1. Introduction
Apples (
Malus domestica) are a well-established, domesticated fruit worldwide, with approximately 86 million metric tons produced annually [
1]. On a fresh basis, apples are among the top fruit varieties grown in the United States—second only to grapes [
2]. Fresh apple consumption is most common among consumers, however, 35% of the apples consumed are processed [
3]. While processed apple products generally include jams, jellies, cider, vinegar, and dried products, most apples are processed for apple juice. There is a 75% juice extraction efficiency in the apple juice industry; thus, 25–30% of the fruit waste remains. Known as the pomace, this leftover fraction is a heterogeneous mixture of skin, flesh, seeds, stems, core, and calyx [
4,
5]. According to the U.S. Apple Association, approximately 33.4 million bushels (701.4 million kg) of apples were produced in 2021–2022 for juice and cider production [
6]. Based on the juice extraction efficiency (75%), an estimated 175,350 metric tons of apple pomace were produced annually [
4]. Apple waste is typically disposed of in landfills, leading to several damaging environmental effects, as disruption to the carbon:nitrogen ratio of soil can occur due to sugar content, organic acids, and microbial fermentation of apple pomace [
7]. The high-water content of apple pomace is also an issue as it can cause water pollution. Given the complications of pomace disposal, various industries have taken advantage of the by-product as it is a rich source of nutrients such as carbohydrates, micronutrients, and phytochemicals [
8]. Further utilization includes pectin extraction, production of enzymes and aroma compounds, cultivation of microbial strains and edible mushrooms, and incorporation into animal feed [
5]. However, the burden remains to prevent the disposal of apple waste into landfills and, ultimately, avoid environmental pollution.
Apples contain health-promoting bioactive constituents. Quercetin derivatives (galactoside, glucoside, rhamnoside), catechin, gallic acid, phloretin, and chlorogenic acid are polyphenolic compounds that are reportedly found in apples [
8]. Studies have reported the antioxidative [
9,
10,
11,
12,
13], antiproliferative [
11,
14,
15,
16,
17], and anti-inflammatory [
18,
19,
20] potential of apple polyphenols. It is important to note that apple phenolic compounds differ in concentration throughout the fruit matrix. For example, while apple flesh and apple peels contain chlorogenic acid, the flesh contains higher concentrations [
21,
22]. Research has also shown apple peels to contain greater amounts of antioxidants and, therefore, greater antioxidant potential compared to apple flesh. In addition, interest in apple seeds as a source of polyphenolic compounds has been investigated and found to be a rich source of quercetin derivatives, phenolic acids, catechin, and phloridzin [
23]. Another constituent with health-promoting properties within the apple is dietary fiber, specifically, the non-digestible soluble polysaccharide pectin. Historically utilized as a commercial thickening and gelling agent, pectin holds potential functional properties which may improve intestinal health. The resistance to gastric digestion enables pectin to reach the host gut and undergo fermentation by microbiota, and ultimately produces short-chain fatty acid (SCFA) metabolites [
24,
25,
26,
27]. Previous studies have shown SCFAs to be beneficial to the gut by promoting enteric epithelial cell proliferation, enhancing gut barrier function, enhancing micronutrient absorption, and favoring the growth of beneficial bacteria over potentially pathogenic bacteria [
28,
29,
30,
31,
32].
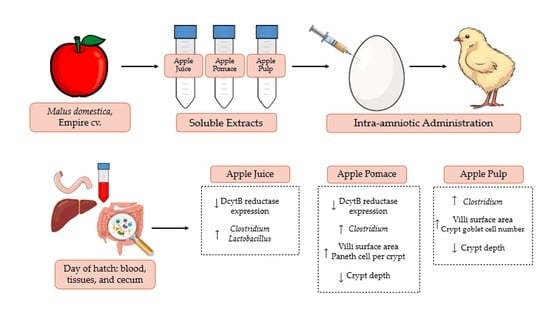
This study aimed to evaluate the in vivo effects of apple juice, pomace, and pulp soluble extracts on intestinal morphology, functionality, and the microbiome using
Gallus gallus as our model. The broiler chicken is an established model employed to evaluate the effects of plant-origin bioactives on intestinal health and the microbiome [
33]. The broiler chicken model exhibits genetic homology, a complex gut microbiota, and notable microbial similarity at the phylum level to human gut microbiota [
34].
