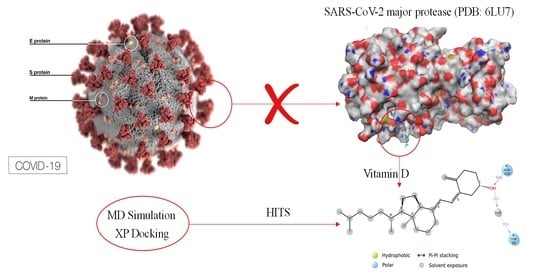
The Efficacious Benefit of 25-Hydroxy Vitamin D to Prevent COVID-19: An In-Silico Study Targeting SARS-CoV-2 Spike Protein
Abstract
:1. Introduction
2. Materials and Methods
2.1. Molecular Docking
2.2. Determination of Active Sites
2.3. Protein Preparation
2.4. Ligand Preparation
2.5. Induced-Fit Molecular Docking
2.6. Molecular Dynamics Simulation
3. Results
3.1. Active Site Determination
3.2. Molecular Docking
3.3. Molecular Dynamics Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence That 25-hydroxy vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients 2020, 12, 988. [Google Scholar] [CrossRef] [Green Version]
- Zhong, N.S.; Zheng, B.J.; Li, Y.M.; Poon, L.L.M.; Xie, Z.H.; Chan, K.H.; Li, P.H.; Tan, S.Y.; Chang, Q.; Xie, J.P.; et al. Epidemiology and Cause of Severe Acute Respiratory Syndrome (SARS) in Guangdong, People’s Republic of China, in February, 2003. Lancet Lond. Engl. 2003, 362, 1353–1358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assiri, A.; McGeer, A.; Perl, T.M.; Price, C.S.; Al Rabeeah, A.A.; Cummings, D.A.T.; Alabdullatif, Z.N.; Assad, M.; Almulhim, A.; Makhdoom, H.; et al. Hospital Outbreak of Middle East Respiratory Syndrome Coronavirus. N. Engl. J. Med. 2013, 369, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Misra, D.P.; Agarwal, V.; Gasparyan, A.Y.; Zimba, O. Rheumatologists’ Perspective on Coronavirus Disease 19 (COVID-19) and Potential Therapeutic Targets. Clin. Rheumatol. 2020, 39, 2055–2062. [Google Scholar] [CrossRef] [Green Version]
- Allegra, A.; Tonacci, A.; Pioggia, G.; Musolino, C.; Gangemi, S. 25-hydroxy vitamin Deficiency as Risk Factor for SARS-CoV-2 Infection: Correlation with Susceptibility and Prognosis. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 9721–9738. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Leng, K.; Lu, Y.; Wen, L.; Qi, Y.; Gao, W.; Chen, H.; Bai, L.; An, X.; Sun, B.; et al. Epidemiological Features and Time-Series Analysis of Influenza Incidence in Urban and Rural Areas of Shenyang, China, 2010–2018. Epidemiol. Infect. 2020, 148, e29. [Google Scholar] [CrossRef] [Green Version]
- Kast, J.I.; McFarlane, A.J.; Głobińska, A.; Sokolowska, M.; Wawrzyniak, P.; Sanak, M.; Schwarze, J.; Akdis, C.A.; Wanke, K. Respiratory Syncytial Virus Infection Influences Tight Junction Integrity. Clin. Exp. Immunol. 2017, 190, 351–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, G.A.; Fanous, H.; Colin, A.A. Viral Strategies Predisposing to Respiratory Bacterial Superinfections. Pediatr. Pulmonol. 2020, 55, 1061–1073. [Google Scholar] [CrossRef]
- Verstuyf, A.; Carmeliet, G.; Bouillon, R.; Mathieu, C. 25-hydroxy vitamin D: A Pleiotropic Hormone. Kidney Int. 2010, 78, 140–145. [Google Scholar] [CrossRef]
- Norman, A.W.; Roth, J.; Orci, L. The 25-hydroxy vitamin D Endocrine System: Steroid Metabolism, Hormone Receptors, and Biological Response (Calcium Binding Proteins)*. Endocr. Rev. 1982, 3, 331–366. [Google Scholar] [CrossRef] [PubMed]
