R.K. Polycystic Ovary Syndrome. Nurs. Clin. North Am. 2018, 53, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhang, C.; Shi, Y.; Zhang, F.; Li, L.; Wang, X.; Ling, Y.; Fu, H.; Dong, W.; Shen, J.; et al. Dysbiosis of Gut Microbiota Associated with Clinical Parameters in Polycystic Ovary Syndrome. Front. Microbiol. 2017, 8, 62. [Google Scholar] [CrossRef] [PubMed]
- Azziz, R.; Carmina, E.; Chen, Z.; Dunaif, A.; Laven, J.S.E.; Legro, R.S.; Lizneva, D.; Natterson-Horowtiz, B.; Teede, H.J.; Yildiz, B.O. Polycystic ovary syndrome. Nat. Rev. Dis. Prim. 2016, 2, 16058. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.C.J.; Lyall, H.; Petrie, J.R.; Gould, G.W.; Connell, J.M.C.; Sattar, N. Low Grade Chronic Inflammation in Women with Polycystic Ovarian Syndrome. J. Clin. Endocrinol. Metab. 2001, 86, 2453–2455. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Dunaif, A. Insulin Resistance and the Polycystic Ovary Syndrome Revisited: An Update on Mechanisms and Implications. Endocr. Rev. 2012, 33, 981–1030. [Google Scholar] [CrossRef] [PubMed]
- Goldrat, O.; Delbaere, A. PCOS: Update and diagnostic approach. Clin. Biochem. 2018, 62, 24–31. [Google Scholar] [CrossRef]
- Dokras, A. Cardiovascular disease risk in women with PCOS. Steroids 2013. [Google Scholar] [CrossRef]
- Shulman, G.I. Ectopic Fat in Insulin Resistance, Dyslipidemia, and Cardiometabolic Disease. N. Engl. J. Med. 2014, 371, 1131–1141. [Google Scholar] [CrossRef]
- Morrison, S.; Goss, A.M.; Azziz, R.; Raju, D.A.; Gower, B.A. Peri-muscular adipose tissue may play a unique role in determining insulin sensitivity/resistance in women with polycystic ovary syndrome. Hum. Reprod. 2016, 32, 185–192. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; Rayner, C.K.; Horowitz, M. Incretins. Pharmacol. Ther. Cough 2015, 233, 137–171. [Google Scholar] [CrossRef]
- Landay, M.; Huang, A.; Azziz, R. Degree of hyperinsulinemia, independent of androgen levels, is an important determinant of the severity of hirsutism in PCOS. Fertil Steril. 2009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferk, P.; Teran, N.; Gersak, K. The (TAAAA)n microsatellite polymorphism in the SHBG gene influences serum SHBG levels in women with polycystic ovary syndrome. Hum. Reprod. 2006, 22, 1031–1036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González, F.; Rote, N.S.; Minium, J.; Kirwan, J.P. Reactive Oxygen Species-Induced Oxidative Stress in the Development of Insulin Resistance and Hyperandrogenism in Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2006, 91, 336–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindheim, L.; Bashir, M.; Munzker, J.; Trummer, C.; Zachhuber, V.; Leber, B.; Horvath, A.; Pieber, T.R.; Gorkiewicz, G.; Stadlbauer, V.; et al. Alterations in Gut Microbiome Composition and Barrier Function Are Associated with Reproductive and Metabolic Defects in Women with Polycystic Ovary Syndrome (PCOS): A Pilot Study. PLoS ONE 2017, 12, e0168390. [Google Scholar] [CrossRef]
- Elorinne, A.-L.; Alfthan, G.; Erlund, I.; Kivimäki, H.; Paju, A.; Salminen, I.; Turpeinen, U.; Voutilainen, S.; Laakso, J. Food and Nutrient Intake and Nutritional Status of Finnish Vegans and Non-Vegetarians. PLoS ONE 2016, 11, e0148235. [Google Scholar] [CrossRef] [Green Version]
- Atkinson, C.; Newton, K.M.; Stanczyk, F.Z.; Westerlind, K.C.; Li, L.; Lampe, J.W. Daidzein-metabolizing phenotypes in relation to serum hormones and sex hormone binding globulin, and urinary estrogen metabolites in premenopausal women in the United States. Cancer Causes Control. 2008, 19, 1085–1093. [Google Scholar] [CrossRef]
- Uchiyama, K.; Naito, Y.; Takagi, T. Intestinal microbiome as a novel therapeutic target for local and systemic inflammation. Pharmacol. Ther. 2019, 199, 164–172. [Google Scholar] [CrossRef]
- Yamagata, K. Soy Isoflavones Inhibit Endothelial Cell Dysfunction and Prevent Cardiovascular Disease. J. Cardiovasc. Pharmacol. 2019, 74, 201–209. [Google Scholar] [CrossRef]
- Yuan, J.-P.; Wang, J.-H.; Liu, X. Metabolism of dietary soy isoflavones to equol by human intestinal microflora—implications for health. Mol. Nutr. Food Res. 2007, 51, 765–781. [Google Scholar] [CrossRef]
- Krizova, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef] [Green Version]
- Esch, H.; Kleider, C.; Scheffler, A.; Lehmann, L. Isoflavones. Nutraceuticals 2016, 465–487. [Google Scholar] [CrossRef]
