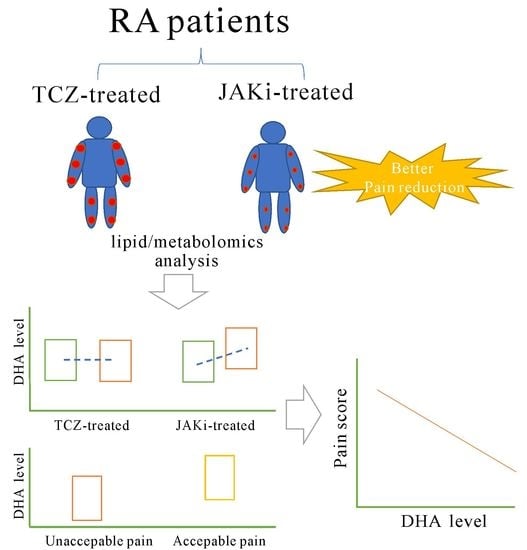
Increased Levels of Omega-3 Fatty Acids and DHA Are Linked to Pain Reduction in Rheumatoid Arthritis Patients Treated with Janus Kinase Inhibitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. The Major Outcome for Pain
2.3. Blood Sample Preparation and Lipid Profiles Measurement
2.4. Determination of Serum Lipid Metabolites by 1H-NMR Lipid/Metabolomics
2.5. Quantification and Replication of the Significant Lipid Metabolites Using ELISA
2.6. Statistical Analysis
3. Results
3.1. Demographic Data and Clinical Characteristics of RA Patients
3.2. Change in Serum Levels of Omega-3 PUFAs and DHA Determined the 1H NMR-Based Lipid/Metabolomics in Patients Treated with 6-Month JAKi or TCZ
3.3. Change of Serum DHA Levels Determined by ELISA in Patients Treated with Six Months’ JAKi or TCZ
3.4. Correlation between the Change of DHA Levels and the Decrement of Pain Score or DAS28 Score in JAKi-Treated Patients
3.5. Linear Regression Analysis for Pain Decrement after JAKi Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Furst, D.E.; Emery, P. Rheumatoid arthritis pathophysiology: Update on emerging cytokine and cytokine-associated cell targets. Rheumatology 2014, 53, 1560–1569. [Google Scholar] [CrossRef] [Green Version]
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef]
- Heiberg, T.; Kvien, T.K. Preferences for improved health examined in 1,024 patients with rheumatoid arthritis: Pain has highest priority. Arthritis Rheum. 2002, 47, 391–397. [Google Scholar] [CrossRef]
- Sanderson, T.; Morris, M.; Calnan, M.; Richards, P.; Hewlett, S. Patient perspective of measuring treatment efficacy: The rheumatoid arthritis patient priorities for pharmacologic interventions outcomes. Arthritis Care Res. 2010, 62, 647–656. [Google Scholar] [CrossRef]
- Walsh, D.A.; McWilliams, D.F. Mechanisms, impact and management of pain in rheumatoid arthritis. Nat. Rev. Rheumatol. 2014, 10, 581–592. [Google Scholar] [CrossRef]
- McWilliams, D.F.; Walsh, D.A. Pain mechanisms in rheumatoid arthritis. Clin. Exp. Rheumatol. 2017, 35, 94–101. [Google Scholar]
- Zhang, A.; Lee, Y.C. Mechanisms for Joint Pain in Rheumatoid Arthritis (RA): From Cytokines to Central Sensitization. Curr. Osteoporos. Rep. 2018, 16, 603–610. [Google Scholar] [CrossRef]
- Meeus, M.; Vervisch, S.; De Clerck, L.S.; Moorkens, G.; Hans, G.; Nijs, J. Central sensitization in patients with rheumatoid arthritis: A systematic literature review. Semin. Arthritis Rheum. 2012, 41, 556–567. [Google Scholar] [CrossRef]
- Crispino, N.; Ciccia, F. JAK/STAT pathway and nociceptive cytokine signalling in rheumatoid arthritis and psoriatic arthritis. Clin. Exp. Rheumatol. 2021, 39, 668–675. [Google Scholar]
- Simon, L.S.; Taylor, P.C.; Choy, E.H.; Sebba, A.; Quebe, A.; Knopp, K.L.; Porreca, F. The Jak/STAT pathway: A focus on pain in rheumatoid arthritis. Semin. Arthritis Rheum. 2021, 51, 278–284. [Google Scholar] [CrossRef]
- Smolen, J.S.; Landewe, R.; Bijlsma, J.; Burmester, G.; Chatzidionysiou, K.; Dougados, M.; Nam, J.; Ramiro, S.; Voshaar, M.; van Vollenhoven, R.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann. Rheum. Dis. 2017, 76, 960–977. [Google Scholar] [CrossRef]