- Slater, G.H.; Ren, C.J.; Siegel, N.; Williams, T.; Barr, D.; Wolfe, B.; Dolan, K.; Fielding, G.A. Serum Fat-Soluble 25-hydroxy vitamin Deficiency and Abnormal Calcium Metabolism after Malabsorptive Bariatric Surgery. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract. 2004, 8, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Hewison, M. 25-hydroxy vitamin D and the Immune System: New Perspectives on an Old Theme. Rheum. Dis. Clin. 2012, 38, 125–139. [Google Scholar] [CrossRef]
- Klepeis, N.E.; Nelson, W.C.; Ott, W.R.; Robinson, J.P.; Tsang, A.M.; Switzer, P.; Behar, J.V.; Hern, S.C.; Engelmann, W.H. The National Human Activity Pattern Survey (NHAPS): A Resource for Assessing Exposure to Environmental Pollutants. J. Expo. Anal. Environ. Epidemiol. 2001, 11, 231–252. [Google Scholar] [CrossRef] [Green Version]
- Nath, H.; Gary, T.; Shepard-Smith, A. The Impact of Environmental Factors on the Transmission and Mortality of COVID-19; Social Science Research Network: Rochester, NY, USA, 2020. [Google Scholar]
- Wortsman, J.; Matsuoka, L.Y.; Chen, T.C.; Lu, Z.; Holick, M.F. Decreased Bioavailability of 25-hydroxy vitamin D in Obesity. Am. J. Clin. Nutr. 2000, 72, 690–693. [Google Scholar] [CrossRef] [Green Version]
- Dusso, A.S.; Brown, A.J.; Slatopolsky, E. 25-hydroxy vitamin D. Am. J. Physiol. Ren. Physiol. 2005, 289, F8–F28. [Google Scholar] [CrossRef]
- Khajavi, A.; Amirhakimi, G.H. The Rachoitic Lung: Pulmonary Findings in 30 Infants and Children with Malnutritional Rickets. Clin. Pediatr. 1977, 16, 36–38. [Google Scholar] [CrossRef]
- Martineau, A.R.; Honecker, F.U.; Wilkinson, R.J.; Griffiths, C.J. 25-hydroxy vitamin D in the Treatment of Pulmonary Tuberculosis. J. Steroid Biochem. Mol. Biol. 2007, 103, 793–798. [Google Scholar] [CrossRef]
- Cannell, J.J.; Vieth, R.; Umhau, J.C.; Holick, M.F.; Grant, W.B.; Madronich, S.; Garland, C.F.; Giovannucci, E. Epidemic Influenza and 25-hydroxy vitamin D. Epidemiol. Infect. 2006, 134, 1129–1140. [Google Scholar] [CrossRef]
- Reichert, T.A. Influenza and the Winter Increase in Mortality in the United States, 1959-1999. Am. J. Epidemiol. 2004, 160, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.T. Toll-Like Receptor Triggering of a 25-hydroxy vitamin D-Mediated Human Antimicrobial Response. Science 2006, 311, 1770–1773. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-T.; Nestel, F.P.; Bourdeau, V.; Nagai, Y.; Wang, Q.; Liao, J.; Tavera-Mendoza, L.; Lin, R.; Hanrahan, J.W.; Mader, S.; et al. Cutting Edge: 1,25-Dihydroxy25-hydroxy vitamin D3 Is a Direct Inducer of Antimicrobial Peptide Gene Expression. J. Immunol. Baltim. Md. 2004, 173, 2909–2912. [Google Scholar] [CrossRef] [Green Version]
- Ginde, A.A.; Mansbach, J.M.; Camargo, C.A. 25-hydroxy vitamin D, Respiratory Infections, and Asthma. Curr. Allergy Asthma Rep. 2009, 9, 81–87. [Google Scholar] [CrossRef]