- Usui, T.; Tochiya, M.; Sasaki, Y.; Muranaka, K.; Yamakage, H.; Himeno, A.; Shimatsu, A.; Inaguma, A.; Ueno, T.; Uchiyama, S.; et al. Effects of naturalS-equol supplements on overweight or obesity and metabolic syndrome in the Japanese, based on sex and equol status. Clin. Endocrinol. 2013, 78, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Messina, M. Soy and Health Update: Evaluation of the Clinical and Epidemiologic Literature. Nutrients 2016, 8, 754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanaka, K.; Higuchi, M.; Ishimi, Y. Anti-Osteoporotic Effect of Soy Isoflavones Intake on Low Bone Mineral Density Caused by Voluntary Exercise and Food Restriction in Mature Female Rats. J. Nutr. Sci. Vitaminol. 2019, 65, 335–342. [Google Scholar] [CrossRef]
- Moradi, M.; Daneshzad, E.; Azadbakht, L. The effects of isolated soy protein, isolated soy isoflavones and soy protein containing isoflavones on serum lipids in postmenopausal women: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2019, 1–15. [Google Scholar] [CrossRef]
- Khani, B.; Mehrabian, F.; Khalesi, E.; Eshraghi, A. Effect of soy phytoestrogen on metabolic and hormonal disturbance of women with polycystic ovary syndrome. J. Res. Med Sci. 2011, 16, 297–302. [Google Scholar]
- Utian, W.H.; Jones, M.; Setchell, K.D.R. S-equol: A Potential Nonhormonal Agent for Menopause-Related Symptom Relief. J. Women’s Heal. 2015, 24, 200–208. [Google Scholar] [CrossRef]
- Halakivi-Clarke, L.; De Assis, S. Fetal origins of breast cancer. Trends Endocrinol. Metab. 2006, 17, 340–348. [Google Scholar] [CrossRef]
- Franke, A.A.; Lai, J.F.; Halm, B.M. Absorption, distribution, metabolism, and excretion of isoflavonoids after soy intake. Arch. Biochem. Biophys. 2014, 559, 24–28. [Google Scholar] [CrossRef] [Green Version]
- Cani, P.D.; Delzenne, N.M.; Amar, J.; Burcelin, R. Role of gut microflora in the development of obesity and insulin resistance following high-fat diet feeding. Pathol. Boil. 2008, 56, 305–309. [Google Scholar] [CrossRef]
- Zhao, X.; Jiang, Y.; Xi, H.; Chen, L.; Feng, X. Exploration of the Relationship Between Gut Microbiota and Polycystic Ovary Syndrome (PCOS): A Review. Geburtshilfe Frauenheilkd 2020, 80, 161–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zubik, L.; Meydani, M. Bioavailability of soybean isoflavones from aglycone and glucoside forms in American women. Am. J. Clin. Nutr. 2003, 77, 1459–1465. [Google Scholar] [CrossRef]
- Rowlands, D.J.; Chapple, S.; Siow, R.C.; Mann, G. Equol-stimulated mitochondrial reactive oxygen species activate endothelial nitric oxide synthase and redox signaling in endothelial cells: Roles for F-actin and GPR30. Hypertension 2011, 57, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Sada, S.; Sakaguchi, H.; Takizawa, M.; Ishida, R.; Tsuboi, T. Bacterial metabolite S-equol modulates glucagon-like peptide-1 secretion from enteroendocrine L cell line GLUTag cells via actin polymerization. Biochem. Biophys. Res. Commun. 2018, 501, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Iino, C.; Shimoyama, T.; Iino, K.; Yokoyama, Y.; Chinda, D.; Sakuraba, H.; Fukuda, S.; Nakaji, S. Daidzein Intake Is Associated with Equol Producing Status through an Increase in the Intestinal Bacteria Responsible for Equol Production. Nutrients 2019, 11, 433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Setchell, K.D.; Cole, S.J. Method of Defining Equol-Producer Status and Its Frequency among Vegetarians. J. Nutr. 2006, 136, 2188–2193. [Google Scholar] [CrossRef] [Green Version]
- Markowiak, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- Pfieffer, M.L. Polycystic ovary syndrome. Nursing 2019, 49, 34–40. [Google Scholar] [CrossRef]
- Azziz, R.; Carmina, E.; Sawaya, M.E. Idiopathic Hirsutism*. Endocr. Rev. 2000, 21, 347–362. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Shi, G. Smoking and microbiome in oral, airway, gut and some systemic diseases. J. Transl. Med. 2019, 17, 225. [Google Scholar] [CrossRef] [Green Version]
- Gomes, A.C.; Hoffmann, C.; Mota, J.F. The human gut microbiota: Metabolism and perspective in obesity. Gut Microbes 2018, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willmann, M.; Vehreschild, M.J.G.T.; Biehl, L.M.; Vogel, W.; Dörfel, D.; Hamprecht, A.; Seifert, H.; Autenrieth, I.B.; Peter, S. Distinct impact of antibiotics on the gut microbiome and resistome: A longitudinal multicenter cohort study. BMC Boil. 2019, 17, 1–18. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Food Frequency Questionnaire at a Glance. Diet Assess Prim; 2017. Available online: https://dietassessmentprimer.cancer.gov/profiles/questionnaire/index.html (accessed on 23 April 2020).