- Olofsson, T.; Wallman, J.K.; Joud, A.; Schelin, M.E.; Ernestam, S.; van Vollenhoven, R.; Saevarsdottir, S.; Lampa, J. Pain over 2 years after start of biological versus conventional combination treatment in early rheumatoid arthritis: Results from the randomised controlled SWEFOT trial. Arthritis Care Res. 2020, 73, 1312–1321. [Google Scholar] [CrossRef]
- Rifbjerg-Madsen, S.; Christensen, A.W.; Christensen, R.; Hetland, M.L.; Bliddal, H.; Kristensen, L.E.; Danneskiold-Samsoe, B.; Amris, K. Pain and pain mechanisms in patients with inflammatory arthritis: A Danish nationwide cross-sectional DANBIO registry survey. PLoS ONE 2017, 12, e0180014. [Google Scholar] [CrossRef]
- Nash, P.; Kerschbaumer, A.; Dorner, T.; Dougados, M.; Fleischmann, R.M.; Geissler, K.; McInnes, I.; Pope, J.E.; van der Heijde, D.; Stoffer-Marx, M.; et al. Points to consider for the treatment of immune-mediated inflammatory diseases with Janus kinase inhibitors: A consensus statement. Ann. Rheum. Dis. 2021, 80, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, S. Tofacitinib: A Review in Rheumatoid Arthritis. Drugs 2017, 77, 1987–2001. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.C.; Keystone, E.C.; van der Heijde, D.; Weinblatt, M.E.; Del Carmen Morales, L.; Reyes Gonzaga, J.; Yakushin, S.; Ishii, T.; Emoto, K.; Beattie, S.; et al. Baricitinib versus Placebo or Adalimumab in Rheumatoid Arthritis. N. Engl. J. Med. 2017, 376, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Coombs, J.H.; Bloom, B.J.; Breedveld, F.C.; Fletcher, M.P.; Gruben, D.; Kremer, J.M.; Burgos-Vargas, R.; Wilkinson, B.; Zerbini, C.A.; Zwillich, S.H. Improved pain, physical functioning and health status in patients with rheumatoid arthritis treated with CP-690,550, an orally active Janus kinase (JAK) inhibitor: Results from a randomised, double-blind, placebo-controlled trial. Ann. Rheum. Dis. 2010, 69, 413–416. [Google Scholar] [CrossRef]
- Wallenstein, G.V.; Kanik, K.S.; Wilkinson, B.; Cohen, S.; Cutolo, M.; Fleischmann, R.M.; Genovese, M.C.; Gomez Reino, J.; Gruben, D.; Kremer, J.; et al. Effects of the oral Janus kinase inhibitor tofacitinib on patient-reported outcomes in patients with active rheumatoid arthritis: Results of two Phase 2 randomised controlled trials. Clin. Exp. Rheumatol. 2016, 34, 430–442. [Google Scholar] [PubMed]
- Strand, V.; Kremer, J.M.; Gruben, D.; Krishnaswami, S.; Zwillich, S.H.; Wallenstein, G.V. Tofacitinib in Combination With Conventional Disease-Modifying Antirheumatic Drugs in Patients With Active Rheumatoid Arthritis: Patient-Reported Outcomes From a Phase III Randomized Controlled Trial. Arthritis Care Res. 2017, 69, 592–598. [Google Scholar] [CrossRef] [Green Version]
- Taylor, P.C.; Lee, Y.C.; Fleischmann, R.; Takeuchi, T.; Perkins, E.L.; Fautrel, B.; Zhu, B.; Quebe, A.K.; Gaich, C.L.; Zhang, X.; et al. Achieving Pain Control in Rheumatoid Arthritis with Baricitinib or Adalimumab Plus Methotrexate: Results from the RA-BEAM Trial. J. Clin. Med. 2019, 8, 831. [Google Scholar] [CrossRef] [Green Version]
- Fautrel, B.; Kirkham, B.; Pope, J.E.; Takeuchi, T.; Gaich, C.; Quebe, A.; Zhu, B.; de la Torre, I.; De Leonardis, F.; Taylor, P.C. Effect of Baricitinib and Adalimumab in Reducing Pain and Improving Function in Patients with Rheumatoid Arthritis in Low Disease Activity: Exploratory Analyses from RA-BEAM. J. Clin. Med. 2019, 8, 1394. [Google Scholar] [CrossRef] [Green Version]