2. Materials and Methods
2.1. Apple Preparation
Empire apples (Malus domestica) were harvested (>2 tons) during the fall of 2021 from multiple trees from the Cornell AgriTech Orchards and processed at the Cornell Food Venture Center Pilot Plant (Geneva, NY, USA). Before processing, Empire apples were destemmed and washed. Apple pulp was made by removing the core and seeds and dicing. The apple pieces were then freeze-dried (Max53, Millrock Technology, Kingston, NY, USA) for 24 h. The dried apple was then ground into a fine powder using a bench-scale processor (Robo Coue; Jackson, MI, USA) at 1500 RPM. Apple juice was made to typical industry standards. For apple juice, whole apples were ground in a hammer mill in a blade configuration. The pulp was then pressed using a pilot-scale hydraulic press (Orchard Equipment Co., Conway, MA, USA) at 1200–1400 PSI. The juice was commercially sterilized by hot-packing into PET bottles at 85 °C and keeping it hot for 2 min. Apple pomace was the resulting pomace from the juice pressing. After juice pressing, the pomace was freeze-dried for 24 h. The dried pomace was ground into a fine powder using a bench-scale grinder. The powders were vacuumed-sealed, and all samples were kept frozen until use.
2.2. Apple Analysis Sample Preparation
Apple samples were extracted under dark conditions utilizing absolute methanol and constant agitation for 2 h. The resulting slurry was centrifuged and decanted to acquire the supernatant. The subsequent isolate and washings were diluted to attain an extract (15% w/v) which was ultimately utilized for the further analysis below.
2.2.1. Polyphenol Analysis
The Folin-Ciocalteu method previously detailed by Waterhouse was utilized to quantify total polyphenol content (TPC) [
35]. Essentially, the Folin-Ciocalteu reagent and the extract were allowed to incubate at room temperature. The sodium carbonate solution was used to quench the reaction and sample absorbance was measured immediately using a UV-visible spectrophotometer (Thermo Fisher; Waltham, MA, USA) at 765 nm. Therefore, TPC was calculated as gallic equivalents (GE) using a standard curve prepared under the same conditions.
2.2.2. Fibrous and Non-Fibrous Carbohydrate Analysis
According to AOAC 962.09, the non-fibrous carbohydrate analysis (NFC) was completed. Acid detergent fiber (ADF) and neutral detergent fiber (NDF) analyses were conducted according to AOAC 973.18. The analysis was performed by Dairy One Co-Op Inc. (Ithaca, NY, USA).
2.3. Extraction of Soluble Apple Contents
Apple powders and juice samples were dissolved and diluted in distilled water to create 6% concentrations. All apple samples were heated via water bath for 1 h at 60 °C, centrifuged (3500 RPM) for 10 min at room temperature, and the supernatant was collected only in the case of the pomace and pulp.
2.4. Animals and Design
Fertile Cornish-cross broiler chicken eggs (n = 55) were provided by a hatchery (Moyer’s chicks, Quakertown, PA, USA). All animal protocols were approved by Cornell University Institutional Animal Care and Use Committee (ethic approval code: 2020-0077). Apple extract powders and juice were diluted with 18 MΩ H2O to acquire the necessary concentration to maintain an osmolarity value of less than 320 Osm. On day 17 of embryonic incubation, viable eggs were weighed and randomly allocated into five groups (n = 12) with a similar weight frequency distribution. After identifying the amniotic fluid by candling, the treatment solution (1 mL) was injected with a 21-gauge needle. Subsequent to injection, the injection site was sealed with cellophane tape. Eggs were placed in hatching baskets for each treatment and equal representation at each incubator location. The five treatment groups consisted as follows: non-injected (NI); 18 MΩ H2O (H2O); 6% apple juice (AJ); 6% apple pomace (APo); and 6% apple pulp (APu). On day 21, exposure to CO2 was used to euthanize hatchlings, and the blood, pectoral muscle, liver, duodenum, and cecum were collected for further analysis.
2.5. Blood Analysis
Blood was collected from the heart using micro-hematocrit heparinized capillary tubes (Fisher Scientific Waltham, MA, USA). Blood glucose concentrations were determined using the Accu-Chek® blood glucose monitor following the manufacturer’s protocol.
