Nutritional Iodine Status in Pregnant Women from Health Area IV in Asturias (Spain): Iodised Salt Is Enough
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Iodine Consumption Questionnaire
- Habitual consumption of iodised salt (yes/no/not known).
- Consumption of dairy products: number of glasses of milk (1 glass = 200 mL), yoghurts consumed per day and daily consumption of cheese (yes/no). One serving of dairy product was taken as a glass of milk, 2 yoghurts or 80 g of soft or 40 g of hard cheese.
- Consumption of iodine supplements (yes/no) and date when iodine supplementation began.
2.3. Urinary Iodine Concentration and Thyroid Function
2.4. Statistical Analysis
3. Results
3.1. Iodine Consumption Questionnaire
3.2. Urinary Iodine Concentration
3.3. Thyroid Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zimmermann, M.B. The Role of Iodine in Human Growth and Development. Semin. Cell Dev. Biol. 2011, 22, 645–652. [Google Scholar] [CrossRef]
- De Escobar, G.M.; Obregón, M.J.; del Rey, F.E. Maternal Thyroid Hormones Early in Pregnancy and Fetal Brain Development. Best Pract. Res. Clin. Endocrinol. Metab. 2004, 18, 225–248. [Google Scholar] [CrossRef]
- Pharoah, P.O.D.; Buttfield, I.H.; Hetzel, B.S. Neurological damage to the fetus resulting from severe iodine deficiency during pregnancy. Lancet 1971, 297, 308–310. [Google Scholar] [CrossRef]
- Delong, G.; Robbins, J.C. Iodine and the Brain; Plenum Press: New York, NY, USA, 1989. [Google Scholar]
- De Escobar, G.M.; Obregón, M.J.; del Rey, F.E. Iodine Deficiency and Brain Development in the First Half of Pregnancy. Public Health Nutr. 2007, 10, 1554–1570. [Google Scholar] [CrossRef] [Green Version]
- Bath, S.C.; Steer, C.D.; Golding, J.; Emmett, P.; Rayman, M.P. Effect of Inadequate Iodine Status in UK Pregnant Women on Cognitive Outcomes in Their Children: Results from the Avon Longitudinal Study of Parents and Children (ALSPAC). Lancet 2013, 382, 331–337. [Google Scholar] [CrossRef]
- Murcia, M.; Espada, M.; Julvez, J.; Llop, S.; Lopez-Espinosa, M.-J.; Vioque, J.; Basterrechea, M.; Riaño, I.; González, L.; Alvarez-Pedrerol, M.; et al. Iodine Intake from Supplements and Diet during Pregnancy and Child Cognitive and Motor Development: The INMA Mother and Child Cohort Study. J. Epidemiol. Commun. Health 2018, 72, 216–222. [Google Scholar] [CrossRef]
- Hynes, K.L.; Otahal, P.; Hay, I.; Burgess, J.R. Mild Iodine Deficiency During Pregnancy Is Associated with Reduced Educational Outcomes in the Offspring: 9-Year Follow-up of the Gestational Iodine Cohort. J. Clin. Endocrinol. Metab. 2013, 98, 1954–1962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markhus, M.; Dahl, L.; Moe, V.; Abel, M.; Brantsæter, A.; Øyen, J.; Meltzer, H.; Stormark, K.; Graff, I.; Smith, L.; et al. Maternal Iodine Status Is Associated with Offspring Language Skills in Infancy and Toddlerhood. Nutrients 2018, 10, 1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Benoist, B.; McLean, E.; Andersson, M.; Rogers, L. Iodine Deficiency in 2007: Global Progress since 2003. Food Nutr. Bull. 2008, 29, 195–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization (WHO); United Nations Children’s Fund (UNICEF); International Council for Control of Iodine Deficiency Disorders (ICCIDD). Indicators for Assessing Iodine Deficiency Disorders and Their Control through Salt Iodization. Available online: https://apps.who.int/iris/handle/10665/70715 (accessed on 15 December 2020).
