Triacylglycerides and Phospholipids from Egg Yolk Differently Influence the Immunostimulating Properties of Egg White Proteins
Abstract
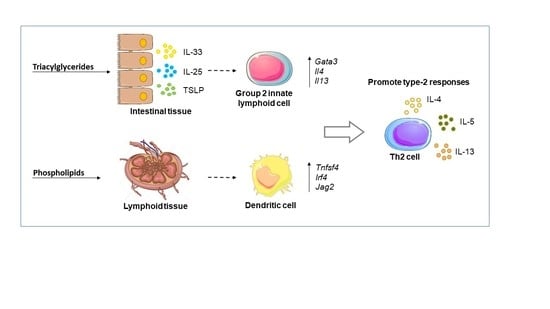
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Experiments in Mice
2.3. Isolation of Cells from Lamina Propria
2.4. Gene Expression
2.5. Flow Cytometry Analyses
2.6. Solubility Experiments
2.7. Statistical Analyses
3. Results
3.1. Egg Yolk Triacylglycerides Promote Type 2 Responses at the Small Intestine Level
3.2. Egg Yolk Phospholipids Induce Th2 Skewing in Lymphoid Intestinal Tissues
3.3. Egg Yolk Phospholipids Reduce the Solubility of Egg White Proteins in a Simulated Duodenal Medium
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nwaru, B.; Hickstein, L.; Panesar, S.S.; Roberts, G.; Muraro, A.; Sheikh, A. Prevalence of common food allergies in Europe: A systematic review and meta-analysis. Allergy 2014, 69, 992–1007. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Holdford, D.; Bilaver, L.; Dyer, A.; Holl, J.L.; Meltzer, D. The economic impact of childhood food allergy in the United States. J. Am. Med. Assoc. Pediatr. 2013, 167, 1026–1031. [Google Scholar] [CrossRef] [Green Version]
- DunnGalvin, A.; Dubois, A.; Blok, B.F.-D.; Hourihane, J. the effects of food allergy on quality of life. Chem. Immunol. Allergy 2015, 101, 235–252. [Google Scholar] [CrossRef]
- Martorell, A.; Alonso, E.; Boné, J.; Echeverría, L.; López, M.; Martín, F.; Nevot, S.; Plaza, A. Position document: IgE-mediated allergy to egg protein. Allergol. Immunopathol. 2013, 41, 320–336. [Google Scholar] [CrossRef] [PubMed]
- Benedé, S.; López-Expósito, I.; Molina, E.; López-Fandiño, R. Egg proteins as allergens and the effects of the food matrix and processing. Food Funct. 2014, 6, 694–713. [Google Scholar] [CrossRef] [PubMed]
- López-Fandiño, R. Role of dietary lipids in food allergy. Crit. Rev. Food Sci. Nutr. 2019, 60, 1797–1814. [Google Scholar] [CrossRef] [PubMed]
- Pablos-Tanarro, A.; Lozano-Ojalvo, D.; Martínez-Blanco, M.; Molina, E.; López-Fandiño, R. Egg yolk provides Th2 adjuvant stimuli and promotes sensitization to egg white allergens in BALB/c mice. Mol. Nutr. Food Res. 2018, 62, 1800057. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rodríguez, L.; Martínez-Blanco, M.; Lozano-Ojalvo, D.; Molina, E.; López-Fandiño, R. Egg yolk augments type 2 immunity by activating innate cells. Eur. J. Nutr. 2020, 59, 3245–3256. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-H.; Angkasekwinai, P.; Lu, N.; Voo, K.S.; Arima, K.; Hanabuchi, S.; Hippe, A.; Corrigan, C.; Dong, C.; Homey, B.; et al. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC–activated Th2 memory cells. J. Exp. Med. 2007, 204, 1837–1847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Wang, Y.; Tang, L.; de Villiers, W.J.; Cohen, D.; Woodward, J.; Finkelman, F.D.; Eckhardt, E.R. Dietary medium-chain triglycerides promote oral allergic sensitization and orally induced anaphylaxis to peanut protein in mice. J. Allergy Clin. Immunol. 2013, 131, 442–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, J.W.; Tjota, M.Y.; Clay, B.S.; Lugt, B.V.; Bandukwala, H.S.; Hrusch, C.L.; Decker, D.C.; Blaine, K.M.; Fixsen, B.R.; Singh, H.; et al. Transcription factor IRF4 drives dendritic cells to promote Th2 differentiation. Nat. Commun. 2013, 4, 2990. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, T.F.; Bao, K.; Dell’Aringa, M.; Ang, W.X.G.; Abraham, S.; Reinhardt, R.L. Cytokine expression by invariant natural killer T cells is tightly regulated throughout development and settings of type-2 inflammation. Mucosal Immunol. 