Vitamin C for Cardiac Protection during Percutaneous Coronary Intervention: A Systematic Review of Randomized Controlled Trials
Abstract
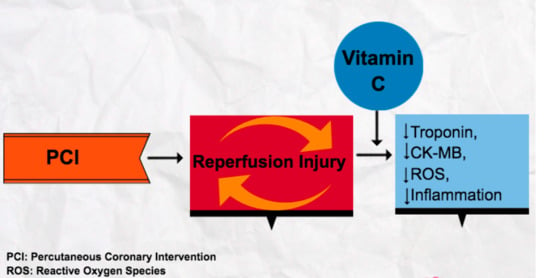
:1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Literature Search
2.3. Quality Assessment of Included Trials
3. Results
3.1. Characteristics of the Trials
3.2. Risk of Bias Analysis
3.3. Effect of VC Administration on Myocardial Injury
3.3.1. Elevation of Troponin
3.3.2. Elevation of CK-MB
3.4. Effect of VC on Cardiac Contractility
3.5. Effect of VC on Infarct Size and Coronary Artery Restenosis
3.6. Effect of VC on Total Antioxidant Status (TAS)
3.7. Effect of VC on Reactive Oxidation Species (ROS)
3.8. Effect of VC on Inflammation Mediators/Markers
3.9. Effect of VC on Reperfusion Indices and Vascular Endothelial Dysfunction
3.10. Overall Results
4. Discussion
4.1. Insights into Mechanisms
4.2. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Cardiovascular Diseases (CVDs). Available online: https://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 17 May 2017).
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef] [PubMed]
- Neri, M.; Riezzo, I.; Pascale, N.; Pomara, C.; Turillazzi, E. Ischemia/Reperfusion Injury following Acute Myocardial Infarction: A Critical Issue for Clinicians and Forensic Pathologists. Mediat. Inflamm. 2017, 2017, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; De Ferranti, S.; Després, J.P.; Fullerton, H.J.; Howard, V.J.; et al. Heart Disease and Stroke Statistics-2015 Update: A Report From the American Heart Association. Circulation 2016, 133, E417. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Fourth Universal Definition of Myocardial Infarction. J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar] [CrossRef]
- Anderson, J.L.; Morrow, D.A. Acute Myocardial Infarction. N. Engl. J. Med. 2017, 376, 2053–2064. [Google Scholar] [CrossRef] [Green Version]
- O’Gara, P.T.; Kushner, F.G.; Ascheim, D.D.; Casey, D.E.; Chung, M.K.; de Lemos, J.A.; Ettinger, S.M.; Fang, J.C.; Fesmire, F.M.; Franklin, B.A.; et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2013, 61, e78. [Google Scholar] [CrossRef] [Green Version]
- Keeley, E.C.; Boura, J.A.; Grines, C.L. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: A quantitative review of 23 randomised trials. Lancet 2003, 361, 13–20. [Google Scholar] [CrossRef]
- Harold, J.G.; Bass, T.A.; Bashore, T.M.; Brindis, R.G.; Brush, J.E.; Burke, J.A.; Dehmer, G.J.; Deychak, Y.A.; Jneid, H.; Jollis, J.G.; et al. ACCF/AHA/SCAI 2013 update of the clinical competence statement on coronary artery interventional procedures: A report of the American College of Cardiology Foundation/American Heart Association/American College of Physicians Task Force on Clinical Competence and Training (Writing Committee to Revise the 2007 Clinical Competence Statement on Cardiac Interventional Procedures). J. Am. Coll. Cardiol. 2013, 62, 357. [Google Scholar]
- Nallamothu, B.K.; Bradley, E.H.; Krumholz, H.M. Time to Treatment in Primary Percutaneous Coronary Intervention. N. Engl. J. Med. 2007, 357, 1631–1638. [Google Scholar] [CrossRef]
- Hayato, H.; Yasuhide, A.; Teruo, N.; Yoshiaki, M.; Yu, K.; Fumiyuki, O.; Kazuhiro, N.; Masashi, F.; Toshiyuki, N.; Michikazu, N.; et al. Three-dimensional assessment of coronary high-intensity plaques with T1-weighted cardiovascular magnetic resonance imaging to predict periprocedural myocardial injury after elective percutaneous coronary intervention. J. Cardiovasc. Magn. Reson. 2020, 22, 1–11. [Google Scholar]