- Zimmermann, M.B.; Jooste, P.L.; Pandav, C.S. Iodine-Deficiency Disorders. Lancet 2008, 372, 1251–1262. [Google Scholar] [CrossRef]
- Pearce, E.N.; Caldwell, K.L. Urinary Iodine, Thyroid Function, and Thyroglobulin as Biomarkers of Iodine Status. Am. J. Clin. Nutr. 2016, 104, 898S–901S. [Google Scholar] [CrossRef] [Green Version]
- Glinoer, D.; Nayer, P.D.; Bourdoux, P.; Lemone, M.; Robyn, C.; Steirteghem, A.V.; Kinthaert, J.; Lejeune, B. Regulation of Maternal Thyroid during Pregnancy*. J. Clin. Endocrinol. Metab. 1990, 71, 276–287. [Google Scholar] [CrossRef]
- Glinoer, D. The Regulation of Thyroid Function in Pregnancy: Pathways of Endocrine Adaptation from Physiology to Pathology. Endocr. Rev. 1997, 18, 404–433. [Google Scholar] [CrossRef]
- Trumpff, C.; De Schepper, J.; Tafforeau, J.; Van Oyen, H.; Vanderfaeillie, J.; Vandevijvere, S. Mild Iodine Deficiency in Pregnancy in Europe and Its Consequences for Cognitive and Psychomotor Development of Children: A Review. J. Trace Elem. Med. Biol. 2013, 27, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.; De Benoist, B.; Delange, F. Iodine Deficiency in Europe: A Continuing Public Health Problem. Available online: www.who.int/nutrition/publications/VMNIS_Iodine_deficiency_in_Europe.pdf (accessed on 20 December 2020).
- De Groot, L.; Abalovich, M.; Alexander, E.K.; Amino, N.; Barbour, L.; Cobin, R.H.; Eastman, C.J.; Lazarus, J.H.; Luton, D.; Mandel, S.J.; et al. Management of Thyroid Dysfunction during Pregnancy and Postpartum: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2012, 97, 2543–2565. [Google Scholar] [CrossRef] [Green Version]
- Alexander, E.K.; Pearce, E.N.; Brent, G.A.; Brown, R.S.; Chen, H.; Dosiou, C.; Grobman, W.A.; Laurberg, P.; Lazarus, J.H.; Mandel, S.J.; et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid 2017, 27, 315–389. [Google Scholar] [CrossRef] [Green Version]
- Donnay, S.; Arena, J.; Lucas, A.; Velasco, I.; Ares, S. Iodine supplementation during pregnancy and lactation. Position statement of the working group on disorders related to iodine deficiency and thyroid dysfunction of the Spanish Society of Endocrinology and Nutrition. Endocrinol. Nutr. 2014, 61, 27–34. [Google Scholar] [CrossRef]
- WHO Secretariat; Andersson, M.; de Benoist, B.; Delange, F.; Zupan, J. Prevention and Control of Iodine Deficiency in Pregnant and Lactating Women and in Children Less than 2-Years-Old: Conclusions and Recommendations of the Technical Consultation. Public Health Nutr. 2007, 10, 1606–1611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The IODEGEST Study Group; Torres, M.T.; Francés, L.; Vila, L.; Manresa, J.M.; Falguera, G.; Prieto, G.; Casamitjana, R.; Toran, P. Iodine Nutritional Status of Women in Their First Trimester of Pregnancy in Catalonia. BMC Pregnancy Childbirth 2017, 17, 249. [Google Scholar] [CrossRef] [Green Version]
- Ollero, M.D.; Martínez, J.P.; Pineda, J.; Toni, M.; Espada, M.; Anda, E. Change over time in the iodine nutritional status of pregnant women from the Pamplona healthcare region. Endocrinol. Diabetes Nutr. 2020, 67, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Murillo-Llorente, M.T.; Fajardo-Montañana, C.; Pérez-Bermejo, M. Reference Values of Thyroid Hormones during the First Trimester of Pregnancy in Valencian Community (Spain) and Their Relationship with Iodine Intake. Nutrients 2020, 12, 1433. [Google Scholar] [CrossRef] [PubMed]
- Suplementación con Yodo y Ácido Fólico Durante el Embarazo y la Lactancia. Resumen y Recomendaciones del Taller Llevado a Cabo en Bilbao el 30 de Octubre de 2012. Available online: https://www.osakidetza.euskadi.eus/contenidos/informacion/publicaciones_informes_estudio/es_pub/adjuntos/Taller_yodo_embarazo_lactancia.pdf (accessed on 20 December 2020).