- Fautrel, B.; Zhu, B.; Taylor, P.C.; van de Laar, M.; Emery, P.; De Leonardis, F.; Kannowski, C.L.; Nicolay, C.; Kadziola, Z.; De La Torre, I.; et al. Comparative effectiveness of improvement in pain and physical function for baricitinib versus adalimumab, tocilizumab and tofacitinib monotherapies in rheumatoid arthritis patients who are naive to treatment with biologic or conventional synthetic disease-modifying antirheumatic drugs: A matching-adjusted indirect comparison. RMD Open 2020, 6, e001131. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.H.; Mishra, P.P.; Mononen, N.; Hilvo, M.; Sievanen, H.; Juonala, M.; Laaksonen, M.; Hutri-Kahonen, N.; Viikari, J.; Kahonen, M.; et al. Lipidomic architecture shared by subclinical markers of osteoporosis and atherosclerosis: The Cardiovascular Risk in Young Finns Study. Bone 2020, 131, 115160. [Google Scholar] [CrossRef]
- Soininen, P.; Kangas, A.J.; Wurtz, P.; Suna, T.; Ala-Korpela, M. Quantitative serum nuclear magnetic resonance metabolomics in cardiovascular epidemiology and genetics. Circ. Cardiovasc. Genet. 2015, 8, 192–206. [Google Scholar] [CrossRef] [Green Version]
- Souto-Carneiro, M.; Toth, L.; Behnisch, R.; Urbach, K.; Klika, K.D.; Carvalho, R.A.; Lorenz, H.M. Differences in the serum metabolome and lipidome identify potential biomarkers for seronegative rheumatoid arthritis versus psoriatic arthritis. Ann. Rheum. Dis. 2020, 79, 499–506. [Google Scholar] [CrossRef] [Green Version]
- Abdulrazaq, M.; Innes, J.K.; Calder, P.C. Effect of omega-3 polyunsaturated fatty acids on arthritic pain: A systematic review. Nutrition 2017, 39–40, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Gotlinger, K.; Hong, S.; Arita, M. Resolvins, docosatrienes, and neuroprotectins, novel omega-3-derived mediators, and their aspirin-triggered endogenous epimers: An overview of their protective roles in catabasis. Prostaglandins Other Lipid Mediat. 2004, 73, 155–172. [Google Scholar] [CrossRef]
- Xu, Z.Z.; Zhang, L.; Liu, T.; Park, J.Y.; Berta, T.; Yang, R.; Serhan, C.N.; Ji, R.R. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat. Med. 2010, 16, 592–597. [Google Scholar] [CrossRef] [Green Version]
- Miles, E.A.; Calder, P.C. Influence of marine n-3 polyunsaturated fatty acids on immune function and a systematic review of their effects on clinical outcomes in rheumatoid arthritis. Br. J. Nutr. 2012, 107, S171–S184. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, R.J.; Katz, J. A meta-analysis of the analgesic effects of omega-3 polyunsaturated fatty acid supplementation for inflammatory joint pain. Pain 2007, 129, 210–223. [Google Scholar] [CrossRef] [Green Version]
- Souto, A.; Salgado, E.; Maneiro, J.R.; Mera, A.; Carmona, L.; Gomez-Reino, J.J. Lipid profile changes in patients with chronic inflammatory arthritis treated with biologic agents and tofacitinib in randomized clinical trials: A systematic review and meta-analysis. Arthritis Rheumatol. 2015, 67, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., 3rd; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann. Rheum. Dis. 2010, 69, 1580–1588. [Google Scholar] [CrossRef]
- Prevoo, M.L.; van ‘t Hof, M.A.; Kuper, H.H.; van Leeuwen, M.A.; van de Putte, L.B.; van Riel, P.L. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995, 38, 44–48. [Google Scholar] [CrossRef] [Green Version]