- Langabeer, J.R.; Henry, T.D.; Kereiakes, D.J.; Dellifraine, J.; Emert, J.; Wang, Z.; Stuart, L.; King, R.; Segrest, W.; Moyer, P.; et al. Growth in percutaneous coronary intervention capacity relative to population and disease prevalence. J. Am. Heart Assoc. 2013, 2, e000370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masoudi, F.A.; Ponirakis, A.; de Lemos, J.A.; Jollis, J.G.; Kremers, M.; Messenger, J.C.; Moore, J.W.M.; Moussa, I.; Oetgen, W.J.; Varosy, P.D.; et al. Trends in U.S. Cardiovascular Care: 2016 Report From 4 ACC National Cardiovascular Data Registries. J. Am. Coll. Cardiol. 2017, 69, 1427–1450. [Google Scholar] [CrossRef] [PubMed]
- Tzlil, G.; Tamir, B.; Yoav, H.; Abid, A.; Hana, V.A.; Ran, K.; Alon, E. Temporal Trends of the Management and Outcome of Patients with Myocardial Infarction According to the Risk for Recurrent Cardiovascular Events. Am. J. Med. 2020, 133, 839–847. [Google Scholar]
- Hess, C.N.; Clare, R.M.; Neely, M.L.; Tricoci, P.; Mahaffey, K.W.; James, S.K.; Alexander, J.H.; Held, C.; Lopes, R.D.; Fox, K.A.A.; et al. Differential occurrence, profile, and impact of first recurrent cardiovascular events after an acute coronary syndrome. Am. Heart J. 2017, 187, 194–203. [Google Scholar] [CrossRef]
- Goldberg, R.J.; Currie, K.; White, K.; Brieger, D.; Steg, P.G.; Goodman, S.G.; Dabbous, O.; Fox, K.A.A.; Gore, J.M. Six-month outcomes in a multinational registry of patients hospitalized with an acute coronary syndrome (The Global Registry of Acute Coronary Events [GRACE]). Am. J. Cardiol. 2004, 93, 288–293. [Google Scholar] [CrossRef]
- Steg, P.G.; Bhatt, D.L.; Wilson, P.W.F.; D’Agostino, R.; Ohman, E.M.; Röther, J.; Liau, C.-S.; Hirsch, A.T.; Mas, J.-L.; Ikeda, Y.; et al. One-Year Cardiovascular Event Rates in Outpatients With Atherothrombosis. JAMA 2007, 297, 1197–1206. [Google Scholar] [CrossRef]
- Morrow, D.A. Cardiovascular Risk Prediction in Patients With Stable and Unstable Coronary Heart Disease. Circulation 2010, 121, 2681–2691. [Google Scholar] [CrossRef] [Green Version]
- Prasad, A.; Singh, M.; Lerman, A.; Lennon, R.J.; Holmes, D.R.; Rihal, C.S. Isolated Elevation in Troponin T After Percutaneous Coronary Intervention Is Associated With Higher Long-Term Mortality. J. Am. Coll. Cardiol. 2006, 48, 1765–1770. [Google Scholar] [CrossRef] [Green Version]
- Prasad, A.; Herrmann, J. Myocardial Infarction Due to Percutaneous Coronary Intervention. N. Engl. J. Med. 2011, 364, 453–464. [Google Scholar] [CrossRef] [Green Version]
- Zeitouni, M.; Silvain, J.; Guedeney, P.; Kerneis, M.; Yan, Y.; Overtchouk, P.; Barthelemy, O.; Hauguel-Moreau, M.; Choussat, R.; Helft, G.; et al. Periprocedural myocardial infarction and injury in elective coronary stenting. Eur. Heart J. 2018, 39, 1100–1109. [Google Scholar] [CrossRef]
- Yellon, D.M.; Hausenloy, D.J. Myocardial reperfusion injury. N. Engl. J. Med. 2007, 357, 1121–1135. [Google Scholar] [CrossRef] [PubMed]
- Otani, H. Ischemic preconditioning: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2008, 10, 207–247. [Google Scholar] [CrossRef] [PubMed]
- Bonnemeier, H.; Wiegand, U.K.H.; Giannitsis, E.; Schulenburg, S.; Hartmann, F.; Kurowski, V.; Bode, F.; Tölg, R.; Katus, H.A.; Richardt, G. Temporal repolarization inhomogeneity and reperfusion arrhythmias in patients undergoing successful primary percutaneous coronary intervention for acute ST-segment elevation myocardial infarction: Impact of admission troponin T. Am. Heart J. 2003, 145, 484–492. [Google Scholar] [CrossRef]