- Delgado, E.; Díaz-Cadórniga, F.J.; Tartón, T.; Bobis, M.L.; Valdés, M.M.; Méndez, A. Eradication of Iodine Deficiency Disorders in Asturias (Spain): 18 Years of Iodized Salt Prophylaxis. Endocrinol. Nutr. 2004, 51, 492–496. [Google Scholar] [CrossRef]
- Riestra, M. Situación Actual de la Nutrición de Yodo en Asturias Tras 28 Años de Yodoprofilaxis con Sal. Ph.D. Thesis, Universidad de Oviedo, Oviedo, Spain, 2017. [Google Scholar]
- Menéndez Torre, E.; Delgado Alvarez, E.; Rabal Artal, A.; Suárez Gutiérrez, L.; Rodríguez Caballero, M.G.; Ares Blanco, J.; Díaz Naya, L.; Fernández Fernández, J.C. Iodine nutrition in pregnant women from Oviedo area. Is iodine supplementation necessary? Endocrinol. Nutr. 2014, 61, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Escudero Gomis, A.I.; Eyaralar Riera, B.; Mosquera Tenreiro, C.; Menéndez Torre, E.L.; Arbesú Fernández, E.; Riaño Galán, I.; García González, M.C.; Caicoya Gómez-Morán, M. Recomendaciones Acerca de la Nutrición Con Yodo en la Etapa Preconcepcional, el Embarazo y la Lactancia. Available online: https://www.astursalud.es/documents/31867/228148/In-forme+nutrici%C3%B3n+con+yodo+en+embarazo+090115.pdf/7f57a964-8b37-6990-a2fa-0282065d11c7 (accessed on 20 December 2020).
- Travers, C.A.; Guttikonda, K.; Norton, C.A.; Lewis, P.R.; Mollart, L.J.; Wiley, V.; Wilcken, B.; Eastman, C.J.; Boyages, S.C. Iodine Status in Pregnant Women and Their Newborns: Are Our Babies at Risk of Iodine Deficiency? Med. J. Aust. 2006, 184, 617–620. [Google Scholar] [CrossRef] [PubMed]
- Bath, S.C.; Rayman, M.P. Iodine Deficiency in the UK: An Overlooked Cause of Impaired Neurodevelopment? Proc. Nutr. Soc. 2013, 72, 226–235. [Google Scholar] [CrossRef] [Green Version]
- Panth, P.; Guerin, G.; DiMarco, N.M. A Review of Iodine Status of Women of Reproductive Age in the USA. Biol. Trace Elem. Res. 2019, 188, 208–220. [Google Scholar] [CrossRef] [Green Version]
- Riestra Fernández, M.; Menéndez Torre, E.; Díaz Cadórniga, F.; Fernández Fernández, J.C.; Delgado Álvarez, E. Iodine nutritional status in Asturian schoolchildren. Endocrinol. Diabetes Nutr. 2017, 64, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Tamarit Encarnación, A.; Vallada Regalado, E.; Clérigues Bonet, V.; Olaso González, G.; Moreno Gálvez, A.; Gandía Balaguer, A. Frequency of the consumption of iodized salt in valencian children of school age studied. Nutr. Clin. Diet. Hosp. 2017, 65–68. [Google Scholar] [CrossRef]
- Donnay, S.; Fajardo, C.; Fernández-García, J.C.; Torres, T.; Bandrés, O.; Domínguez, J.R.; Menéndez, E.; Serrano, J.; Torrejón, S.; López, I.; et al. Diagnosis, Treatment, and Management of Gestational Hypothyroidism. The TIROGEST Study. Endocrinol. Diabetes Nutr. 2020, 67, 36–42. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Assessment of Iodine Deficiency Disorders and Monitoring Their Elimination: A Guide for Programme Managers. Available online: https://apps.who.int/iris/bitstream/handle/10665/43781/9789241595827_eng.pdf?sequence=1 (accessed on 1 December 2020).