- Ledingham, J.; Deighton, C.; British Society for Rheumatology Standards, G.; Audit Working, G. Update on the British Society for Rheumatology guidelines for prescribing TNFalpha blockers in adults with rheumatoid arthritis (update of previous guidelines of April 2001). Rheumatology 2005, 44, 157–163. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, F.; Michaud, K. Assessment of pain in rheumatoid arthritis: Minimal clinically significant difference, predictors, and the effect of anti-tumor necrosis factor therapy. J. Rheumatol. 2007, 34, 1674–1683. [Google Scholar]
- Svensson, C.I. Interleukin-6: A local pain trigger? Arthritis Res. 2010, 12, 145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, D.; Kong, L.Y.; Cai, J.; Li, S.; Liu, X.D.; Han, J.S.; Xing, G.G. Interleukin-6-mediated functional upregulation of TRPV1 receptors in dorsal root ganglion neurons through the activation of JAK/PI3K signaling pathway: Roles in the development of bone cancer pain in a rat model. Pain 2015, 156, 1124–1144. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, M.; Masuda, T.; Kitano, J.; Shimoyama, H.; Tozaki-Saitoh, H.; Inoue, K. IFN-gamma receptor signaling mediates spinal microglia activation driving neuropathic pain. Proc. Natl. Acad. Sci. USA 2009, 106, 8032–8037. [Google Scholar] [CrossRef] [Green Version]
- Raoof, R.; Willemen, H.; Eijkelkamp, N. Divergent roles of immune cells and their mediators in pain. Rheumatology 2018, 57, 429–440. [Google Scholar] [CrossRef] [Green Version]
- Schaible, H.G. Nociceptive neurons detect cytokines in arthritis. Arthritis Res. 2014, 16, 470. [Google Scholar] [CrossRef] [Green Version]
- Veselinovic, M.; Vasiljevic, D.; Vucic, V.; Arsic, A.; Petrovic, S.; Tomic-Lucic, A.; Savic, M.; Zivanovic, S.; Stojic, V.; Jakovljevic, V. Clinical Benefits of n-3 PUFA and -Linolenic Acid in Patients with Rheumatoid Arthritis. Nutrients 2017, 9, 325. [Google Scholar] [CrossRef] [PubMed]
- Lourdudoss, C.; Di Giuseppe, D.; Wolk, A.; Westerlind, H.; Klareskog, L.; Alfredsson, L.; van Vollenhoven, R.F.; Lampa, J. Dietary Intake of Polyunsaturated Fatty Acids and Pain in Spite of Inflammatory Control Among Methotrexate-Treated Early Rheumatoid Arthritis Patients. Arthritis Care Res. 2018, 70, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Lampa, J. Pain without inflammation in rheumatic diseases. Best Pr. Res. Clin. Rheumatol. 2019, 33, 101439. [Google Scholar] [CrossRef] [PubMed]




| JAKi-Treated Patients (n = 24) | TCZ-Treated Patients (n = 12) | |
|---|---|---|
| Age at entry, years | 60.5 (55.5–65.3) | 59.0 (50.3–64.0) |
| Age at disease onset, years | 53.0 (48.0–59.3) | 51.0 (44.0–56.5) |
| Disease duration, years | 5.0 (4.0–6.5) | 7.0 (4.8–8.5) |
| Proportion of women | 18 (75.0%) | 10 (83.3%) |
| BMI, kg/m2 | 23.6 (21.1–25.8) | 22.7 (21.1–25.7) |
| RF positivity, at baseline | 15 (62.5%) | 8 (66.7%) |
| ACPA positivity, at baseline | 15 (62.5%) | 9 (75.0%) |
| DAS28 at baseline | 6.77 (6.23–7.13) | 7.04 (5.98–7.36) |
| DAS28 at week 24 | 3.12 (3.05–3.40) ** | 3.14 (3.08–3.30) ** |
| Change of DAS28 (ΔDAS28) | 3.37 (3.12–3.98) | 3.64 (2.94–4.10) |
| Tender joint count at baseline | 15 (10–22) | 15 (12–19) |
| Tender joint count at week 24 | 2 (2–3) ** | 4 (3–9) ** |
| Swollen joint count at baseline | 10 (5–12) | 9 (7–14) |
| Swollen joint count at week 24 | 2 (1–3) ** | 2 (2–5) ** |
| Pain scores at baseline b | 87.5 (85.0–90.9) | 80.0 (80.0–85.0) |
| Pain scores at week 24 b | 17.5 (15.0–45.0) ** | 32.5 (15.0–46.3) ** |
| Change of pain scores (Δpain scores) | 70.0 (36.9–75.0) | 52.5 (33.8–70.0) |