2016, 9, 597–609. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Tian, Y.; Yan, R.; Wang, C.; Niu, F.; Yang, Y. Study on a novel process for the separation of phospholipids, triacylglycerol and cholesterol from egg yolk. J. Food Sci. Technol. 2014, 52, 4586–4592. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Alcalá, L.M.; Fontecha, J. Major lipid classes separation of buttermilk, and cows, goats and ewes milk by high performance liquid chromatography with an evaporative light scattering detector focused on the phospholipid fraction. J. Chromatogr. A 2010, 1217, 3063–3066. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Saiz, R.; Martos, G.; Carrillo, W.; López-Fandiño, R.; Molina, E. Susceptibility of lysozyme to in-vitro digestion and immunoreactivity of its digests. Food Chem. 2011, 127, 1719–1726. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Mumm, J.B.; Herbst, R.; Kolbeck, R.; Wang, Y. IL-22 Increases permeability of intestinal epithelial tight junctions by enhancing claudin-2 expression. J. Immunol. 2017, 199, 3316–3325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johansson, M.E.V.; Larsson, J.M.H.; Hansson, G.C. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc. Natl. Acad. Sci. USA 2011, 108, 4659–4665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.J.; Wang, H.; Starrett, G.J.; Phuong, V.; Jameson, S.C.; Hogquist, K.A. Tissue-specific distribution of iNKT cells impacts their cytokine response. Immunity 2015, 43, 566–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, D.; Llop-Guevara, A.; Walker, T.D.; Flader, K.; Goncharova, S.; Boudreau, J.E.; Moore, C.L.; In, T.S.; Waserman, S.; Coyle, A.J.; et al. IL-33, but not thymic stromal lymphopoietin or IL-25, is central to mite and peanut allergic sensitization. J. Allergy Clin. Immunol. 2013, 131, 187–200.e8. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Wang, Y.H.; Duramad, O.; Hori, T.; Delespesse, G.J.; Watanabe, N.; Qin, F.X.; Yao, Z.; Cao, W.; Liu, Y.J. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J. Exp. Med. 2005, 202, 1213–1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, G.-N.; Jiang, D.-S.; Li, H. Interferon regulatory factors: At the crossroads of immunity, metabolism, and disease. Biochim. Biophys. Acta (BBA) 2015, 1852, 365–378. [Google Scholar] [CrossRef] [Green Version]
- Amsen, D.; Helbig, C.; Backer, R.A. Notch in T Cell Differentiation: All Things Considered. Trends Immunol. 2015, 36, 802–814. [Google Scholar] [CrossRef]
- Weaver, C.T.; Hatton, R.; Mangan, P.R.; Harrington, L.E. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu. Rev. Immunol. 2007, 25, 821–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stier, M.T.; Peebles, R.S. Innate lymphoid cells and allergic disease. Ann. Allergy Asthma Immunol. 2017, 119, 480–488. [Google Scholar] [CrossRef] [Green Version]
- Yanagawa, Y.; Suzuki, M.; Matsumoto, M.; Togashi, H. Prostaglandin E2 enhances IL-33 production by dendritic cells. Immunol. Lett. 2011, 141, 55–60. [Google Scholar] [CrossRef]
- Griesenauer, B.; Paczesny, S. The ST2/IL-33 axis in immune cells during inflammatory diseases. Front. Immunol. 2017, 8, 475. [Google Scholar] [CrossRef] [PubMed]
- Kostewicz, E.S.; Brauns, U.; Becker, R.; Dressman, J.B. Forecasting the oral absorption behavior of poorly soluble weak bases using solubility and dissolution studies in biorelevant media. Pharm. Res. 2002, 19, 345–349. [Google Scholar] [CrossRef]