- Lee, S.M.; Lewis, J.; Buss, D.H.; Holcombe, G.D.; Lawrance, P.R. Iodine in British Foods and Diets. Br. J. Nutr. 1994, 72, 435–446. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, L.B.; Ovesen, L.; Bülow, I.; Jørgensen, T.; Knudsen, N.; Laurberg, P.; Perrild, H. Dietary Iodine Intake and Urinary Iodine Excretion in a Danish Population: Effect of Geography, Supplements and Food Choice. Br. J. Nutr. 2002, 87, 61–69. [Google Scholar] [CrossRef]
- Girelli, M.E.; Coin, P.; Mian, C.; Nacamulli, D.; Zambonin, L.; Piccolo, M.; Vianello-Dri, A.; Gottardo, F.; Busnardo, B. Milk Represents an Important Source of Iodine in Schoolchildren of the Veneto Region, Italy. J. Endocrinol. Investig. 2004, 27, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.I. Iodine, Milk, and the Elimination of Endemic Goitre in Britain: The Story of an Accidental Public Health Triumph. J. Epidemiol. Commun. Health 1997, 51, 391–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donnay, S.; Vila, L. Task force on disorders related to iodine deficiency and thyroid dysfunction. Eradication of iodine deficiency in Spain. Close, but not there yet. Endocrinol. Nutr. 2012, 59, 471–473. [Google Scholar] [CrossRef]
- Bath, S.C.; Walter, A.; Taylor, A.; Wright, J.; Rayman, M.P. Iodine Deficiency in Pregnant Women Living in the South East of the UK: The Influence of Diet and Nutritional Supplements on Iodine Status. Br. J. Nutr. 2014, 111, 1622–1631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arrizabalaga, J.J.; Jalón, M.; Espada, M.; Cañas, M.; Latorre, P.M. Iodine concentration in ultra-high temperature pasteurized cow’s milk. Applications in clinical practice and in community nutrition. Med. Clin. 2015, 145, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Lago-Sampedro, A.; García-Escobar, E.; Rubio-Martín, E.; Pascual-Aguirre, N.; Valdés, S.; Soriguer, F.; Goday, A.; Calle-Pascual, A.; Castell, C.; Menéndez, E.; et al. Dairy Product Consumption and Metabolic Diseases in the [email protected] Study. Nutrients 2019, 11, 262. [Google Scholar] [CrossRef] [Green Version]
- Adalsteinsdottir, S.; Tryggvadottir, E.A.; Hrolfsdottir, L.; Halldorsson, T.I.; Birgisdottir, B.E.; Hreidarsdottir, I.T.; Hardardottir, H.; Arohonka, P.; Erlund, I.; Gunnarsdottir, I. Insufficient Iodine Status in Pregnant Women as a Consequence of Dietary Changes. Food Nutr. Res. 2020, 64. [Google Scholar] [CrossRef] [Green Version]
- McMullan, P.; Hamill, L.; Doolan, K.; Hunter, A.; McCance, D.; Patterson, C.; Smyth, P.; Woodside, J.V.; Mullan, K. Iodine Deficiency among Pregnant Women Living in Northern Ireland. Clin. Endocrinol. 2019, 91, 639–645. [Google Scholar] [CrossRef]
- Censi, S.; Watutantrige-Fernando, S.; Groccia, G.; Manso, J.; Plebani, M.; Faggian, D.; Mion, M.M.; Venturini, R.; Andrisani, A.; Casaro, A.; et al. The Effects of Iodine Supplementation in Pregnancy on Iodine Status, Thyroglobulin Levels and Thyroid Function Parameters: Results from a Randomized Controlled Clinical Trial in a Mild-to-Moderate Iodine Deficiency Area. Nutrients 2019, 11, 2639. [Google Scholar] [CrossRef] [Green Version]
- Castilla, A.M.; Murcia, M.; Arrizabalaga, J.J.; Espada, M.; Julvez, J.; Basterrechea, M.; Alvarez-Pedrerol, M.; Estarlich, M.; Moreno, E.; Guxens, M.; et al. Comparison of Urinary Iodine Levels in Women of Childbearing Age during and after Pregnancy. Eur. J. Nutr. 2018, 57, 1807–1816. [Google Scholar] [CrossRef]