- Zweier, J.L.; Talukder, M.A. The role of oxidants and free radicals in reperfusion injury. Cardiovasc. Res. 2006, 70, 181–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Z.-Q. Oxidative stress-elicited myocardial apoptosis during reperfusion. Curr. Opin. Pharmacol. 2004, 4, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Duilio, C.; Ambrosio, G.; Kuppusamy, P.; Dipaula, A.; Becker, L.C.; Zweier, J. Neutrophils are primary source of O-2 radicals during reperfusion after prolonged myocardial ischemia. Am. J. Physiol. Heart Circul. Physiol. 2001, 280, H2649–H2657. [Google Scholar] [CrossRef] [Green Version]
- Galang, N.; Sasaki, H.; Maulik, N. Apoptotic cell death during ischemia/reperfusion and its attenuation by antioxidant therapy. Toxicology 2000, 148, 111–118. [Google Scholar] [CrossRef]
- Guan, W.; Osanai, T.; Kamada, T.; Ishizaka, H.; Hanada, H.; Okumura, K. Time course of free radical production after primary coronary angioplasty for acute myocardial infarction and the effect of vitamin C. Jpn. Circ. J. 1999, 63, 924–928. [Google Scholar] [CrossRef] [Green Version]
- Kasap, S.; Gönenç, A.; Sener, D.E.; Hisar, I. Serum cardiac markers in patients with acute myocardial infarction: Oxidative stress, C-reactive protein and N-terminal probrain natriuretic Peptide. J. Clin. Biochem. Nutr. 2007, 41, 50–57. [Google Scholar] [CrossRef] [Green Version]
- Muzáková, V.; Kandár, R.; Vojtísek, P.; Skalický, J.; Cervinková, Z. Selective antioxidant enzymes during ischemia/reperfusion in myocardial infarction. Physiol. Res. 2000, 49, 315–322. [Google Scholar]
- Li, C.; Jackson, R.M. Reactive species mechanisms of cellular hypoxia-reoxygenation injury. Am. J. Physiol.-Cell Physiol. 2002, 282, C227–C241. [Google Scholar] [CrossRef] [Green Version]
- Gasparetto, C.; Malinverno, A.; Culacciati, D.; Gritti, D.; Prosperini, P.G.; Specchia, G.; Ricevuti, G. Antioxidant vitamins reduce oxidative stress and ventricular remodeling in patients with acute myocardial infarction. Int. J. Immunopathol. Pharmacol. 2005, 18, 487–496. [Google Scholar] [CrossRef] [Green Version]
- Basili, S.; Pignatelli, P.; Tanzilli, G.; Mangieri, E.; Carnevale, R.; Nocella, C.; Di Santo, S.; Pastori, D.; Ferroni, P.; Violi, F. Anoxia-reoxygenation enhances platelet thromboxane A2 production via reactive oxygen species-generated NOX2: Effect in patients undergoing elective percutaneous coronary intervention. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1766–1771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lykkesfeldt, J.; Tveden-Nyborg, P. The Pharmacokinetics of Vitamin C. Nutrients 2019, 11, 2412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tribble, D.L. AHA Science Advisory. Antioxidant consumption and risk of coronary heart disease: Emphasison vitamin C, vitamin E, and beta-carotene: A statement for healthcare professionals from the American Heart Association. Circulation 1999, 99, 591–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, D.M.; Raman, S.; McEneny, J.; Young, I.S.; Parham, K.L.; Hullin, D.A.; Davies, B.; McKeeman, G.; McCord, J.M.; Lewis, M.H. Vitamin C prophylaxis promotes oxidative lipid damage during surgical ischemia-reperfusion. Free Radic. Biol. Med. 2006, 40, 591–600. [Google Scholar] [CrossRef]