- Burnett, G.R.; Wickham, M.; Fillery-Travis, A.; Robertson, J.A.; Belton, P.S.; Gilbert, S.M.; Tatham, A.; Shewry, P.R.; Mills, E.N.C. Interaction between protein allergens and model gastric emulsions. Biochem. Soc. Trans. 2002, 30, 916–918. [Google Scholar] [CrossRef] [Green Version]
- Martos, G.; López-Fandiño, R.; Molina, E. Immunoreactivity of hen egg allergens: Influence on in vitro gastrointestinal digestion of the presence of other egg white proteins and of egg yolk. Food Chem. 2013, 136, 775–781. [Google Scholar] [CrossRef]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in Gut Microbiota Control Metabolic Endotoxemia-Induced Inflammation in High-Fat Diet-Induced Obesity and Diabetes in Mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef] [Green Version]
- Price, D.B.; Ackland, M.L.; Burks, W.; Knight, M.; Suphioglu, C. Peanut allergens alter intestinal barrier permeability and tight junction localisation in Caco-2 Cell cultures1. Cell. Physiol. Biochem. 2014, 33, 1758–1777. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Ojalvo, D.; Martínez-Blanco, M.; Pérez-Rodríguez, L.; Molina, E.; Peláez, C.; Requena, T.; López-Fandiño, R. Egg white peptide-based immunotherapy enhances vitamin A metabolism and induces RORγt+ regulatory T cells. J. Funct. Foods 2019, 52, 204–211. [Google Scholar] [CrossRef]
- Blázquez, A.B.; Berin, M.C. Gastrointestinal Dendritic Cells Promote Th2 Skewing via OX40L. J. Immunol. 2008, 180, 4441–4450. [Google Scholar] [CrossRef] [PubMed]
- Roth-Walter, F.; Berin, M.C.; Arnaboldi, P.; Escalante, C.R.; Dahan, S.; Rauch, J.; Jensen-Jarolim, E.; Mayer, L. Pasteurization of milk proteins promotes allergic sensitization by enhancing uptake through Peyer’s patches. Allergy 2008, 63, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Hussain, M.M. Intestinal lipid absorption. Am. J. Physiol. Metab. 2009, 296, E1183–E1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohn, J.S.; Kamili, A.; Wat, E.; Chung, R.W.S.; Tandy, S. Dietary phospholipids and intestinal cholesterol absorption. Nutrition 2010, 2, 116–127. [Google Scholar] [CrossRef] [Green Version]
- Kuang, H.; Yang, F.; Zhang, Y.; Wang, T.; Chen, G. The impact of egg nutrient composition and its consumption on cholesterol homeostasis. Cholesterol 2018, 2018, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Iwamura, C.; Nakayama, T. Role of NKT cells in allergic asthma. Curr. Opin. Immunol. 2010, 22, 807–813. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Rodríguez, L.; Martínez-Blanco, M.; Lozano-Ojalvo, D.; Fontecha, J.; Molina, E.; Benedé, S.; López-Fandiño, R. Triacylglycerides and Phospholipids from Egg Yolk Differently Influence the Immunostimulating Properties of Egg White Proteins. Nutrients 2021, 13, 3301. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13103301
Pérez-Rodríguez L, Martínez-Blanco M, Lozano-Ojalvo D, Fontecha J, Molina E, Benedé S, López-Fandiño R. Triacylglycerides and Phospholipids from Egg Yolk Differently Influence the Immunostimulating Properties of Egg White Proteins. Nutrients. 2021; 13(10):3301. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13103301
Chicago/Turabian StylePérez-Rodríguez, Leticia, Mónica Martínez-Blanco, Daniel Lozano-Ojalvo, Javier Fontecha, Elena Molina, Sara Benedé, and Rosina López-Fandiño. 2021. "Triacylglycerides and Phospholipids from Egg Yolk Differently Influence the Immunostimulating Properties of Egg White Proteins" Nutrients 13, no. 10: 3301. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13103301