- Korevaar, T.I.M.; Schalekamp-Timmermans, S.; de Rijke, Y.B.; Visser, W.E.; Visser, W.; de Muinck Keizer-Schrama, S.M.P.F.; Hofman, A.; Ross, H.A.; Hooijkaas, H.; Tiemeier, H.; et al. Hypothyroxinemia and TPO-Antibody Positivity Are Risk Factors for Premature Delivery: The Generation R Study. J. Clin. Endocrinol. Metab. 2013, 98, 4382–4390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sitoris, G.; Veltri, F.; Kleynen, P.; Cogan, A.; Belhomme, J.; Rozenberg, S.; Pepersack, T.; Poppe, K. The Impact of Thyroid Disorders on Clinical Pregnancy Outcomes in a Real-World Study Setting. Thyroid 2020, 30, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Haddow, J.E.; Palomaki, G.E.; Allan, W.C.; Williams, J.R.; Knight, G.J.; Gagnon, J.; O’Heir, C.E.; Mitchell, M.L.; Hermos, R.J.; Waisbren, S.E.; et al. Maternal Thyroid Deficiency during Pregnancy and Subsequent Neuropsychological Development of the Child. N. Engl. J. Med. 1999, 341, 549–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansen, T.A.; Korevaar, T.I.M.; Mulder, T.A.; White, T.; Muetzel, R.L.; Peeters, R.P.; Tiemeier, H. Maternal Thyroid Function during Pregnancy and Child Brain Morphology: A Time Window-Specific Analysis of a Prospective Cohort. Lancet Diabetes Endocrinol. 2019, 7, 629–637. [Google Scholar] [CrossRef]
- Vila, L.; Velasco, I.; González, S.; Morales, F.; Sánchez, E.; Lailla, J.M.; Martinez-Astorquiza, T.; Puig-Domingo, M. Detection of Thyroid Dysfunction in Pregnant Women: Universal Screening Is Justified. Endocrinol. Nutr. 2012, 59, 547–560. [Google Scholar] [CrossRef]
- Ortega Carpio, A.; Vázquez Rico, I.; Castaño López, M.A.; Duarte González, L.; Montilla Álvaro, M.; Ruiz Reina, A. Thyrotropin reference ranges during pregnancy in the province of Huelva, Spain. Semergen 2018, 44, 372–379. [Google Scholar] [CrossRef]
- Arrobas Velilla, T.; González Rodríguez, C.; Barco Sánchez, A.; Castaño López, M.; Perea-Carrasco, R.; Pascual Salvador, E.; Limón Padilla, J. Deficiencia Nutricional de Yodo En Gestantes Pertenecientes al Distrito Sanitario Sierra de Huelva-Andévalo, Sur de España. Rev. Investig. Clin. 2011, 63, 467–474. [Google Scholar]
- Glinoer, D. Maternal Thyroid Function in Pregnancy. J. Endocrinol. Investig. 1993, 16, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.M.; Laurberg, P.; Iversen, E.; Knudsen, P.R.; Gregersen, H.E.; Rasmussen, O.S.; Larsen, K.R.; Eriksen, G.M.; Johannesen, P.L. Amelioration of Some Pregnancy-Associated Variations in Thyroid Function by Iodine Supplementation. J. Clin. Endocrinol. Metab. 1993, 77, 1078–1083. [Google Scholar] [CrossRef]
- Glinoer, D.; De Nayer, P.; Delange, F.; Lemone, M.; Toppet, V.; Spehl, M.; Grün, J.P.; Kinthaert, J.; Lejeune, B. A Randomized Trial for the Treatment of Mild Iodine Deficiency during Pregnancy: Maternal and Neonatal Effects. J. Clin. Endocrinol. Metab. 1995, 80, 258–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nøhr, S.B.; Jørgensen, A.; Pedersen, K.M.; Laurberg, P. Postpartum Thyroid Dysfunction in Pregnant Thyroid Peroxidase Antibody-Positive Women Living in an Area with Mild to Moderate Iodine Deficiency: Is Iodine Supplementation Safe? J. Clin. Endocrinol. Metab. 2000, 85, 3191–3198. [Google Scholar] [CrossRef] [PubMed]