- MacLaughlin, J.; Holick, M.F. Aging Decreases the Capacity of Human Skin to Produce 25-hydroxy vitamin D3. J. Clin. Invest. 1985, 76, 1536–1538. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez, O.M.; Farwell, W.R.; Kermah, D.; Taylor, E.N. Racial Differences in the Relationship between 25-hydroxy vitamin D, Bone Mineral Density, and Parathyroid Hormone in the National Health and Nutrition Examination Survey. Osteoporos. Int. 2011, 22, 1745–1753. [Google Scholar] [CrossRef] [Green Version]
- Iruretagoyena, M.; Hirigoyen, D.; Naves, R.; Burgos, P.I. Immune Response Modulation by 25-hydroxy vitamin D: Role in Systemic Lupus Erythematosus. Front. Immunol. 2015, 6, 513. [Google Scholar] [CrossRef] [Green Version]
- Bolland, M.J.; Grey, A.; Avenell, A.; Gamble, G.D.; Reid, I.R. Calcium Supplements with or without 25-hydroxy vitamin D and Risk of Cardiovascular Events: Reanalysis of the Women’s Health Initiative Limited Access Dataset and Meta-Analysis. BMJ 2011, 342, d2040. [Google Scholar] [CrossRef] [Green Version]
- Brenner, H.; Holleczek, B.; Schöttker, B. 25-hydroxy vitamin D Insufficiency and Deficiency and Mortality from Respiratory Diseases in a Cohort of Older Adults: Potential for Limiting the Death Toll during and beyond the COVID-19 Pandemic? Nutrients 2020, 12, 2488. [Google Scholar] [CrossRef] [PubMed]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. 25-hydroxy vitamin D Supplementation to Prevent Acute Respiratory Tract Infections: Systematic Review and Meta-Analysis of Individual Participant Data. BMJ 2017, 356, i6583. [Google Scholar] [CrossRef] [Green Version]
- Urashima, M.; Segawa, T.; Okazaki, M.; Kurihara, M.; Wada, Y.; Ida, H. Randomized Trial of 25-hydroxy vitamin D Supplementation to Prevent Seasonal Influenza A in Schoolchildren. Am. J. Clin. Nutr. 2010, 91, 1255–1260. [Google Scholar] [CrossRef] [PubMed]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors Associated with COVID-19-Related Death Using Open SAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef]
- Ilie, P.C.; Stefanescu, S.; Smith, L. The Role of 25-hydroxy vitamin D in the Prevention of Coronavirus Disease 2019 Infection and Mortality. Aging Clin. Exp. Res. 2020, 32, 1195–1198. [Google Scholar] [CrossRef]
- D’Avolio, A.; Avataneo, V.; Manca, A.; Cusato, J.; De Nicolò, A.; Lucchini, R.; Keller, F.; Cantù, M. 25-Hydroxy25-hydroxy vitamin D Concentrations Are Lower in Patients with Positive PCR for SARS-CoV-2. Nutrients 2020, 12, 1359. [Google Scholar] [CrossRef]
- Zhou, J.; Du, J.; Huang, L.; Wang, Y.; Shi, Y.; Lin, H. Preventive Effects of 25-hydroxy vitamin D on Seasonal Influenza A in Infants: A Multicenter, Randomized, Open, Controlled Clinical Trial. Pediatr. Infect. Dis. J. 2018, 37, 749–754. [Google Scholar] [CrossRef]
- Urashima, M.; Mezawa, H.; Noya, M.; Camargo, C.A. Effects of 25-hydroxy vitamin D Supplements on Influenza A Illness during the 2009 H1N1 Pandemic: A Randomized Controlled Trial. Food Funct. 2014, 5, 2365–2370. [Google Scholar] [CrossRef]