| ESR, mm/1st h, at baseline | 32 (23–49) | 37 (23–46) |
| ESR, mm/1st h, at week 24 | 16 (11–24) ** | 8 (4–10) * |
| Change of ESR (ΔESR), mm/1st h | 21 (7–30) | 30 (18–38) |
| CRP, mg/dL, at baseline | 1.79 (0.98–2.75) | 1.61 (0.78–3.93) |
| CRP, mg/dL, at week 24 | 0.17 (0.06–0.57) ** | 0.02 (0.02–0.08) * |
| Change of CRP (ΔCRP), mg/dL | 1.14 (0.46–2.53) | 1.59 (0.76–3.65) |
| WBC (×103/mm3) at baseline | 7.6 (6.5–9.9) | 5.8 (4.9–7.8) |
| WBC (×103/mm3) at week 24 | 6.4 (5.4–8.4) * | 5.0 (4.6–5.8) |
| Neutrophil (%) at baseline | 72.6 (60.3–74.9) | 60.2 (51.5–70.4) |
| Neutrophil (%) at week 24 | 62.8 (58.8–65.9) | 47.5 (41.3–53.1) * |
| Lymphocyte (%) at baseline | 19.0 (15.5–21.5) | 26.7 (19.2–31.5) |
| Lymphocyte (%) at week 24 | 26.7 (24.3–28.6) | 34.3 (32.7–38.1) |
| TC, mg/dL, at baseline | 179.5 (161.8–208.8) | 206.0 (181.5–221.5) |
| TC, mg/dL, at week 24 | 193.0 (175.0–213.5) | 212.0 (183.3–230.3) |
| HDL-C, mg/dL, at baseline | 56.5 (52.2–67.0) | 58.7 (46.6–70.7) |
| HDL-C, mg/dL, at week 24 | 60.4 (51.6–71.0) | 56.1 (48.5–62.9) |
| TG, mg/dL, at baseline | 79.0 (51.8–127.5) | 81.5 (69.8–123.3) |
| TG, mg/dL, at week 24 | 83.0 (59.5–131.0) | 113.0 (82.0–148.0) |
| LDL-C, mg/dL, at baseline | 104.5 (88.9–123.6) | 123.7 (103.1–137.3) |
| LDL-C, mg/dL, at week 24 | 103.6 (97.6–113.3) | 131.6 (106.2–142.2) |
| Concomitant corticosteroids | 10 (41.7%) | 6 (50.0%) |
| Concomitant methotrexate | 15 (62.5%) | 8 (66.7%) |
| Hypertension | 6 (25.0%) | 4 (33.3%) |
| Diabetes mellitus | 2 (8.3%) | 0 (0.0%) |
| Ever smoking | 3 (12.5%) | 1 (8.3%) |
| Univariate Regression Analysis | ||||
| B | 95% CI | p Value | ||
| Change in DHA | −1.284 | −1.581 | −0.988 | <0.0001 |
| Change in ESR | 0.383 | −0.066 | 0.831 | 0.0904 |
| Change in CRP | 0.867 | −1.366 | 3.099 | 0.4285 |
| Change in DAS28 | 5.215 | −3.994 | 14.425 | 0.2528 |
| Age at entry | −0.319 | −1.066 | 0.429 | 0.3862 |
| Gender | 20.139 | 3.506 | 36.772 | 0.0199 |
| Multiple Regression Analysis | ||||
| B | 95% CI | p value | ||
| Change in DHA | −1.148 | −1.532 | −0.765 | <0.0001 |
| Change in ESR | 0.057 | −0.295 | 0.409 | 0.7362 |
| Change in CRP | 0.399 | −0.928 | 1.726 | 0.5327 |
| Change in DAS28 | −0.413 | −7.358 | 6.532 | 0.9013 |
| Age at entry | −0.101 | −0.505 | 0.303 | 0.6046 |
| Gender | 8.250 | −2.538 | 19.038 | 0.1245 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.-K.; Chen, P.-K.; Chen, C.-C.; Chang, S.-H.; Chen, C.-H.; Chen, D.-Y. Increased Levels of Omega-3 Fatty Acids and DHA Are Linked to Pain Reduction in Rheumatoid Arthritis Patients Treated with Janus Kinase Inhibitors. Nutrients 2021, 13, 3050. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13093050
Chang C-K, Chen P-K, Chen C-C, Chang S-H, Chen C-H, Chen D-Y. Increased Levels of Omega-3 Fatty Acids and DHA Are Linked to Pain Reduction in Rheumatoid Arthritis Patients Treated with Janus Kinase Inhibitors. Nutrients. 2021; 13(9):3050. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13093050
Chicago/Turabian StyleChang, Ching-Kun, Po-Ku Chen, Chia-Ching Chen, Shih-Hsin Chang, Chu-Huang Chen, and Der-Yuan Chen. 2021. "Increased Levels of Omega-3 Fatty Acids and DHA Are Linked to Pain Reduction in Rheumatoid Arthritis Patients Treated with Janus Kinase Inhibitors" Nutrients 13, no. 9: 3050. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13093050