- Censi, S.; Manso, J.; Barollo, S.; Mondin, A.; Bertazza, L.; De Marchi, M.; Mian, C. Food and Nutrition Hygiene Services (SIAN), on behalf ofthe Changing Dietary Habits in Veneto Region over Two Decades: Still a Long Road to Go to Reach an Iodine-Sufficient Status. Nutrients 2020, 12, 2399. [Google Scholar] [CrossRef]
- Romano, R.; Jannini, E.A.; Pepe, M.; Grimaldi, A.; Olivieri, M.; Spennati, P.; Cappa, F.; D’Armiento, M. The Effects of Iodoprophylaxis on Thyroid Size during Pregnancy. Am. J. Obstet. Gynecol. 1991, 164, 482–485. [Google Scholar] [CrossRef]
- Antonangeli, L.; Maccherini, D.; Cavaliere, R.; Di Giulio, C.; Reinhardt, B.; Pinchera, A.; Aghini-Lombardi, F. Comparison of Two Different Doses of Iodide in the Prevention of Gestational Goiter in Marginal Iodine Deficiency: A Longitudinal Study. Eur. J. Endocrinol. 2002, 147, 29–34. [Google Scholar] [CrossRef] [Green Version]
- Liesenkötter, K.P.; Göpel, W.; Bogner, U.; Stach, B.; Grüters, A. Earliest Prevention of Endemic Goiter by Iodine Supplementation during Pregnancy. Eur. J. Endocrinol. 1996, 134, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Santiago, P.; Velasco, I.; Muela, J.A.; Sánchez, B.; Martínez, J.; Rodriguez, A.; Berrio, M.; Gutierrez-Repiso, C.; Carreira, M.; Moreno, A.; et al. Infant Neurocognitive Development Is Independent of the Use of Iodised Salt or Iodine Supplements given during Pregnancy. Br. J. Nutr. 2013, 110, 831–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murcia, M.; Rebagliato, M.; Iniguez, C.; Lopez-Espinosa, M.-J.; Estarlich, M.; Plaza, B.; Barona-Vilar, C.; Espada, M.; Vioque, J.; Ballester, F. Effect of Iodine Supplementation During Pregnancy on Infant Neurodevelopment at 1 Year of Age. Am. J. Epidemiol. 2011, 173, 804–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verhagen, N.J.E.; Gowachirapant, S.; Winichagoon, P.; Andersson, M.; Melse-Boonstra, A.; Zimmermann, M.B. Iodine Supplementation in Mildly Iodine-Deficient Pregnant Women Does Not Improve Maternal Thyroid Function or Child Development: A Secondary Analysis of a Randomized Controlled Trial. Front. Endocrinol. 2020, 11, 572984. [Google Scholar] [CrossRef]
- Velasco, I.; Carreira, M.; Santiago, P.; Muela, J.A.; García-Fuentes, E.; Sánchez-Muñoz, B.; Garriga, M.J.; González-Fernández, M.C.; Rodríguez, A.; Caballero, F.F.; et al. Effect of Iodine Prophylaxis during Pregnancy on Neurocognitive Development of Children during the First Two Years of Life. J. Clin. Endocrinol. Metab. 2009, 94, 3234–3241. [Google Scholar] [CrossRef] [Green Version]
- Berbel, P.; Mestre, J.L.; Santamaría, A.; Palazón, I.; Franco, A.; Graells, M.; González-Torga, A.; de Escobar, G.M. Delayed Neurobehavioral Development in Children Born to Pregnant Women with Mild Hypothyroxinemia During the First Month of Gestation: The Importance of Early Iodine Supplementation. Thyroid 2009, 19, 511–519. [Google Scholar] [CrossRef]
- Taylor, P.N.; Okosieme, O.E.; Dayan, C.M.; Lazarus, J.H. Therapy of Endocrine Disease: Impact of Iodine Supplementation in Mild-to-Moderate Iodine Deficiency: Systematic Review and Meta-Analysis. Eur. J. Endocrinol. 2014, 170, R1–R15. [Google Scholar] [CrossRef] [Green Version]