- Rodrigo, R.; Prieto, J.; Castillo, R. Cardioprotection against ischaemia/reperfusion by vitamins C and E plus n-3 fatty acids: Molecular mechanisms and potential clinical applications. Clin. Sci. 2013, 124, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Shafaei-Bajestani, N.; Talasaz, A.; Salarifar, M.; Pourhosseini, H.; Sadri, F.; Jalali, A. Potential role of Vitamin C intracoronary administration in preventing cardiac injury after primary percutaneous coronary intervention in patients with ST-elevation myocardial infarction. J. Res. Pharm. Pract. 2019, 8, 75–82. [Google Scholar]
- Valls, N.; Gormaz, J.G.; Aguayo, R.; Gonzalez, J.; Brito, R.; Hasson, D.; Libuy, M.; Ramos, C.; Carrasco, R.; Prieto, J.C.; et al. Amelioration of persistent left ventricular function impairment through increased plasma ascorbate levels following myocardial infarction. Redox Rep. 2016, 21, 75–83. [Google Scholar]
- Wang, Z.J.; Hu, W.K.; Liu, Y.Y.; Shi, D.M.; Cheng, W.J.; Guo, Y.H.; Yang, Q.; Zhao, Y.X.; Zhou, Y.J. The effect of intravenous vitamin C infusion on periprocedural myocardial injury for patients undergoing elective percutaneous coronary intervention. Can. J. Cardiol. 2014, 30, 96–101. [Google Scholar] [CrossRef]
- Pignatelli, P.; Tanzilli, G.; Carnevale, R.; Di Santo, S.; Loffredo, L.; Celestini, A.; Proietti, M.; Tovaglia, P.; Mangieri, E.; Basili, S.; et al. Ascorbic acid infusion blunts CD40L upregulation in patients undergoing coronary stent. Cardiovasc. Ther. 2011, 29, 385–394. [Google Scholar] [CrossRef]
- Basili, S.; Tanzilli, G.; Mangieri, E.; Raparelli, V.; Di Santo, S.; Pignatelli, P.; Violi, F. Intravenous ascorbic acid infusion improves myocardial perfusion grade during elective percutaneous coronary intervention: Relationship with oxidative stress markers. JACC Cardiovasc. Interv. 2010, 3, 221–229. [Google Scholar] [CrossRef] [Green Version]
- Ramos, C.; Brito, R.; Gonzalez-Montero, J.; Valls, N.; Gormaz, J.G.; Prieto, J.C.; Aguayo, R.; Puentes, A.; Noriega, V.; Pereira, G.; et al. Effects of a novel ascorbate-based protocol on infarct size and ventricle function in acute myocardial infarction patients undergoing percutaneous coronary angioplasty. Arch. Med. Sci. 2017, 13, 558–567. [Google Scholar] [CrossRef]
- Hu, X.; Yuan, L.; Wang, H.; Li, C.; Cai, J.; Hu, Y.; Ma, C. Efficacy and safety of vitamin C for atrial fibrillation after cardiac surgery: A meta-analysis with trial sequential analysis of randomized controlled trials. Int. J. Surg. 2017, 37, 58–64. [Google Scholar] [CrossRef]
- Shi, R.; Li, Z.H.; Chen, D.; Wu, Q.C.; Zhou, X.L.; Tie, H.T. Sole and combined vitamin C supplementation can prevent postoperative atrial fibrillation after cardiac surgery: A systematic review and meta-analysis of randomized controlled trials. Clin. Cardiol. 2018, 41, 871–878. [Google Scholar] [CrossRef] [Green Version]
- Ali-Hassan-Sayegh, S.; Mirhosseini, S.J.; Rezaeisadrabadi, M.; Dehghan, H.R.; Sedaghat-Hamedani, F.; Kayvanpour, E.; Popov, A.-F.; Liakopoulos, O.J. Antioxidant supplementations for prevention of atrial fibrillation after cardiac surgery: An. updated comprehensive systematic review and meta-analysis of 23 randomized controlled trials. Interact. Cardiovasc. Thorac. Surg. 2014, 18, 646–654. [Google Scholar] [CrossRef] [Green Version]
- Baker, L.W.; Coleman, I.C. Meta-analysis of ascorbic acid for prevention of postoperative atrial fibrillation after cardiac surgery. Am. J. Health-Syst. Pharm. 2016, 73, 2056–2066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [Green Version]