- Messina, M.; Nagata, C.; Wu, A.H. Estimated Asian Adult Soy Protein and Isoflavone Intakes. Nutr. Cancer 2006, 55, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Grace, P.B.; Mistry, N.S.; Carter, M.H.; Leathem, A.J.; Teale, P. High throughput quantification of phytoestrogens in human urine and serum using liquid chromatography/tandem mass spectrometry (LC–MS/MS). J. Chromatogr. B 2007, 853, 138–146. [Google Scholar] [CrossRef]
- Kozich, J.J.; Westcott, S.L.; Baxter, N.; Highlander, S.; Schloss, P.D. Development of a Dual-Index Sequencing Strategy and Curation Pipeline for Analyzing Amplicon Sequence Data on the MiSeq Illumina Sequencing Platform. Appl. Environ. Microbiol. 2013, 79, 5112–5120. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [Green Version]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G. Greengenes, a Chimera-Checked 16S rRNA Gene Database and Workbench Compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef] [Green Version]
- Afgan, E.; Baker, D.; Batut, B.; Beek, M.V.D.; Bouvier, D.; Čech, M.; Chilton, J.; Clements, D.; Coraor, N.; A Grüning, B.; et al. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Res. 2018, 46, W537–W544. [Google Scholar] [CrossRef] [Green Version]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; I Gordon, J.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335. [Google Scholar] [CrossRef] [Green Version]
- Coordinators, N.R.; Agarwala, R.; Barrett, T.; Beck, J.; A Benson, D.; Bollin, C.; Bolton, E.; Bourexis, D.; Brister, J.R.; Bryant, S.H.; et al. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2017, 46, D8–D13. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakrzewski, M.; Proietti, C.; Ellis, J.; Hasan, S.; Brion, M.-J.; Berger, B.; Krause, L. Calypso: A user-friendly web-server for mining and visualizing microbiome-environment interactions. Bioinformatics 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langille, M.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Thurber, R.L.V.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Boil. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogata, H.; Goto, S.; Sato, K.; Fujibuchi, W.; Bono, H.; Kanehisa, M. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 1999, 27, 29–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, R.A. The use of multiple measurements in taxonomic problems. Ann. Eugen. 1936, 7, 179–188. [Google Scholar] [CrossRef]
- Aleix, M. PCA versus LDA. IEEE Trans. Pattern Anal. Mach. Intell. 2001, 23, 228–233. [Google Scholar] [CrossRef] [Green Version]
- Wooldridge, J.M. Introductory Econometrics: A Modern Approach. Econ Anal. 2003. [Google Scholar] [CrossRef]
- Wolters, M.; Hahn, A. Soy isoflavones—A therapy for menopausal symptoms? Wien. Med. Wochenschr. 2004, 154, 334–341. [Google Scholar] [CrossRef]
- Hedlund, T.E.; Johannes, W.U.; Miller, G.J. Soy isoflavonoid equol modulates the growth of benign and malignant prostatic epithelial cells in vitro. Prostate 2002, 54, 68–78. [Google Scholar] [CrossRef]
- Decroos, K.; Vanhemmens, S.; Cattoir, S.; Boon, N.; Verstraete, W. Isolation and characterisation of an equol-producing mixed microbial culture from a human faecal sample and its activity under gastrointestinal conditions. Arch. Microbiol. 2004, 183, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, S.-M.; Wahala, K.; Liukkonen, K.-H.; Aura, A.-M.; Poutanen, K.; Adlercreutz, H. Studies of the In Vitro Intestinal Metabolism of Isoflavones Aid in the Identification of Their Urinary Metabolites. J. Agric. Food Chem. 2004, 52, 2640–2646. [Google Scholar] [CrossRef] [PubMed]
- Bowey, E.; Adlercreutz, H.; Rowland, I. Metabolism of isoflavones and lignans by the gut microflora: A study in germ-free and human flora associated rats. Food Chem. Toxicol. 2003, 41, 631–636. [Google Scholar] [CrossRef]
- Hedlund, T.E.; Maroni, P.D.; Ferucci, P.G.; Dayton, R.; Barnes, S.; Jones, K.; Moore, R.; Ogden, L.G.; Wähälä, K.; Sackett, H.M.; et al. Long-term dietary habits affect soy isoflavone metabolism and accumulation in prostatic fluid in caucasian men. J. Nutr. 2005, 135, 1400–1406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Constantinou, A.; White, B.E.; Tonetti, D.; Yang, Y.; Liang, W.; Li, W.; Van Breemen, R.B. The soy isoflavone daidzein improves the capacity of tamoxifen to prevent mammary tumours. Eur. J. Cancer 2005, 41, 647–654. [Google Scholar] [CrossRef]