- Rebagliato, M.; Murcia, M.; Alvarez-Pedrerol, M.; Espada, M.; Fernandez-Somoano, A.; Lertxundi, N.; Navarrete-Munoz, E.-M.; Forns, J.; Aranbarri, A.; Llop, S.; et al. Iodine Supplementation during Pregnancy and Infant Neuropsychological Development: INMA Mother and Child Cohort Study. Am. J. Epidemiol. 2013, 177, 944–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gowachirapant, S.; Jaiswal, N.; Melse-Boonstra, A.; Galetti, V.; Stinca, S.; Mackenzie, I.; Thomas, S.; Thomas, T.; Winichagoon, P.; Srinivasan, K.; et al. Effect of Iodine Supplementation in Pregnant Women on Child Neurodevelopment: A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Diabetes Endocrinol. 2017, 5, 853–863. [Google Scholar] [CrossRef]
- Nazeri, P.; Shariat, M.; Azizi, F. Effects of Iodine Supplementation during Pregnancy on Pregnant Women and Their Offspring: A Systematic Review and Meta-Analysis of Trials over the Past 3 Decades. Eur. J. Endocrinol. 2021, 184, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Abel, M.; Ystrom, E.; Caspersen, I.; Meltzer, H.; Aase, H.; Torheim, L.; Askeland, R.; Reichborn-Kjennerud, T.; Brantsæter, A. Maternal Iodine Intake and Offspring Attention-Deficit/Hyperactivity Disorder: Results from a Large Prospective Cohort Study. Nutrients 2017, 9, 1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moleti, M.; Di Bella, B.; Giorgianni, G.; Mancuso, A.; De Vivo, A.; Alibrandi, A.; Trimarchi, F.; Vermiglio, F. Maternal Thyroid Function in Different Conditions of Iodine Nutrition in Pregnant Women Exposed to Mild-Moderate Iodine Deficiency: An Observational Study: Iodine Nutrition in Pregnancy. Clin. Endocrinol. 2011, 74, 762–768. [Google Scholar] [CrossRef]
- Zhou, S.J.; Anderson, A.J.; Gibson, R.A.; Makrides, M. Effect of Iodine Supplementation in Pregnancy on Child Development and Other Clinical Outcomes: A Systematic Review of Randomized Controlled Trials. Am. J. Clin. Nutr. 2013, 98, 1241–1254. [Google Scholar] [CrossRef]
- De-Regil, L.M.; Harding, K.B.; Peña-Rosas, J.P.; Webster, A.C. Iodine supplementation for women during the preconception, pregnancy and postpartum period. In Cochrane Database System Reviews; The Cochrane Collaboration, Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2015; p. CD011761. [Google Scholar]
- Dineva, M.; Fishpool, H.; Rayman, M.P.; Mendis, J.; Bath, S.C. Systematic Review and Meta-Analysis of the Effects of Iodine Supplementation on Thyroid Function and Child Neurodevelopment in Mildly-to-Moderately Iodine-Deficient Pregnant Women. Am. J. Clin. Nutr. 2020, 112, 389–412. [Google Scholar] [CrossRef] [PubMed]
- Abel, M.H.; Brandlistuen, R.E.; Caspersen, I.H.; Aase, H.; Torheim, L.E.; Meltzer, H.M.; Brantsaeter, A.L. Language Delay and Poorer School Performance in Children of Mothers with Inadequate Iodine Intake in Pregnancy: Results from Follow-up at 8 Years in the Norwegian Mother and Child Cohort Study. Eur. J. Nutr. 2019, 58, 3047–3058. [Google Scholar] [CrossRef] [Green Version]