- Arihiro, S.; Nakashima, A.; Matsuoka, M.; Suto, S.; Uchiyama, K.; Kato, T.; Mitobe, J.; Komoike, N.; Itagaki, M.; Miyakawa, Y.; et al. Randomized Trial of 25-hydroxy vitamin D Supplementation to Prevent Seasonal Influenza and Upper Respiratory Infection in Patients with Inflammatory Bowel Disease. Inflamm. Bowel. Dis. 2019, 25, 1088–1095. [Google Scholar] [CrossRef]
- Loeb, M.; Dang, A.D.; Thiem, V.D.; Thanabalan, V.; Wang, B.; Nguyen, N.B.; Tran, H.T.M.; Luong, T.M.; Singh, P.; Smieja, M.; et al. Effect of 25-hydroxy vitamin D Supplementation to Reduce Respiratory Infections in Children and Adolescents in Vietnam: A Randomized Controlled Trial. Influenza Other Respir. Viruses. 2019, 13, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Gruber-Bzura, B.M. 25-hydroxy vitamin D and Influenza—Prevention or Therapy? Int. J. Mol. Sci. 2018, 19, 2419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansueto, P.; Seidita, A.; Vitale, G.; Gangemi, S.; Iaria, C.; Cascio, A. 25-hydroxy vitamin D Deficiency in HIV Infection: Not Only a Bone Disorder. BioMed. Res. Int. 2015, 2015, 735615. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Al Anouti, F.; Moukayed, M. Targeted 25-Hydroxy25-hydroxy vitamin D Concentration Measurements and 25-hydroxy vitamin D3 Supplementation Can Have Important Patient and Public Health Benefits. Eur. J. Clin. Nutr. 2020, 74, 366–376. [Google Scholar] [CrossRef]
- Norman, K.; Pichard, C.; Lochs, H.; Pirlich, M. Prognostic Impact of Disease-Related Malnutrition. Clin. Nutr. Edinb. Scotl. 2008, 27, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Caccialanza, R.; Laviano, A.; Lobascio, F.; Montagna, E.; Bruno, R.; Ludovisi, S.; Corsico, A.G.; Di Sabatino, A.; Belliato, M.; Calvi, M.; et al. Early Nutritional Supplementation in Non-Critically Ill Patients Hospitalized for the 2019 Novel Coronavirus Disease (COVID-19): Rationale and Feasibility of a Shared Pragmatic Protocol. Nutrition 2020, 74, 110835. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Rong, L.; Nian, W.; He, Y. Review Article: Gastrointestinal Features in COVID-19 and the Possibility of Faecal Transmission. Aliment. Pharmacol. Ther. 2020, 51, 843–851. [Google Scholar] [CrossRef]
- Panarese, A.; Pesce, F.; Porcelli, P.; Riezzo, G.; Iacovazzi, P.A.; Leone, C.M.; De Carne, M.; Rinaldi, C.M.; Shahini, E. Chronic Functional Constipation Is Strongly Linked to 25-hydroxy vitamin D Deficiency. World J. Gastroenterol. 2019, 25, 1729–1740. [Google Scholar] [CrossRef]
- Kong, J.; Zhu, X.; Shi, Y.; Liu, T.; Chen, Y.; Bhan, I.; Zhao, Q.; Thadhani, R.; Li, Y.C. VDR Attenuates Acute Lung Injury by Blocking Ang-2-Tie-2 Pathway and Renin-Angiotensin System. Mol. Endocrinol. Baltim. Md. 2013, 27, 2116–2125. [Google Scholar] [CrossRef] [PubMed]
- Iddir, M.; Brito, A.; Dingeo, G.; Fernandez Del Campo, S.S.; Samouda, H.; La Frano, M.R.; Bohn, T. Strengthening the Immune System and Reducing Inflammation and Oxidative Stress through Diet and Nutrition: Considerations during the COVID-19 Crisis. Nutrients 2020, 12, 1562. [Google Scholar] [CrossRef]