- Duncan, A.M.; E Merz-Demlow, B.; Xu, X.; Phipps, W.R.; Kurzer, M.S. Premenopausal equol excretors show plasma hormone profiles associated with lowered risk of breast cancer. Cancer Epidemiol. Biomark. Prev. 2000, 9, 581–586. [Google Scholar]
- Lampe, J.W.; Karr, S.C.; Hutchins, A.M.; Slavin, J.L. Urinary equol excretion with a soy challenge: Influence of habitual diet. Proc. Soc. Exp. Boil. Med. 1998, 217, 335–339. [Google Scholar] [CrossRef]
- Atkinson, C.; Skor, H.E.; Fitzgibbons, E.D.; Scholes, D.; Chen, C.; Wahala, K.; Schwartz, S.; Lampe, J.W. Urinary equol excretion in relation to 2-hydroxyestrone and 16α-hydroxyestrone concentrations: An observational study of young to middle-aged women. J. Steroid Biochem. Mol. Boil. 2003, 86, 71–77. [Google Scholar] [CrossRef]
- Atkinson, C.; Berman, S.; Humbert, O.; Lampe, J.W. In Vitro Incubation of Human Feces with Daidzein and Antibiotics Suggests Interindividual Differences in the Bacteria Responsible for Equol Production. J. Nutr. 2004, 134, 596–599. [Google Scholar] [CrossRef] [Green Version]
- Rowland, I.R.; Wiseman, H.; Sanders, T.; Adlercreutz, H.; Bowey, E.A. Interindividual Variation in Metabolism of Soy Isoflavones and Lignans: Influence of Habitual Diet on Equol Production by the Gut Microflora. Nutr. Cancer 2000, 36, 27–32. [Google Scholar] [CrossRef]
- Muthyala, R.S.; Ju, Y.H.; Sheng, S.; Williams, L.D.; Doerge, D.R.; Katzenellenbogen, B.S.; Katzenellenbogen, J.A. Equol, a natural estrogenic metabolite from soy isoflavones: Convenient preparation and resolution of R- and S-equols and their differing binding and biological activity through estrogen receptors alpha and beta. Bioorganic Med. Chem. 2004. [Google Scholar] [CrossRef] [PubMed]
- Teede, H.J.; Misso, M.L.; Costello, M.; Dokras, A.; Laven, J.; Moran, L.J.; Piltonen, T.T.; Norman, R.; Andersen, M.; Azziz, R.; et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum. Reprod. 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindheim, L.; Bashir, M.; Münzker, J.; Trummer, C.; Zachhuber, V.; Pieber, T.R.; Gorkiewicz, G.; Obermayer-Pietsch, B. The Salivary Microbiome in Polycystic Ovary Syndrome (PCOS) and Its Association with Disease-Related Parameters: A Pilot Study. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Shortt, C.; Hasselwander, O.; Meynier, A.; Nauta, A.; Fernández, E.N.; Putz, P.; Rowland, I.; Swann, J.; Türk, J.; Vermeiren, J.; et al. Systematic review of the effects of the intestinal microbiota on selected nutrients and non-nutrients. Eur. J. Nutr. 2017, 57, 25–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pyrczak, F.; Oh, D.M. Making Sense of Statistics, 7th ed.; Routledge: New York, NY, USA, 2018. [Google Scholar]
- Salkind, N. Encyclopedia of Research Design; SAGE Publications, Inc.: Thousand Oaks, CA, USA, 2010. [Google Scholar] [CrossRef]
- Gao, X.; Liu, K.; Huang, F.; Zhang, N.; Guo, X.; Wang, M.; Liu, B. Positive and negative regulation of insulin action by genistein in the endothelium. J. Nutr. Biochem. 2013, 24, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, M.; Sugiya, M.; Iijima, H.; Nakagome, I.; Hirono, S.; Tsuda, T. Genistein and daidzein, typical soy isoflavones, inhibit TNF-α-mediated downregulation of adiponectin expression via different mechanisms in 3T3-L1 adipocytes. Mol. Nutr. Food Res. 2012, 56, 1783–1793. [Google Scholar] [CrossRef]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001. [Google Scholar] [CrossRef]
- Horiuchi, H.; Usami, A.; Shirai, R.; Harada, N.; Ikushiro, S.; Sakaki, T.; Nakano, Y.; Inui, H.; Yamaji, R. S -Equol Activates cAMP Signaling at the Plasma Membrane of INS-1 Pancreatic β-Cells and Protects against Streptozotocin-Induced Hyperglycemia by Increasing β-Cell Function in Male Mice. J. Nutr. 2017, 147, 1631–1639. [Google Scholar] [CrossRef] [Green Version]
- Vrbikova, J.; Hill, M.; Bendlová, B.; Grimmichova, T.; Dvořáková, K.; Vondra, K.; Pacini, G. Incretin levels in polycystic ovary syndrome. Eur. J. Endocrinol. 2008, 159, 121–127. [Google Scholar] [CrossRef]
- Maktabi, M.; Jamilian, M.; Asemi, Z. Magnesium-Zinc-Calcium-Vitamin D Co-supplementation Improves Hormonal Profiles, Biomarkers of Inflammation and Oxidative Stress in Women with Polycystic Ovary Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Boil. Trace Element Res. 2017, 182, 21–28. [Google Scholar] [CrossRef]