- Cochrane.org. Cochrane Methods Bias. Available online: https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials (accessed on 18 February 2020).
- Rodrigo, R.; Hasson, D.; Prieto, J.C.; Dussaillant, G.; Ramos, C.; Leon, L.; Garate, J.; Valls, N.; Gormaz, J.G. The effectiveness of antioxidant vitamins C and E in reducing myocardial infarct size in patients subjected to percutaneous coronary angioplasty (PREVEC Trial): Study protocol for a pilot randomized double-blind controlled trial. Trials 2014, 15, 192. [Google Scholar] [CrossRef] [Green Version]
- Hicks, J.J.; Montes-Cortes, D.H.; Cruz-Dominguez, M.P.; Medina-Santillan, R.; Olivares-Corichi, I.M. Antioxidants decrease reperfusion induced arrhythmias in myocardial infarction with ST-elevation. Front. Biosci. 2007, 12, 2029–2037. [Google Scholar] [CrossRef] [Green Version]
- Jaxa-Chamiec, T.; Bednarz, B.; Drozdowska, D.; Gessek, J.; Gniot, J.; Janik, K.; Kawka-Urbanek, T.; Maciejewski, P.; Ogorek, M.; Szpajer, M. Antioxidant effects of combined vitamins C and E in acute myocardial infarction. The randomized, double-blind, placebo controlled, multicenter pilot Myocardial Infarction and VITamins (MIVIT) trial. Kardiol. Pol. 2005, 62, 344–350. [Google Scholar]
- Panczenko-Kresowska, B.; Ziemlanski, S.; Rudnicki, S.; Wojtulewicz, L.; Przepiorka, M. The influence of vitamin C and e or beta-carotene on peroxidative processes in persons with myocardial ischemia. Pol. Merkur. Lek. 1998, 4, 12–15. [Google Scholar]
- Tardif, J.C.; Cote, G.; Lesperance, J.; Bourassa, M.; Lambert, J.; Doucet, S.; Bilodeau, L.; Nattel, S.; de Guise, P. Probucol and multivitamins in the prevention of restenosis after coronary angioplasty. Multivitamins and Probucol Study Group. N. Engl. J. Med. 1997, 337, 365–372. [Google Scholar] [CrossRef]
- Safaei, N.; Babaei, H.; Azarfarin, R.; Jodati, A.R.; Yaghoubi, A.; Sheikhalizadeh, M.A. Comparative effect of grape seed extract. Ann Card. Anaesth. 2017, 20, 45–51. [Google Scholar]
- Emadi, N.; Nemati, M.H.; Ghorbani, M.; Allahyari, E. The Effect of High.-Dose Vitamin C on Biochemical Markers of Myocardial Injury in Coronary Artery Bypass Surgery. Braz. J. Cardiovasc. Surg. 2019, 34, 517–524. [Google Scholar] [CrossRef]
- Sherman, D.L.; Keaney, J.F.; Biegelsen, E.S.; Duffy, S.J.; Coffman, J.D.; Vita, J.A. Pharmacological concentrations of ascorbic acid are required for the beneficial effect on endothelial vasomotor function in hypertension. Hypertension 2000, 35, 936–941. [Google Scholar] [CrossRef] [Green Version]
- Taddei, S.; Virdis, A.; Ghiadoni, L.; Magagna, A.; Salvetti, A. Vitamin C improves endothelium-dependent vasodilation by restoring nitric oxide activity in essential hypertension. Circulation 1998, 97, 2222–2229. [Google Scholar] [CrossRef] [Green Version]
- Ang, A.; Pullar, J.M.; Currie, M.J.; Vissers, M.C.M. Vitamin C and immune cell function in inflammation and cancer. Biochem. Soc. Trans. 2018, 46, 1147–1159. [Google Scholar] [CrossRef] [Green Version]
- Ockaili, R.; Natarajan, R.; Salloum, F.; Fisher, B.J.; Jones, D.; Fowler, A.A., 3rd; Kukreja, R.C. HIF-1 activation attenuates postischemic myocardial injury: Role for heme oxygenase-1 in modulating microvascular chemokine generation. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H542–H548. [Google Scholar] [CrossRef] [Green Version]