- Mahdavi, A.M. A Brief Review of Interplay between 25-hydroxy vitamin D and Angiotensin-Converting Enzyme 2: Implications for a Potential Treatment for COVID-19. Rev. Med. Virol. 2020, 30, e2119. [Google Scholar] [CrossRef]
- Panarese, A.; Shahini, E. Letter: Covid-19, and 25-hydroxy vitamin D. Aliment. Pharmacol. Ther. 2020, 51, 993–995. [Google Scholar] [CrossRef] [PubMed]
- Marik, P.E.; Kory, P.; Varon, J. Does 25-hydroxy vitamin D Status Impact Mortality from SARS-CoV-2 Infection? Med. Drug Discov. 2020, 6, 100041. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, T.; Yao, L.; Xing, Y.; Zhao, X.; Fu, J.; Xue, X. Chronic 25-hydroxy vitamin D Deficiency Induces Lung Fibrosis through Activation of the Renin-Angiotensin System. Sci. Rep. 2017, 7, 3312. [Google Scholar] [CrossRef] [PubMed]
- Tsujino, I.; Ushikoshi-Nakayama, R.; Yamazaki, T.; Matsumoto, N.; Saito, I. Pulmonary Activation of 25-hydroxy vitamin D3 and Preventive Effect against Interstitial Pneumonia. J. Clin. Biochem. Nutr. 2019, 65, 245–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez, N.; Aguilar-Jimenez, W.; Rugeles, M.T. The Potential Protective Role of 25-hydroxy vitamin D Supplementation on HIV-1 Infection. Front. Immunol. 2019, 10, 2291. [Google Scholar] [CrossRef] [PubMed]
- Hanff, T.C.; Harhay, M.O.; Brown, T.S.; Cohen, J.B.; Mohareb, A.M. Is There an Association Between COVID-19 Mortality and the Renin-Angiotensin System? A Call for Epidemiologic Investigations. Clin. Infect. Dis. 2020, 71, 870–874. [Google Scholar] [CrossRef] [Green Version]
- Mohammad, S.; Mishra, A.; Ashraf, M.Z. Emerging Role of 25-hydroxy vitamin D and Its Associated Molecules in Pathways Related to Pathogenesis of Thrombosis. Biomolecules 2019, 9, 649. [Google Scholar] [CrossRef] [Green Version]
- Entrenas Castillo, M.; Entrenas Costa, L.M.; Vaquero Barrios, J.M.; Alcalá Díaz, J.F.; López Miranda, J.; Bouillon, R.; Quesada Gomez, J.M. Effect of Calcifediol Treatment and Best Available Therapy versus Best Available Therapy on Intensive Care Unit Admission and Mortality among Patients Hospitalized for COVID-19: A Pilot Randomized Clinical Study. J. Steroid Biochem. Mol. Biol. 2020, 203, 105751. [Google Scholar] [CrossRef]
- Su, W.; Chen, Y.; Wang, C.; Ding, X.; Rwibasira, G.; Kong, Y. Human Cathelicidin LL-37 Inhibits Platelet Aggregation and Thrombosis via Src/PI3K/Akt Signaling. Biochem. Biophys. Res. Commun. 2016, 473, 283–289. [Google Scholar] [CrossRef]
- Zhang, J.; McCullough, P.A.; Tecson, K.M. 25-hydroxy vitamin D Deficiency in Association with Endothelial Dysfunction: Implications for Patients with COVID-19. Rev. Cardiovasc. Med. 2020, 21, 339–344. [Google Scholar] [CrossRef]
- Tian, Y.; Rong, L. Letter: Covid-19, and 25-hydroxy vitamin D. Authors’ Reply. Aliment. Pharmacol. Ther. 2020, 51, 995–996. [Google Scholar] [CrossRef] [Green Version]
- Weir, E.K.; Thenappan, T.; Bhargava, M.; Chen, Y. Does 25-hydroxy vitamin D Deficiency Increase the Severity of COVID-19? Clin. Med. 2020, 20, e107–e108. [Google Scholar] [CrossRef]