- Kadoura, S.; Alhalabi, M.; Nattouf, A.H. Effect of Calcium and Vitamin D Supplements as an Adjuvant Therapy to Metformin on Menstrual Cycle Abnormalities, Hormonal Profile, and IGF-1 System in Polycystic Ovary Syndrome Patients: A Randomized, Placebo-Controlled Clinical Trial. Adv. Pharmacol. Sci. 2019, 2019, 9680390. [Google Scholar] [CrossRef] [PubMed]
- Shojaeian, Z.; Sadeghi, R.; Roudsari, R.L. Calcium and vitamin D supplementation effects on metabolic factors, menstrual cycles and follicular responses in women with polycystic ocvary syndrome: A systematic review and meta-analysis. Casp. J. Intern. Med. 2019, 10, 359–369. [Google Scholar]
- Hooper, L.; Ryder, J.J.; Kurzer, M.S.; Lampe, J.W.; Messina, M.J.; Phipps, W.R.; Cassidy, A. Effects of soy protein and isoflavones on circulating hormone concentrations in pre- and post-menopausal women: A systematic review and meta-analysis. Hum. Reprod. Update 2009, 15, 423–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romualdi, D.; Costantini, B.; Campagna, G.; Lanzone, A.; Guido, M. Is there a role for soy isoflavones in the therapeutic approach to polycystic ovary syndrome? Results from a pilot study. Fertil. Steril. 2008, 90, 1826–1833. [Google Scholar] [CrossRef] [PubMed]
- Jamilian, M.; Asemi, Z. The Effects of Soy Isoflavones on Metabolic Status of Patients with Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2016, 101, 3386–3394. [Google Scholar] [CrossRef] [Green Version]
- Rajaei, S.; Alihemmati, A.; Abedelahi, A. Antioxidant effect of genistein on ovarian tissue morphology, oxidant and antioxidant activity in rats with induced polycystic ovary syndrome. Int. J. Reprod. Biomed. 2019, 17, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Farkhad, S.A.; Khazali, H. Therapeutic effects of isoflavone-aglycone fraction from soybean (Glycine max L. Merrill) in rats with estradiol valerate-induced polycystic ovary syndrome as an inflammatory state. Gynecol. Endocrinol. 2019, 35, 1078–1083. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Chi, X.X. Estrogenic properties of genistein acting on FSHR and LHR in rats with PCOS. Pol. J. Veter- Sci. 2019, 22, 83–90. [Google Scholar] [CrossRef]
- Chi, X.-X.; Zhang, T.; Chu, X.-L.; Zhen, J.-L.; Zhang, D.-J. The regulatory effect of Genistein on granulosa cell in ovary of rat with PCOS through Bcl-2 and Bax signaling pathways. J. Veter- Med Sci. 2018, 80, 1348–1355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motlekar, N.; Khan, M.; Youan, B.-B.C. Preparation and characterization of genistein containing poly(ethylene glycol) microparticles. J. Appl. Polym. Sci. 2006, 101, 2070–2078. [Google Scholar] [CrossRef]
- Huang, A.-S.; Hsieh, O.A.-L.; Chang, S.S. Characterization of the Nonvolatile Minor Constituents Responsible for the Objectionable Taste of Defatted Soybean Flour. J. Food Sci. 1982, 47, 19–23. [Google Scholar] [CrossRef]
- Risal, S.; Pei, Y.; Lu, H.; Manti, M.; Fornes, R.; Pui, H.-P.; Zhao, Z.; Massart, J.; Ohlsson, C.; Lindgren, E.; et al. Prenatal androgen exposure and transgenerational susceptibility to polycystic ovary syndrome. Nat. Med. 2019, 25, 1894–1904. [Google Scholar] [CrossRef] [PubMed]
- Siemienowicz, K.J.; Filis, P.; Shaw, S.; Douglas, A.; Thomas, J.; Mulroy, S.; Howie, F.; Fowler, P.A.; Duncan, W.C.; Rae, M.T. Fetal androgen exposure is a determinant of adult male metabolic health. Sci. Rep. 2019, 9, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palomba, S.; Falbo, A.; Chiossi, G.; Muscogiuri, G.; Fornaciari, E.; Orio, F.; Tolino, A.; Colao, A.; La Sala, G.B.; Zullo, F. Lipid profile in nonobese pregnant women with polycystic ovary syndrome: A prospective controlled clinical study. Steroids 2014, 88, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Wilhelmson, A.S.; Rodriguez, M.L.; Stubelius, A.; Fogelstrand, P.; Johansson, I.; Buechler, M.B.; Lianoglou, S.; Kapoor, V.N.; Johansson, M.E.; Fagman, J.B.; et al. Testosterone is an endogenous regulator of BAFF and splenic B cell number. Nat. Commun. 2018, 9, 2067. [Google Scholar] [CrossRef]
- Fagman, J.B.; Wilhelmson, A.S.; Motta, B.M.; Pirazzi, C.; Alexanderson, C.; De Gendt, K.; Verhoeven, G.; Holmäng, A.; Anesten, F.; Jansson, J.-O.; et al. The androgen receptor confers protection against diet-induced atherosclerosis, obesity, and dyslipidemia in female mice. FASEB J. 2014, 29, 1540–1550. [Google Scholar] [CrossRef]