- Morel, O.; Jesel, L.; Hugel, B.; Douchet, M.P.; Zupan, M.; Chauvin, M.; Freyssinet, J.M.; Toti, F. Protective effects of vitamin C on endothelium damage and platelet activation during myocardial infarction in patients with sustained generation of circulating microparticles. J. Thromb. Haemost. 2003, 1, 171–177. [Google Scholar] [CrossRef]
- Hill, A.; Wendt, S.; Benstoem, C.; Neubauer, C.; Meybohm, P.; Langlois, P.; Adhikari, N.K.; Heyland, D.K.; Stoppe, C. Vitamin C to Improve Organ. Dysfunction in Cardiac Surgery Patients-Review and Pragmatic Approach. Nutrients 2018, 10, 974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashor, A.W.; Lara, J.; Mathers, J.C.; Siervo, M. Effect of vitamin C on endothelial function in health and disease: A systematic review and meta-analysis of randomised controlled trials. Atherosclerosis 2014, 235, 9–20. [Google Scholar] [CrossRef] [PubMed]


| Trial | Country | Diag | Sample Size (Ctr, VC) | Age (Mean (±SD) Years | Sex (Male) n (%) | Vitamin C | Additional Therapy to VC Group (Yes/No) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ctr | VC | Ctr | VC | Route | Dose (g) | Time before PCI (min) | ||||||
| 1 | Shafaei et al. (2019) [39] | Iran | ACS | 252 (126,126) | 57.18 ± 10.4 | 58.64 ± 10.41 | 97 (76.9) | 104 (82.5) | IV, IC | 3 | 0 | No |
| 2 | Ramos et al. (2017) a [44] | Chile | ACS | 67 (41,26) | 56.16 ± 8.51 | 59.2 ± 11.98 | NA | NA | IV | 56 | 30 | Yes e |
| 3 | Valls et al. (2016) a [40] | Chile | ACS | 43 (21,22) | 57.1 ± 7.2 | 59.8 ± 13.3 | 19 (95.2) | 20 (90.9) | IV | 56 | 60 | Yes e |
| 4 | Wang et al. (2014) [41] | China | SA | 532 (267,265) | 58.0 ± 10.1 | 57.9 ± 10.4 | 182 (68.1) | 192 (72) | IV | 3 | 360 | No |
| 5 | Basili et al. (2010–2011) c | Italy | SA | 56 (28,28) | 68 ± 9 | 66 ± 8 | 23 (82) | 24 (86) | IV | 1 | 60 | No |
| 6 | Gasparetto et al. (2005) [33] | Italy | ACS | 98 (49,49) | 40–86 years | 74.4% Males | IV | 1 | 60 | Yes f | ||
| 7 | Guan et al. (1999) d [29] | Japan | ACS | 21 (11,10) | 68 ± 4 | 68 ± 4 | 8(72) | 6 (60) | IV | 2 | 0 | No |
| 8 | Tardif et al. (1997) b [55] | Canada | SA | 116 (62,54) | 60.3 ± 8.4 | 57.7 ± 11.1 | 61 (77) | 66 (84) | PO | 1 | 720 | Yes g |
| Trial [Ref] | Troponin < 24 h | CK-MB < 24 h | LVEF < 15 Days or 3 mo | Infarct Size < 15 Days or 3 mo | TLVV (mL), 7 or 30 Days | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ctr | VC | p | Ctr | VC | p | Ctr | VC | p | Ctr | VC | p | Ctr | VC | p | |
| Shafaei et al. (2019) [39] | 7.5 ng/L a b | 7.1 ng/L a b | 0.003 | 3.98 ng/L b | 3.52 ng/L b | 0.00 | |||||||||
| Ramos et al. (2017) [44] | 387 u/L (189.0–725.0) | 335 u/L (134.0–478.0) | 0.66 | 49.1% c (41.0–59.4) | 47.3% c (40.0–56.4) | 0.54 | 21.5% c (17.0–34.2) | 17.0% c (13.0–36.0) | 0.66 | ||||||
| 47.5% d (38.0–61.7) | 54.6% d (39.9–64.8) | 0.41 | 19.0% d (14.0–34.0) | 21.0% d (14.0–34.0) | 0.96 | ||||||||||
| Valls et al. (2016) [40] | 48.0% c b (56.6–33.3) | 56% c b (44.0–58.6) | NS g | ||||||||||||
| NA g | NA g | NS g | 44% d b (34.0–56.0) | 63% d b (50.0–68.0) | 0.05 | ||||||||||
| Wang et al. (2014) [41] | 0.04 ng/mL (0.02–1.12) | 0.03 ng/mL (0.03–0.06) | 0.02 | 6.1 ng/mL (4.4–6.4) | 4.9 ng/mL (4.1–5.7) | 0.001 | |||||||||
| Pignatelli et al. h (2011) [42] | 54.1 ± 4.7% | 58.3 ± 2.9% | 0.03 | ||||||||||||
| Basili et al. h (2010) [43] | △0.027 ng/mL (0.05 to 0.032) | △0.008 ng/mL (0.02 to 0.013) | 0.08 | ||||||||||||
| Gasparetto et al. (2005) [33] | 125.12 ± 29.8 e | 119.4 ± 29.4 e | 0.05 | ||||||||||||