- Rhodes, J.M.; Subramanian, S.; Laird, E.; Kenny, R.A. Editorial: Low Population Mortality from COVID-19 in Countries South of Latitude 35 Degrees North Supports 25-hydroxy vitamin D as a Factor Determining Severity. Aliment. Pharmacol. Ther. 2020, 51, 1434–1437. [Google Scholar] [CrossRef]
- Jakovac, H. COVID-19 and 25-hydroxy vitamin D-Is There a Link and an Opportunity for Intervention? Am. J. Physiol. Endocrinol. Metab. 2020, 318, E589. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.J.; Murphy, K.E.; Fernandez, M.L. Impact of Obesity and Metabolic Syndrome on Immunity. Adv. Nutr. 2016, 7, 66–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carter, S.J.; Baranauskas, M.N.; Fly, A.D. Considerations for Obesity, 25-hydroxy vitamin D, and Physical Activity Amid the COVID-19 Pandemic. Obes. Silver Spring Md. 2020, 28, 1176–1177. [Google Scholar] [CrossRef] [PubMed]
- Dhama, K.; Sharun, K.; Tiwari, R.; Dadar, M.; Malik, Y.S.; Singh, K.P.; Chaicumpa, W. COVID-19, an Emerging Coronavirus Infection: Advances and Prospects in Designing and Developing Vaccines, Immunotherapeutics, and Therapeutics. Hum. Vaccines Immunother. 2020, 16, 1232–1238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, I.D.; Crofts, C.A.P.; DiNicolantonio, J.J.; Malhotra, A.; Elliott, B.; Kyriakidou, Y.; Brookler, K.H. Relationships between Hyperinsulinaemia, Magnesium, 25-hydroxy vitamin D, Thrombosis and COVID-19: Rationale for Clinical Management. Open Heart. 2020, 7, e001356. [Google Scholar] [CrossRef]
- La Vignera, S.; Cannarella, R.; Condorelli, R.A.; Torre, F.; Aversa, A.; Calogero, A.E. Sex-Specific SARS-CoV-2 Mortality: Among Hormone-Modulated ACE2 Expression, Risk of Venous Thromboembolism and Hypovitaminosis D. Int. J. Mol. Sci. 2020, 21, 2948. [Google Scholar] [CrossRef]
- Walker, R.F.; Zakai, N.A.; MacLehose, R.F.; Cowan, L.T.; Adam, T.J.; Alonso, A.; Lutsey, P.L. Association of Testosterone Therapy with Risk of Venous Thromboembolism Among Men with and without Hypogonadism. JAMA Intern. Med. 2020, 180, 190–197. [Google Scholar] [CrossRef]
- Salciccia, S.; Del Giudice, F.; Gentile, V.; Mastroianni, C.M.; Pasculli, P.; Di Lascio, G.; Ciardi, M.R.; Sperduti, I.; Maggi, M.; De Berardinis, E.; et al. Interplay between Male Testosterone Levels and the Risk for Subsequent Invasive Respiratory Assistance among COVID-19 Patients at Hospital Admission. Endocrine 2020, 70, 206–210. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, Y.; Yang, Z. The Cytokine Storm of Severe Influenza and Development of Immunomodulatory Therapy. Cell. Mol. Immunol. 2016, 13, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Labudzynskyi, D.; Shymanskyy, I.; Veliky, M. Role of 25-hydroxy vitamin D3 in Regulation of Interleukin-6 and Osteopontin Expression in Liver of Diabetic Mice. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 2916–2919. [Google Scholar]
- Gopal Samy, B.; Xavier, L. Molecular Docking Studies on Antiviral Drugs for SARS. Int. J. Adv. Res. Comput. Sci. Softw. Eng. 2015, 5, 75–79. [Google Scholar]