2.6. Pectoral Glycogen
The pectoral muscle was collected on the day of the hatch. Glycogen analysis was completed as previously described [
36,
37,
38]. Briefly, 20 mg of the sample was homogenized in 8% perchloric acid and centrifuged at 12,000 rpm (4 °C) for 15 min. The supernatant was removed, and 1.0 mL of petroleum ether was added. Following mixing, the petroleum ether fraction was discarded, and the remaining sample layer was transferred to a new container with the color reagent (300 µL). Samples were read in an ELISA reader at 450 nm, and glycogen content was analyzed based on the standard curve. Total glycogen content in the pectoral sample was identified as the product of multiplying tissue weight by the amount of glycogen per 1 g of wet tissue.
2.7. Total RNA Extraction from Duodenum and Liver Tissue Samples
Total RNA was extracted from 30 mg of duodenal and liver tissue samples (
n = 5) as previously described [
39,
40,
41,
42,
43]. Briefly, the Qiagen Rneasy Mini Kit (Rneasy Mini Kit, Qiagen Inc., Valencia, CA, USA) was used. All protocols were carried out according to the manufacturer and under Rnase-free conditions. RNA was quantified by absorbance at A 260/280. The integrity of 18S ribosomal rRNA was verified by 1.5% agarose gel electrophoresis, followed by ethidium bromide staining. RNA was stored at −80 °C until further use.
2.8. Real-Time Polymerase Chain Reaction (RT-PCR)
cDNA was created from the extracted RNA by a 20 µL reverse transcriptase (RT) reaction. To complete the reaction, the BioRad C1000 touch thermocycler using the Improm-II Reverse Transcriptase Kit (Catalog #A1250; Promega, Madison, WI, USA) was utilized. The cDNA concentration was measured by Nanodrop (Thermo Fisher Scientific, Waltham, MA, USA) at an absorbance of 260 nm and 280 nm using an extinction coefficient of 33 (for single-stranded DNA). Genomic DNA contamination was assessed by a real-time RT-PCR assay for the reference gene samples.
The RT-PCR primers were designed based on relevant gene sequences from the GenBank database using the Real-Time Primer Design Tool software (IDT DNA, Coralvilla, IA, USA), as detailed previously [
44].
Table 1 indicates the primer sequences used in accordance with iron, zinc, and vitamin A metabolism, immune response, and brush border membrane functionality. The reference gene used was the
Gallus gallus primer 18S rRNA. BLAST searches against the genomic National Center for Biotechnology Information (NCBI) database were applied to verify primer specificity.
2.9. Microbial Samples and Intestinal Contents DNA Isolation
Cecum samples were weighed and placed in sterile tubes containing PBS. Subsequently, the samples were vortexed with sterile glass beads for 3 min. All protocols were completed as previously described [
42,
45,
46,
47,
48,
49].
2.10. Primer Design and PCR Amplification of Bacterial 16S rRNA
Bifidobacterium, Lactobacillus, Escherichia coli, Clostridium, Klebsiella, and L. plantarum primers were used. 16S rRNA was the universal primer and internal standard. Therefore, the proportions of each bacterial group are presented. PCR products were applied to 1.5% agarose gel with ethidium bromide stain and quantified with Gel-Pro analyzer version 3.0 (Media Cybernetics LP, Rockville, MD, USA).
2.11. Histomorphological Examination
On the day of the hatch, proximal duodenal samples were collected. Subsequently, the samples were soaked in 4% (
v/
v) buffered formaldehyde, dehydrated, cleared, and embedded in paraffin. Several sections were cut with a 5 µm thickness and placed on glass slides. Intestinal sections were then deparaffinized in xylene and rehydrated in a series of graded alcohol. Ultimately, the slides were stained with Alcian Blue/Periodic acid-Schiff and investigated by light microscopy using EPIX XCAP software (Standard version, Olympus, Waltham, MA, USA). The following features were measured in the duodenum: villus surface area, crypt depth, villus and crypt goblet diameter, crypt goblet cell number and type, and Paneth cell number and diameter within the crypt, as previously described [
42,
43,
49,
50,
51]. Per treatment group, five biological samples (
n = 5) (four segments each) were analyzed. Ten randomly selected villi and crypts were measured and analyzed, and cell measurements and counts were completed in ten randomly selected villi or crypts per segment. The following equation was utilized to calculate the villus surface area:
in which
VW is the mean of three villus width measurements, and
VL is the villus length.