- Li, S.; Chu, Q.; Ma, J.; Sun, Y.; Tao, T.; Huang, R.; Liao, Y.; Yue, J.; Zheng, J.; Wang, L.; et al. Discovery of Novel Lipid Profiles in PCOS: Do Insulin and Androgen Oppositely Regulate Bioactive Lipid Production? J. Clin. Endocrinol. Metab. 2016, 102, 810–821. [Google Scholar] [CrossRef] [Green Version]
- Binder, C.J.; Papac-Milicevic, N.; Witztum, J.L. Innate sensing of oxidation-specific epitopes in health and disease. Nat. Rev. Immunol. 2016, 16, 485–497. [Google Scholar] [CrossRef]
- Busch, C.J.; Binder, C.J. Malondialdehyde epitopes as mediators of sterile inflammation. Biochim. Biophys. Acta (BBA) Mol. Cell Boil. Lipids 2017, 1862, 398–406. [Google Scholar] [CrossRef]
- Ko, T.-F.; Tsai, H.-S.; Lin, S.-M.; Liu, C.-D.; Learn, S.-P.; Chiou, R.Y. GC-MS Determined Distribution of Urinary Equol Producers as Affected by Age, Gender, and Repeated Ingestions of Soymilk. J. Food Sci. 2010, 75. [Google Scholar] [CrossRef]
- Franke, A.A.; Lai, J.F.; Pagano, I.; Morimoto, Y.; Maskarinec, G. Equol production changes over time in pre-menopausal women. Br. J. Nutr. 2011, 107, 1201–1206. [Google Scholar] [CrossRef] [PubMed]
- Nakatsu, C.H.; Armstrong, A.; Clavijo, A.P.; Martin, B.R.; Barnes, S.; Weaver, C.M. Fecal Bacterial Community Changes Associated with Isoflavone Metabolites in Postmenopausal Women after Soy Bar Consumption. PLoS ONE 2014, 9, e108924. [Google Scholar] [CrossRef] [PubMed]
- Rajan, R.K.; Balaji, B. Soy isoflavones exert beneficial effects on letrozole-induced rat polycystic ovary syndrome (PCOS) model through anti-androgenic mechanism. Pharm. Boil. 2016, 55, 242–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkinson, C.; Frankenfeld, C.L.; Lampe, J.W. Gut Bacterial Metabolism of the Soy Isoflavone Daidzein: Exploring the Relevance to Human Health. Exp. Boil. Med. 2005, 230, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Torres, P.J.; Siakowska, M.; Banaszewska, B.; Pawelczyk, L.; Duleba, A.J.; Kelley, S.T.; Thackray, V.G. Gut Microbial Diversity in Women With Polycystic Ovary Syndrome Correlates With Hyperandrogenism. J. Clin. Endocrinol. Metab. 2018, 103, 1502–1511. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2013, 505, 559–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashyap, P.C.; Marcobal, A.; Ursell, L.K.; Larauche, M.; Duboc, H.; Earle, K.A.; Sonnenburg, E.D.; Ferreyra, J.A.; Higginbottom, S.K.; Million, M.; et al. Complex interactions among diet, gastrointestinal transit, and gut microbiota in humanized mice. Gastroenterology 2013, 144, 967–977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tilocca, B.; Burbach, K.; Heyer, C.M.E.; Hoelzle, L.E.; Mosenthin, R.; Stefanski, V.; Camarinha-Silva, A.; Seifert, J. Dietary changes in nutritional studies shape the structural and functional composition of the pigs’ fecal microbiome—from days to weeks. Microbiome 2017, 5, 144. [Google Scholar] [CrossRef] [Green Version]
- Bonder, M.-J.; Tigchelaar, E.F.; Cai, X.; Trynka, G.; Cenit, M.C.; Hrdlickova, B.; Zhong, H.; Vatanen, T.; Gevers, D.; Wijmenga, C.; et al. The influence of a short-term gluten-free diet on the human gut microbiome. Genome Med. 2016, 8, 45. [Google Scholar] [CrossRef]
- Ze, X.; Duncan, S.H.; Louis, P.; Flint, H.J. Ruminococcus bromii is a keystone species for the degradation of resistant starch in the human colon. ISME J. 2012, 6, 1535–1543. [Google Scholar] [CrossRef]
- Wang, K.; Liao, M.; Zhou, N.; Bao, L.; Ma, K.; Zheng, Z.; Wang, Y.; Liu, C.; Wang, W.; Wang, J.; et al. Parabacteroides distasonis Alleviates Obesity and Metabolic Dysfunctions via Production of Succinate and Secondary Bile Acids. Cell Rep. 2019, 26, 222–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nallabelli, N.; Patil, P.P.; Pal, V.K.; Singh, N.; Jain, A.; Patil, P.B.; Grover, V.; Korpole, S. Biochemical and genome sequence analyses of Megasphaera sp. strain DISK18 from dental plaque of a healthy individual reveals commensal lifestyle. Sci. Rep. 2016, 6, 33665. [Google Scholar] [CrossRef] [PubMed]