- Joseph, T.M.; Mahapatra, D.K. 5-Lipoxygenase and Phospholipase A2 inhibitory potentials of alcoholic extract of Cyperus rotundus: In vitro and in silico study. Res. Rev. J. Pharmacol. 2018, 8, 1–5. [Google Scholar]
- Garikapati, M.; Adzhigirey, M.; Day, T.; Annabhimoju, R.; Sherman, W. Protein and Ligand Preparation: Parameters, Protocols, and Influence on Virtual Screening Enrichments. J. Comput. Aided Mol. Des. 2013, 27, 221–234. [Google Scholar] [CrossRef]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra Precision Glide: Docking and Scoring Incorporating a Model of Hydrophobic Enclosure for Protein−Ligand Complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kallingal, A.; ThachanKundil, V.; Ayyolath, A.; Muringayil Joseph, T.; Kar Mahapatra, D.; Haponiuk, J.T.; Variyar, E.J. Identification of sustainable trypsin active-site inhibitors from Nigrospora sphaerica strain AVA-1. J. Basic. Microbiol. 2021, 61, 709–720. [Google Scholar] [CrossRef]
- Joseph, T.M.; Mahapatra, D.K. InSilico Molecular Docking of Cuminaldehyde and its Bioconverted Ligands as Lipoxygenase and Cyclooxygenase-2 Inhibitors. Inventi. Impact. Mol. Modeling. 2017, 2017, 118–121. [Google Scholar]
- Deng, X.; StJohn, S.E.; Osswald, H.L.; O’Brien, A.; Banach, B.S.; Sleeman, K.; Ghosh, A.K.; Mesecar, A.D.; Baker, S.C. Coronaviruses resistant to a 3C-like protease inhibitor are attenuated for replication and pathogenesis, revealing a low genetic barrier but high fitness cost of resistance. J. Virol. 2014, 88, 11886–11898. [Google Scholar] [CrossRef] [Green Version]
- Taylor, R.D.; MacCoss, M.; Lawson, A.D. Rings in drugs: Miniperspective. J. Med. Chem. 2014, 57, 5845–5859. [Google Scholar] [CrossRef]
- Craik, D.J.; Fairlie, D.P.; Liras, S.; Price, D. The future of peptide-based drugs. Chem. Biol. Drug Des. 2013, 81, 136–147. [Google Scholar] [CrossRef]
- Qayyum, S.; Mohammad, T.; Slominski, R.M.; Hassan, M.I.; Tuckey, R.C.; Raman, C.; Slominski, A.T. 25-hydroxy vitamin D and lumisterol novel metabolites can inhibit SARS-CoV-2 replication machinery enzymes. Am. J. Physiol. Endocrinol. Metab. 2021, 321, E246–E251. [Google Scholar] [CrossRef]
- Tiwari, A.; Singh, G.; Choudhir, G.; Motiwale, M.; Joshi, N.; Sharma, V.; Srivastava, R.K.; Sharma, S.; Tutone, M.; Singour, P.K. Deciphering the Potential of Pre and Pro-25-hydroxy vitamin D of Mushrooms against Mpro and PLpro Proteases of COVID-19: An In Silico Approach. Molecules 2022, 27, 5620. [Google Scholar] [CrossRef]
- Al-Mazaideh, G.M.; Shalayel, M.H.; Al-Swailmi, F.K.; Aladaileh, S.H. 25-hydroxy vitamin D is a New Promising Inhibitor to the Main Protease (Mpro) of COVID-19 by Molecular Docking. J. Pharm. Res. Int. 2021, 33, 86–191. [Google Scholar]
- Shalayel, M.H.; Al-Mazaideh, G.M.; Aladaileh, S.H.; Al-Swailmi, F.K.; Al-Thiabat, M.G. 25-hydroxy vitamin D is a potential inhibitor of COVID-19: In silico molecular docking to the binding site of SARS-CoV-2 endoribonuclease Nsp15. Pak. J. Pharm. Sci. 2020, 33, 2179–2186. [Google Scholar]