| 132.0 ± 33.5 f | 123.4 ± 21.6 f | 0.05 | |||||||||||||
| Trial [Ref] | Measure | Baseline | 0 h | 6–8 h | 48 h | 1 Month | Discharge | |
|---|---|---|---|---|---|---|---|---|
| Ramos et al. (2017) [44] | Vit C | ctr | 0.02 (0.02–0.050) | 0.02 (0.01–0.03) | 0.02(0.01–0.04) | 0.03 (0.01–0.05) | ||
| (mmol/L) | VC | 0.04 (0.02–0.09) | 9.63 (6.25–11.64) | 0.72 (0.23–2.43) | 0.02 (0.01–0.05) | |||
| p | NS | <0.0001 | <0.0001 | NS | ||||
| FRAP | ctr | 304.1 (213.0–429.6) | 310.3 (227.7–400.0) | 287 (250.2–391.2) | 352.2 (220.0–371.8) | |||
| (umol/L) | VC | 271.7 (200.2–396.4) | 8050 (50275.0–11418.0) | 1080 (699.9–2062.0) | 378.2 (257.9–464.3) | |||
| p | NS | <0.0001 | <0.0001 | NS | ||||
| GSH | ctr | 3.59 (3.21–4.54) | 3.92 (3.26–5.28) | 4.13 (3.48–5.06) | 4.02 (3.34–4.43) | |||
| (mmol/L) | VC | 4.11 (3.61–6.56) | 3.71 (1.75–4.60) | 2.59 (1.61–4.15) | 3.87 (3.55–4.86) | |||
| p | NS | 0.0149 | 0.0198 | NS | ||||
| Valls et al. (2016) [40] | Vit C | ctr | 0.03 ± 0.02 | 0.03 ± 0.04 | 0.03 ± 0.03 | 0.02 ± 0.01 | ||
| (mmol/L) | VC | 0.1 ± 0.20 | 9.79 ± 3.87 | 1.79 ± 1.51 | 0.06 ± 0.06 | |||
| p | NS | <0.01 | <0.01 | NS | ||||
| FRAP | ctr | NA | 1× | 1× | NA | |||
| (umol/L) | VC | NA | 29× | 4.8× | NA | |||
| p | NS | <0.01 | <0.01 | NS | ||||
| GSH b | ctr | 4.2 ± 1.54 | 4.7 ± 2.2 | 4.9 ± 2.4 | 4.4 ± 2.0 | |||
| (mmol/L) | VC | 5 ± 2.29 | 3 ± 2.1 | 2.4 ± 1.76 | 3.8 ± 0.7 | |||
| p | NS | NS | <0.01 | NS | ||||
| Gasparetto et at (2005) [33] | TAS | ctr | 419.79 ± 34.26 | 526.47 ± 44.24 | 598.47 ± 54.99 | |||
| (umol/L) | VC | 401.95 ± 19.02 | 737.65 ± 51.15 | 647.38 ± 5 4.33 | ||||
| p | NS | <0.01 | NS |
| Trial [Ref] | Measure | Baseline | 0–1 h | 1–2 h | 6–8 h | 48 h | 1 Month | |
|---|---|---|---|---|---|---|---|---|
| Valls et al. (2016) [40] | 8-isoprotane b (pg/mL) | ctr | 24.0 ± 16.0 | 28.0 ± 18.0 | 17.1 ± 7.0 | |||
| VC | 24.0 ± 10.4 | 47 ± 23.0 | 26 ± 13.0 | |||||
| p | NS | <0.05 | NS | |||||
| Wang et al. (2014) [41] | 8-OHdG (ng/mL) | ctr | 3.8 ± 1.2 | 2.4 ± 1.0 | ||||
| VC | 3.6 ± 1.2 | 4.1 ± 1.1 | ||||||
| p | NS | <0.001 | ||||||
| Basili et al. (2011) c [34] | TxB2 b (ng/mL) | ctr | 23 ± 2.0 | 26 ± 1.1 | 26.5 ± 4.0 | |||
| VC | 24 ± 3.5 | 21 ± 3.3 | 23 ± 3.5 | |||||
| p | NS | <0.05 | <0.05 | |||||
| sNOX2-dp b (pg/mL) | ctr | 19 ± 1.0 | 21.8 ± 0.2 | 22 ± 1.8 | ||||
| VC | 21.4 ± 1.2 | 18.4 ± 1.4 | 19 ± 2.3 | |||||
| p | NS | 0.05 | <0.05 | |||||
| Pignatelli et al. c (2011) [42] | 8-OHdG (ng/mL) | ctr | 3.7 ± 1.3 | 4.2 ± 1.1 | 4.6 ± 1.0 | |||
| VC | 3.7 ± 1.1 | 2.6 ± 1.1 | 2.7 ± 0.87 | |||||
| p | NS | <0.0001 | <0.0001 | |||||
| hs-crp (mg/L) | ctr | 1.25 (0.80–2.00) | 1.3 (0.75–2.0) | 1.25 (0.95–2.10) | ||||
| VC | 1.0 (0.71–1.90) | 1 (0.67–1.60) | 1.37 (1.0–2.0) | |||||
| p | 0.451 | NS | NS | |||||
| TNFα (pg/mL) | ctr | 40 (40.0–50.0) | 40 (40.0–50.0) | 46.5 (40.0–58.0) | ||||
| VC | 42.5 (35.0–50.0) | 45 (40.0–60.0) | 43.5 (36.5–58.5) | |||||
| p | 0.735 | NS | NS | |||||
| sCD40L (ng/mL) | ctr | 2.3 ± 1.2 | 3.2 ± 1.5 | 3.4 ± 1.7 | ||||
| VC | 2.3 ± 1.4 | 2.2 ± 1.1 | 2.4 ± 1.0 | |||||
| p | 0.95 | 0.0057 | 0.016 | |||||
| CD40L (MF) | ctr | 4.3 ± 0.78 | 5.1 ± 1.3 | 5.4 ± 1.2 | ||||
| VC | 4.2 ± 0.88 | 3.8 ± 1.3 | 3.8 ± 1.1 | |||||
| p | 0.0724 | 0.0008 | 0.0001 | |||||
| Basili et al. c (2010) [43] | 8-iso-PGF2a (pg/mL) | ctr | 126 (85.0 to 170.0) | 50 (20.7 to 102.5) | ||||
| VC | 142.5 (85.5 to 187.5) | 161.5 (117.5 to 190.0) | ||||||