2.12. Statistical Analysis
In this paper, values are portrayed as the mean values ± standard error means. Experimental treatments and controls for the intra-amniotic administration were assigned with approximately equal weight distribution. Tested parameters were analyzed for normal distribution and equal variance through a Shapiro-Wilk test. If the test was accepted, a one-way analysis for variance (ANOVA) was utilized. ANOVA was used to analyze the results, followed by a Duncan post-hoc test to determine significance based on p-values (p < 0.05). For all statistical evaluations, software SPSS version 20.0 was utilized.
4. Discussion
The Empire apple variety is a cross between McIntosh (
Malus domestica “McIntosh”) and Red Delicious (
Malus domestica “Red Delicious”) cultivars and is native to New York state [
52]. According to the United States Apple Association, Empire apples are among the top-produced apples in the nation [
6,
53]. Here, we have investigated the effects of Empire apple juice (AJ), pomace (APo), and pulp (APu) extracts via intra-amniotic administration on micronutrient absorption, intestinal immune response, gut morphology, and cecal bacterial populations. To our knowledge, this is the first study to examine such physiological effects of the Empire apple cultivar.
Body weight, blood glucose, and glycogen content (
Table 3) did not differ throughout the treatment and control groups. As apples contain select macro- and micronutrients, consumption of this fruit and weight gain prevention have been studied in previous animal trials [
54]. While Cho et al. (2013) reported apple pomace and juice supplementation reduced (
p < 0.05) body weight gain in Sprague-Dawley rats [
55], and Samout et al. (2016) found apple pectin to exert anti-obesity effects in Wistar rats [
56], changes in body weight did not occur in our study. We hypothesize that this may be the case as treatment groups were a single dose in a naïve system, while the aforementioned studies utilized overweight models over a prolonged period.
The intra-amniotic administration of apple juice and pomace extracts reduced the gene expression of DcytB reductase relative to the controls (
Figure 1A). Located in the proximal duodenum, DcytB functions to reduce ferric dietary iron to the bioavailable form (Fe
2+) for uptake into the enterocyte [
57]. No significant changes occurred in the remaining iron metabolism proteins (DMT1, Ferroportin, and Hepcidin). Nonetheless, the reduced expression of DcytB potentially suggests an improvement in iron absorption into the enterocyte [
58,
59]. Shah et al. (2003) reported that apple juice enhances iron bioavailability in American children of 3 to 6 years of age [
60]. The soluble apple fractions did not enhance zinc transporter proteins and vitamin A metabolism proteins, yet these results revealed no adverse effects of a single-dose administration. We expected to observe an anti-inflammatory effect of the apple treatments due to the naturally occurring phenolics in apples (represented in
Table 2), which possess antioxidative properties (e.g., chlorogenic acid, quercetin glycosides, catechin) [
21,
22,
23]. However,
Figure 3 reveals no effect of the apple treatments on inflammatory cytokine expression (NF-κB, TNF-α, IL6). Given the reported anti-inflammatory potential of apples [
10,
18,
19,
20], the lack of agreement with this study’s results can be attributed to the short exposure time and concentrations administered. Conversely, our results reveal that apple pomace extract did not stimulate a negative intestinal immune response. Apple seeds are known to generate toxic cyanogenic glycosides upon grinding and have been a factor of concern when upscaling apple pomace for consumption. In a recent study, using Fisher rats, Ravn-Haren et al. investigated the effects of a different cultivar (Shampion) apple pomace with and without seeds [
61]. It was reported that apple pomace, regardless of seed content, did not elevate alanine aminotransferase, a liver toxicity biomarker [
61].