- Payling, L.M.; Juniper, D.T.; Drake, C.; Rymer, C.; Givens, D.I. Effect of Milk Type and Processing on Iodine Concentration of Organic and Conventional Winter Milk at Retail: Implications for Nutrition. Food Chem. 2015, 178, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Arrizabalaga, J.J.; Jalón, M.; Espada, M.; Cañas, M.; Latorre, P.M. Iodine contents in conventional ultra-high temperature (UHT) processed cow milk: Changes over the year and regional differences. Implications for epidemiological studies on iodine nutritional status. Endocrinol. Diabetes Nutr. 2020, 67, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Vila, L.; Lucas, A.; Donnay, S.; de la Vieja, A.; Wengrovicz, S.; Santiago, P.; Bandrés, O.; Velasco, I.; Garcia-Fuentes, E.; Ares, S.; et al. The nutrition of iodine in Spain. Needs for the future. Endocrinol. Diabetes Nutr. 2020, 67, 61–69. [Google Scholar] [CrossRef] [PubMed]


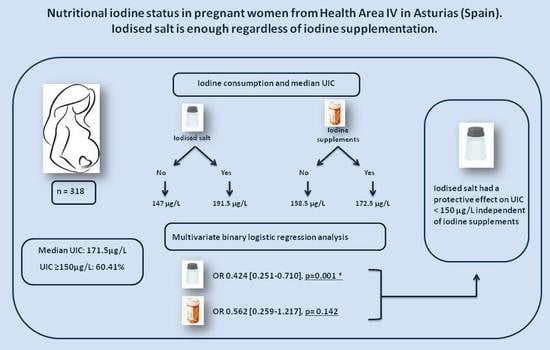
| UIC ≥ 150 μg/L | UIC < 150 μg/L | p | UIC (μg/L) a | p | ||
|---|---|---|---|---|---|---|
| Iodised salt | No | 55 (48.7%) | 58 (51.3%) | 0.001 | 147 (102–206) | 0.016 |
| Yes | 97 (69.3%) | 43 (30.7%) | 191.5 (131.5–285) | |||
| Dairy Products b | <2 servings | 78 (56.1%) | 61 (43.9%) | 0.269 | 168 (96–258) | 0.48 |
| ≥2 servings | 84 (62.7%) | 50 (37.3%) | 172 (122.25–255.75) | |||
| Iodine supplements | No | 21 (53.9%) | 18 (46.1%) | 0.455 | 158.5 (113–199.5) | 0.027 |
| Yes | 157 (60.1%) | 104 (39.9%) | 172.5 (116–285.75) |
| Β | OR | 95% CI d | p | ||
|---|---|---|---|---|---|
| Model 1 | |||||
| Iodised salt | No | Ref. | |||
| Yes | −0.857 | 0.424 | 0.251–0.710 | 0.001 | |
| Dairy products | <2 serving | Ref. | |||
| ≥2 serving | −0.264 | 0.768 | 0.439–1.337 | 0.353 | |
| Iodine supplement | No | Ref. | |||
| Yes | −0.576 | 0.562 | 0.259–1.217 | 0.142 | |
| Model 2 | |||||
| Iodised salt | No | Ref. | |||
| Yes | −0.905 | 0.404 | 0.237–0.683 | 0.001 | |
| Glasses of milk a | 0 servings | Ref. | |||
| ≥1 servings | −0.655 | 0.519 | 0.266–1.007 | 0.053 | |
| Yoghurts b | 0 yoghurts | Ref. | |||
| ≥1 yoghurts | −0.193 | 0.824 | 0.467–1.461 | 0.506 | |
| Cheese c | No | Ref. | |||
| Yes | −0.257 | 0.784 | 0.404–1.486 | 0.462 | |
| Iodine supplement | No | Ref. | |||
| Yes | −0.670 | 0.512 | 0.240–1.085 | 0.080 | |
| TSH ≥ 2.5 mIU/L | TSH < 2.5 mIU/L | p | ||
|---|---|---|---|---|
| Iodised salt | No | 10 (26.3%) | 28 (73.7%) | 0.13 |
| Yes | 19 (42.2%) | 26 (57.8%) | ||
| Dairy products | <2 servings | 16 (35.6%) | 29 (64.4%) | 0.806 |
| ≥2 servings | 16 (38.1%) | 26 (61.9%) | ||
| Iodine supplement | No | 7 (70.0%) | 3 (30.0%) | 0.031 |
| Yes | 25 (31.6%) | 54 (68.4%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Martínez, S.; Riestra-Fernández, M.; Martínez-Morillo, E.; Avello-Llano, N.; Delgado-Álvarez, E.; Menéndez-Torre, E.L. Nutritional Iodine Status in Pregnant Women from Health Area IV in Asturias (Spain): Iodised Salt Is Enough. Nutrients 2021, 13, 1816. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13061816
González-Martínez S, Riestra-Fernández M, Martínez-Morillo E, Avello-Llano N, Delgado-Álvarez E, Menéndez-Torre EL. Nutritional Iodine Status in Pregnant Women from Health Area IV in Asturias (Spain): Iodised Salt Is Enough. Nutrients. 2021; 13(6):1816. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13061816
Chicago/Turabian StyleGonzález-Martínez, Silvia, María Riestra-Fernández, Eduardo Martínez-Morillo, Noelia Avello-Llano, Elías Delgado-Álvarez, and Edelmiro Luis Menéndez-Torre. 2021. "Nutritional Iodine Status in Pregnant Women from Health Area IV in Asturias (Spain): Iodised Salt Is Enough" Nutrients 13, no. 6: 1816. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13061816