- Maghsoudi, S.; Taghavi Shahraki, B.; Rameh, F.; Nazarabi, M.; Fatahi, Y.; Akhavan, O.; Rabiee, M.; Mostafavi, E.; Lima, E.C.; Saeb, M.R.; et al. A review on computer-aided chemogenomics and drug repositioning for rational COVID-19 drug discovery. Chem. Biol. Drug Des. 2022, 100, 699–721. [Google Scholar] [CrossRef]
- Aliabadi, H.A.M.; Eivazzadeh-Keihan, R.; Beig Parikhani, A.; Fattahi Mehraban, S.; Maleki, A.; Fereshteh, S.; Bazaz, M.; Zolriasatein, A.; Bozorgnia, B.; Rahmati, S.; et al. COVID-19: A systematic review and update on prevention, diagnosis, and treatment. MedComm 2022, 3, e115. [Google Scholar]
- Rabiee, N.; Akhavan, O.; Fatahi, Y.; Ghadiri, A.M.; Kiani, M.; Makvandi, P.; Rabiee, M.; Nicknam, M.H.; Saeb, M.R.; Varma, R.S.; et al. CaZnO-based nanoghosts for the detection of ssDNA, pCRISPR and recombinant SARS-CoV-2 spike antigen and targeted delivery of doxorubicin. Chemosphere 2022, 306, 135578. [Google Scholar] [CrossRef] [PubMed]
- Rabiee, N.; Fatahi, Y.; Ahmadi, S.; Abbariki, N.; Ojaghi, A.; Rabiee, M.; Radmanesh, F.; Dinarvand, R.; Bagherzadeh, M.; Mostafavi, E.; et al. Bioactive hybrid metal-organic framework (MOF)-based nanosensors for optical detection of recombinant SARS-CoV-2 spike antigen. Sci. Total Environ. 2022, 825, 153902. [Google Scholar] [CrossRef]
- Al-Hazmi, H.E.; Shokrani, H.; Shokrani, A.; Jabbour, K.; Abida, O.; Khadem, S.S.M.; Habibzadeh, S.; Sonawane, S.H.; Saeb, M.R.; Bonilla-Petriciolet, A.; et al. Recent advances in aqueous virus removal technologies. Chemosphere 2022, 12, 135441. [Google Scholar] [CrossRef] [PubMed]







| Residue | Amino Acid | Distance H-A | Distance D-A | Donor Angle |
|---|---|---|---|---|
| 143A | GLY | 2.00 | 2.87 | 145.90 |
| 144A | SER | 3.65 | 3.99 | 104.01 |
| 163A | HIS | 1.77 | 2.37 | 116.24 |
| 164A | HIS | 1.85 | 2.80 | 161.75 |
| 166A | GLU | 2.60 | 3.48 | 144.50 |
| Drug | Average Binding Energy (kcal/mol) | Coulomb | vdW | Lipo | Bond | Packing | Solve GB |
|---|---|---|---|---|---|---|---|
| 25-hydroxy vitamin D | −57.947 | −12.462 | 33.426 | −17.876 | −0.815 | 0.00 | 27.28 |
| Lopinavir | −58.193 | −12.677 | 35.818 | −18.394 | −1.33 | 0.33 | 33.72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joseph, T.M.; Suresh, A.M.; Kar Mahapatra, D.; Haponiuk, J.; Thomas, S. The Efficacious Benefit of 25-Hydroxy Vitamin D to Prevent COVID-19: An In-Silico Study Targeting SARS-CoV-2 Spike Protein. Nutrients 2022, 14, 4964. https://0-doi-org.brum.beds.ac.uk/10.3390/nu14234964
Joseph TM, Suresh AM, Kar Mahapatra D, Haponiuk J, Thomas S. The Efficacious Benefit of 25-Hydroxy Vitamin D to Prevent COVID-19: An In-Silico Study Targeting SARS-CoV-2 Spike Protein. Nutrients. 2022; 14(23):4964. https://0-doi-org.brum.beds.ac.uk/10.3390/nu14234964
Chicago/Turabian StyleJoseph, Tomy Muringayil, Akshay Maniyeri Suresh, Debarshi Kar Mahapatra, Józef Haponiuk, and Sabu Thomas. 2022. "The Efficacious Benefit of 25-Hydroxy Vitamin D to Prevent COVID-19: An In-Silico Study Targeting SARS-CoV-2 Spike Protein" Nutrients 14, no. 23: 4964. https://0-doi-org.brum.beds.ac.uk/10.3390/nu14234964