Intestinal barrier integrity is vital to gastrointestinal functionality and health, and tight junction proteins play a crucial role in maintaining the luminal structure [
62,
63]. Located in luminal epithelial cells, tight junctions, such as claudin and occludin regulate the permeability of ions, water, and macronutrients [
64,
65,
66]. Expression of the tight junction protein occludin (OCLN) was significantly reduced by apple juice administration (
Figure 3), which suggests an increase in epithelial permeability. Apple juice is known to naturally contain high amounts of simple sugars such as glucose and fructose [
67,
68]. High-sugar diets have reportedly increased intestinal barrier permeability, although the direct mechanism is unclear [
69]. One proposed mechanism is through the intestinal microbiome, as diets rich in simple sugars have been linked to disrupting the balance of gut microbes, causing dysbiosis [
69]. Dysbiosis can be characterized by an increase in the
Firmicutes/
Bacteroidetes ratio [
69,
70,
71]. AJ increased the abundance of
Clostridium and
Klebsiella, as depicted in
Figure 4. The
Clostridium genus comprises commensal bacteria within the
Firmicutes phylum that can exert pathogenic effects under dysbiosis conditions. Essentially, the overgrowth of opportunistic species within the
Clostridium and
Klebsiella may lead to the degradation of the intestinal barrier [
72,
73,
74] or render severe infection [
75]. Conversely, while the beneficial bacteria
Bifidobacterium decreased abundance in AJ,
Lactobacillus increased abundance.
Lactobacillus is a lactic acid-producing bacteria within the
Firmicutes phylum; thus, our results suggest a possible selective stimulation of
Firmicute proliferation by AJ.
Figure 4 reveals the increased abundance of
Clostridium within APo and APu relative to the NI control. According to our results in
Figure 3 and
Table 4,
Table 5 and
Table 6, it is possible that the
Clostridium genera exerted a beneficial effect as an induced effect on intestinal permeability and inflammatory cytokine expression was not observed. Therefore, we hypothesize that valuable species of
Clostridia were increased.
Clostridium is an SCFA-producing genus that has been reported to grow in abundance through pectin fermentation [
76,
77,
78]. APo and APu had the greatest amount of non-fiber carbohydrates (including pectin) (
Table 2), villi surface area (
Table 4), and acidic goblet cells within the crypt (
Table 5). A previous study reported that apple-derived pectin-fed rats increased
Clostridium species abundance four-fold, whilst also increasing butyrate levels [
79]. Butyrate is a short-chain fatty acid produced upon carbohydrate fermentation by
Clostridium that can lower intestinal pH [
78], which could explain the increased count of acidic goblet cells for APo and APu. Dufourny et al. (2021) previously assessed the effects of apple pomace on intestinal morphology and microbiota in weaned piglets. They found the pomace to increase
Clostridia abundance and duodenal and ileal villi length [
80]. Our results agree with the significant findings of this study. This further establishes the role of apple pomace to modulate Clostridia groups in both animal models which leads to improvements in gut health and intestinal homeostasis.
Shortened crypt depth was observed in all apple treatment groups (
Table 4). Shortened crypt depths are morphological evidence for improved intestinal health as it suggests a slower intestinal epithelial cell turnover rate, allowing sufficient time for enterocytes to differentiate and function at capacity [
81,
82,
83]. Within intestinal crypts, goblet cells play a role in maintaining the gut epithelial layer as they secrete mucus and mucin glycoproteins that function as a protective layer along the intestinal lumen [
84]. A previous study found apple polysaccharide isolated from Fuji apple pomace to stimulate the enhancement of gut epithelial integrity by goblet cell autophagy [
85]. Our study observed that APu and APo had the greatest crypt goblet cell count per unit area, respectively. In addition, APu had a significantly lower crypt goblet cell diameter. This finding likely suggests lower mucus content since intestinal crypt goblet cells only secrete mucus upon stimulation [
84] Our findings suggest that the Empire apple has a level of effect on goblet cells located in the crypt. However, further studies should be completed to elucidate the potential impact. Moreover, Paneth cells are in the small intestinal crypts and function in the gut immunological response—secreting antimicrobial peptides and immunomodulating proteins to maintain intestinal homeostasis [
86,
87,
88]. Crypt Paneth cell count per unit area was greatest (
p < 0.05) in APo, APu, and AJ, respectively, compared to H
2O control (
Table 6). Yet, the Paneth cell diameter of APo treatment was similar (
p > 0.05) to APu and AJ, and lower (
p > 0.05) than the H
2O control. Based on these observations and a recent review that summarized the impacts of dietary fiber on host gastrointestinal immune response, it seems that the Empire apple may stimulate Paneth cell function and improve intestinal immune response [
89].