| p | NS | <0.0001 | ||||||
| Gasparetto et al. (2005) [33] | ROM (U.CARR) | ctr | 295.4 ± 36.30 | 335.6 ± 35.8 | 377.3 ± 39.0 | |||
| VC | 308.6 ± 40.30 | 307.5 ± 47.1 | 369.1 ± 42.3 | |||||
| p | NS | <0.05 | <NS |
| Trial | Measure | Baseline | 0 h | 48 h | 1 Month | |
|---|---|---|---|---|---|---|
| Valls et al. (2016) [40] | TIMI (TMPG of 2–3) | ctr | 79% | |||
| VC | 95% | |||||
| p | <0.01 | |||||
| TIMI (TMPG of 0–1) | ctr | 21% | ||||
| VC | 5% | |||||
| p | <0.01 | |||||
| Basili et al. (2010) [43] | %△ cTFC | ctr | 40.2 | −23% | ||
| (frames/s) | VC | 36.1 | −41% | |||
| p | NS | <0.0001 | ||||
| TIMI (TMPG < 2) | ctr | 89% | 32% | |||
| VC | 86% | 4% | ||||
| p | NS | <0.01 | ||||
| TIMI (TMPG = 3) | ctr | 39% | ||||
| VC | 79% | |||||
| p | <0.01 | |||||
| Gasparetto et al. (2005) [33] | sVCAM-1 | ctr | 1.44 ± 0.7 | 2.03 ± 0.5 | 2.13 ± 0.8 | |
| (ug/mL) | VC | 1.53 ± 0.6 | 1.63 ± 0.7 | 1.86 ± 0.9 | ||
| p | NS | <0.01 | NS |
| Studies | Trial Enrollment (Ctr, VC) | Positive Outcomes | Negative Outcomes | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Measure | Outcome Types | Outcome Count | Enrollment | Measure | Outcome Types | Outcome Count | Enrollment | |||
| 1 | Shafaei et al. (2019) [39] | 252 (126,126) | Troponin, CK-MB | Myocardial injury | 1 | 252 | 0 | |||
| 2 | Ramos et al. (2017) [44] | 67 (41,26) | VC, FRAP, GSH | Antioxidants | 1 | 67 | CK-MB, LVEF, Infarct size | Myocardial injury, Cardiac contractility, Infarct size | 3 | 67 |
| 3 | Valls et al. (2016) [40] | 43 (21,22) | LVEF, VC, FRAP, GSH, 8-isoprotane, TIMI | Cardiac contractility Antioxidants ROS, Reperfusion | 4 | 43 | CK-MB, LVEF | Myocardial injury, Cardiac contractility | 2 | 43 |
| 4 | Wang et al. (2014) [41] | 532 (267,265) | Troponin, CK-MB, 8-OHdG | Myocardial injury, ROS | 2 | 532 | 0 | |||
| 5 | Basili et al. (2010–2011) a | 56 (28,28) | TxB2, sNOX2-dp, LVEF, 8-OHdG, sCD40L, 8-oxo-PGF2a, cTFC, TIMI | Inflammation, Cardiac contractility, ROS, ROS, Reperfusion | 6 | 56 | hs-crp, TNFa, Troponin, | Inflammation, Myocardial injury | 2 | 56 |
| 6 | Gasparetto et al. (2005) [33] | 98 (49,49) | TLVV, TAS, ROS, sVCAM-1 | Cardiac contractility, Antioxidant, ROS, Endothelial dysfunction | 4 | 98 | 0 | |||
| 7 | Guan et al. (1999) [29] | 21 (11,10) | 0 | 8-epi-PGF2a in urine | ROS | 1 | 21 | |||
| 8 | Tardif et al. (1997) [55] | 116 (62,54) | 0 | Coronary artery restenosis | Coronary artery restenosis | 1 | 116 | |||
| Total | 1185(605, 580) | 18 | 1048 | 9 | 303 | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, S.A.; Bhattacharjee, S.; Ghani, M.O.A.; Walden, R.; Chen, Q.M. Vitamin C for Cardiac Protection during Percutaneous Coronary Intervention: A Systematic Review of Randomized Controlled Trials. Nutrients 2020, 12, 2199. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12082199
Khan SA, Bhattacharjee S, Ghani MOA, Walden R, Chen QM. Vitamin C for Cardiac Protection during Percutaneous Coronary Intervention: A Systematic Review of Randomized Controlled Trials. Nutrients. 2020; 12(8):2199. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12082199
Chicago/Turabian StyleKhan, Sher Ali, Sandipan Bhattacharjee, Muhammad Owais Abdul Ghani, Rachel Walden, and Qin M. Chen. 2020. "Vitamin C for Cardiac Protection during Percutaneous Coronary Intervention: A Systematic Review of Randomized Controlled Trials" Nutrients 12, no. 8: 2199. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12082199