- Shetty, S.A.; Marathe, N.P.; Lanjekar, V.; Ranade, D.; Shouche, Y.S. Comparative Genome Analysis of Megasphaera sp. Reveals Niche Specialization and Its Potential Role in the Human Gut. PLoS ONE 2013, 8, e79353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Sun, Z.; Jiang, S.; Bai, X.; Ma, C.; Peng, Q.; Chen, K.; Chang, H.; Fang, T.; Zhang, H. Probiotic Bifidobacterium lactis V9 Regulates the Secretion of Sex Hormones in Polycystic Ovary Syndrome Patients through the Gut-Brain Axis. mSystems 2019, 4, e00017-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flint, H.J.; Scott, K.P.; Duncan, S.H.; Louis, P.; Forano, E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes 2012, 3, 289–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harvey, L.; Arnold, B.S.; Lawrence, Z.; Pau, L.M.; David, B.; James, D. Molecular Cell Biology, 4th ed.; W. H. Freeman: New York, NY, USA, 2000. [Google Scholar] [CrossRef] [Green Version]
- Wright, E.M.; Martı́n, M.G.; Turk, E. Intestinal absorption in health and disease—sugars. Best Pr. Res. Clin. Gastroenterol. 2003, 17, 943–956. [Google Scholar] [CrossRef]
- Seelinger, G.; Merfort, I.; Wölfle, U.; Schempp, C. Anti-carcinogenic Effects of the Flavonoid Luteolin. Molecules 2008, 13, 2628–2651. [Google Scholar] [CrossRef]
- Ramos, S. Cancer chemoprevention and chemotherapy: Dietary polyphenols and signalling pathways. Mol. Nutr. Food Res. 2008, 52, 507–526. [Google Scholar] [CrossRef]
- Ingram, D.; Sanders, K.; Kolybaba, M.; Lopez, D. Case-control study of phyto-oestrogens and breast cancer. Lancet 1997, 350, 990–994. [Google Scholar] [CrossRef]
- Zern, T.L.; Fernandez, M.-L. Cardioprotective Effects of Dietary Polyphenols. J. Nutr. 2005, 135, 2291–2294. [Google Scholar] [CrossRef] [Green Version]
- Miret, S.; Simpson, R.J.; McKie, A.T. Physiology and molecular biology of dietary iron absorption. Ann. Rev. Nutr. 2003, 23, 283–301. [Google Scholar] [CrossRef] [PubMed]
- Fleet, J.C.; Schoch, R.D. Molecular mechanisms for regulation of intestinal calcium absorption by vitamin D and other factors. Crit. Rev. Clin. Lab. Sci. 2010, 47, 181–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szczuko, M.; Skowronek, M.; Zapałowska-Chwyć, M.; Starczewski, A. Quantitative Assessment of Nutrition in Patients With the Polycystic Ovary Syndrome (Pcos). Rocz Panstw Zakl Hig. 2016, 67, 419–426. [Google Scholar] [PubMed]
- Eslamian, G.; Hekmatdoost, A. Nutrient Patterns and Risk of Polycystic Ovary Syndrome. J. Reprod. Infertil. 2019, 20, 161–168. [Google Scholar] [PubMed]
- Tremellen, K.; Pearce, K. Dysbiosis of Gut Microbiota (DOGMA)—A novel theory for the development of Polycystic Ovarian Syndrome. Med Hypotheses 2012, 79, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Rojas, J.; Chávez, M.; Olivar, L.C.; Rojas, M.; Morillo, J.; Mejias, J.; Calvo, M.; Bermúdez, V. Polycystic Ovary Syndrome, Insulin Resistance, and Obesity: Navigating the Pathophysiologic Labyrinth. Int. J. Reprod. Med. 2014, 2014, 1–17. [Google Scholar] [CrossRef]
- Orio, F.; Muscogiuri, G.; Ascione, A.; Marciano, F.; Volpe, A.; La Sala, G.; Savastano, S.; Colao, A.; Palomba, S. Effects of physical exercise on the female reproductive system. Minerva Endocrinol. 2013, 38, 305–319. [Google Scholar]
- Stepto, N.K.; Hiam, D.; Gibson-Helm, M.; Cassar, S.; Harrison, C.L.; Hutchison, S.K.; E Joham, A.; Canny, B.; Moreno-Asso, A.; Strauss, B.J.; et al. Exercise and insulin resistance in PCOS: Muscle insulin signalling and fibrosis. Endocr. Connect. 2020, 9, 346–359. [Google Scholar] [CrossRef] [Green Version]
- Llaneza, P.; Gonzalez-Gonzalez, C.; Fernandez-Iñarrea, J.; Alonso, A.; Diaz-Fernandez, M.J.; Arnott, I.; Barriendos, J.F. Soy isoflavones, Mediterranean diet, and physical exercise in postmenopausal women with insulin resistance. Menopause 2010, 17, 372–378. [Google Scholar] [CrossRef]
- Giolo, J.S.; Costa, J.; Junior, J.P.D.C.; Pajuaba, A.C.A.M.; Taketomi, E.A.; De Souza, A.V.; Caixeta, D.C.; Peixoto, L.G.; De Oliveira, E.P.; Everman, S.; et al. The Effects of Isoflavone Supplementation Plus Combined Exercise on Lipid Levels, and Inflammatory and Oxidative Stress Markers in Postmenopausal Women. Nutrients 2018, 10, 424. [Google Scholar] [CrossRef] [Green Version]
- Kazemi, M.; Pierson, R.A.; McBreairty, L.E.; Chilibeck, P.D.; Zello, G.A.; Chizen, D.R. A randomized controlled trial of a lifestyle intervention with longitudinal follow-up on ovarian dysmorphology in women with polycystic ovary syndrome. Clin. Endocrinol. 2020, 92, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Nybacka, Å.; Carlström, K.; Ståhle, A.; Nyren, S.; Hellström, P.M.; Hirschberg, A.L. Randomized comparison of the influence of dietary management and/or physical exercise on ovarian function and metabolic parameters in overweight women with polycystic ovary syndrome. Fertil. Steril. 2011, 96, 1508–1513. [Google Scholar] [CrossRef]
- Wang, B.; Xu, H.; Hu, X.; Ma, W.; Zhang, J.; Li, Y.; Yu, M.; Zhang, Y.; Li, X.; Ye, X. Synergetic inhibition of daidzein and regular exercise on breast cancer in bearing-4T1 mice by regulating NK cells and apoptosis pathway. Life Sci. 2020, 245, 117387. [Google Scholar] [CrossRef] [PubMed]
- Riesco, E.; Aubertin-Leheudre, M.; Maltais, M.L.; Audet, M.; Dionne, I.J. Synergic effect of phytoestrogens and exercise training on cardiovascular risk profile in exercise-responder postmenopausal women. Menopause 2010, 17, 1035–1039. [Google Scholar] [CrossRef] [PubMed]







| Reference Range | Controls (n = 20) | PCOS (n = 24) | ||||
|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | p-Value | ||
| Age | 32 | 12.0 | 27 | 5.9 | 0.003 ** | |
| Anthropometric parameters | ||||||
| Body mass index | 18.5–25.0 # | 22.3 | 4.10 | 24.9 | 11.75 | 0.147 |
| Waist to hip ratio | <0.85 # | 0.80 | 0.06 | 0.82 | 0.077 | 0.439 |
| Metabolic parameters | ||||||
| Fasting glucose (mmol/L) | <7.0 † | 4.5 | 0.50 | 4.7 | 0.59 | 0.209 |
| AUC glucose (mmolh/L) | § | 10.2 | 4.52 | 10.9 | 3.61 | 0.273 |
| Fasting insulin (pmol/L) | 20.9–173.8 | 41.4 | 51.08 | 84.4 | 55.25 | 0.022 * |
| AUC insulin (mmolh/L) | § | 353 | 427.3 | 691 | 562.0 | 0.009 ** |
| HOMA2-IR | <2.0 | 0.8 | 1.05 | 1.7 | 1.20 | 0.027 * |
| Total cholesterol (mmol/L) | <5.2 | 4.6 | 0.64 | 4.5 | 1.13 | 0.699 |
| LDL cholesterol (mmol/L) | <3.4 | 2.3 | 0.83 | 2.3 | 1.14 | 0.144 |
| HDL cholesterol (mmol/L) | >1.0 | 2.0 | 0.42 | 1.7 | 0.49 | 0.006 ** |
| Triglycerides (mmol/L) | <1.65 | 0.59 | 0.25 | 0.74 | 0.24 | 0.010 * |
| Serum sex hormones | ||||||
| FSH (IU/L) | 0.5–61.2 ‡ | 9.2 | 8.11 | 7.5 | 2.73 | 0.178 |
| LH (IU/L) | 2.0–22.0 ‡ | 5.8 | 9.34 | 9.3 | 8.60 | 0.042 * |
| LH:FSH ratio | § | 1.2 | 1.19 | 1.5 | 1.06 | 0.035 * |
| SHBG (nmol/L) | 18–144 | 78 | 29.40 | 43 | 41.50 | <0.001 *** |
| AMH (pmol/L) | 1.4–65.2 | 26.8 | 22.42 | 61.1 | 52.59 | 0.0002 *** |
| Total testosterone (nmol/L) | 0.37–2.1 | 1.1 | 0.56 | 1.3 | 0.77 | 0.002 ** |
| DHT (nmol/L) | § | 0.34 | 0.24 | 0.46 | 0.528 | 0.096 |
| Androstenedione (nmol/L) | 0.89–7.5 | 2.6 | 1.61 | 4.2 | 2.69 | 0.0003 *** |
| DHEA (nmol/L) | § | 13.7 | 11.37 | 21.4 | 12.40 | 0.015* |
| DHEAS (µmol/L) | § | 3.3 | 3.74 | 4.9 | 2.35 | 0.073 |
| Free testosterone (pmol/L) | § | 10.6 | 5.86 | 20.9 | 13.00 | <0.0001 *** |
| Free DHT (pmol/L) | § | 1.3 | 1.03 | 3.0 | 2.19 | <0.0001 *** |
| PCOS assessment | # of cases | % of cases | # of cases | % of cases | p-value | |
| Oligo-/amenorrhea | 1 | 5 | 17 | 71 | <0.0001 *** | |
| Hirsutism | 1 | 5 | 11 | 46 | 0.003 ** | |
| PCOM | 0 | 0 | 22 | 96 | <0.0001 *** | |
| Dietary assessment | Median | IQR | Median | IQR | p-value | |
| Total points | 69 | 27.0 | 68 | 31.8 | 0.318 | |
| Grains (% of total points) | 19 | 9.4 | 17 | 8.9 | 0.025 * | |
| Dairy (% of total points) | 13 | 8.6 | 13 | 10.2 | 0.502 | |
| Meat/fish (% of total points) | 6 | 6.4 | 7 | 6.8 | 0.649 | |
| Fruits/vegetables (% of total points) | 17 | 11.4 | 19 | 12.6 | 0.856 | |
| Fats (% of total points) | 10 | 6.0 | 12 | 5.2 | 0.258 | |
| Soy (% of total points) | 1 | 1.9 | 1 | 2.5 | 0.658 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haudum, C.; Lindheim, L.; Ascani, A.; Trummer, C.; Horvath, A.; Münzker, J.; Obermayer-Pietsch, B. Impact of Short-Term Isoflavone Intervention in Polycystic Ovary Syndrome (PCOS) Patients on Microbiota Composition and Metagenomics. Nutrients 2020, 12, 1622. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12061622
Haudum C, Lindheim L, Ascani A, Trummer C, Horvath A, Münzker J, Obermayer-Pietsch B. Impact of Short-Term Isoflavone Intervention in Polycystic Ovary Syndrome (PCOS) Patients on Microbiota Composition and Metagenomics. Nutrients. 2020; 12(6):1622. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12061622
Chicago/Turabian StyleHaudum, Christoph, Lisa Lindheim, Angelo Ascani, Christian Trummer, Angela Horvath, Julia Münzker, and Barbara Obermayer-Pietsch. 2020. "Impact of Short-Term Isoflavone Intervention in Polycystic Ovary Syndrome (PCOS) Patients on Microbiota Composition and Metagenomics" Nutrients 12, no. 6: 1622. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12061622