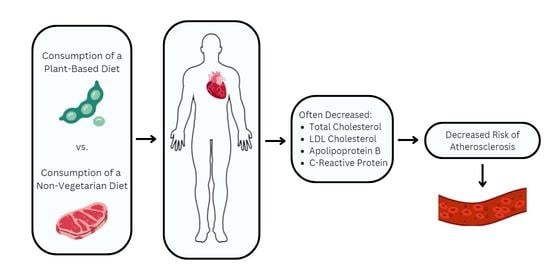
Plant-Based Diets and Lipid, Lipoprotein, and Inflammatory Biomarkers of Cardiovascular Disease: A Review of Observational and Interventional Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Process
- Population: The chosen population was adults aged 18 years or older because primordial prevention of atherosclerosis and CVD is important and should be prioritised for adults of all ages. In addition, dietary effects on biomarkers of CVD are not age dependent.
- Intervention/Exposure: Only studies looking at PBDs administered as an intervention or those habitually following PBDs (i.e., vegans or vegetarians), or PBD scores/indices as measured by food frequency questionnaire, were eligible for inclusion because the objective of this review is to summarise the literature on plant-based dietary patterns and biomarkers of CVD. Vegan diets were defined as exclusively PBDs, whereas vegetarian diets were defined as consisting of plant foods, while permitting any amount of dairy and/or eggs, and trace amounts of meat and/or fish (<1 serving/d), so as to include cohort studies where the vegetarian groups consumed negligible amounts of either meat or fish.
- Comparison: Intervention and cohort studies that compared PBDs versus other dietary patterns were included to highlight differences between them. PBD scores/indices, compared by quintiles or assessed by continuous measures, were included to observe the effects of eating a more or less PBD, and not necessarily a fully PBD, on established biomarkers of CVD.
- Outcomes: Lipid, lipoprotein, and inflammatory outcomes were eligible for inclusion to ensure that the associations between PBDs, administered as an intervention or followed habitually, and important biomarkers of CVD were captured.
- Study type: Low-quality study types, e.g., case reports/series were excluded.
2.2. Data Extraction
3. Results
3.1. Randomised Controlled Trials of Plant-Based Diets and the Lipid Profile
3.1.1. Vegan Dietary Interventions and the Lipid Profile
3.1.2. Vegetarian Dietary Interventions and the Lipid Profile
3.1.3. Summary of Randomised Controlled Trials Investigating Vegan and Vegetarian Dietary Interventions and the Lipid Profile
| Reference | Country | Population (n) | Sex | Age (Years) | Intervention (n) | Study Length/Design | Outcomes | * Results | Significance |
|---|---|---|---|---|---|---|---|---|---|
| Acharya et al. [42] | USA | Overweight/obese (143) | M/F | LOV-D: 45.2; STD-D: 43.5 | LOV-D (64) vs. STD-D (79) | 6 months (parallel) | TC, LDL-C, HDL-C, TGs, | Changes from baseline (%): LOV-D: TC: −4.7, LDL-C: −6.1, HDL-C: −5.5, TGs: −3.8. STD-D: TC: −1.2, LDL-C: −4.2, HDL-C: −3.0, TGs: −1.26 | Both diets lowered lipid outcomes from baseline, but differences between diets were non-significant (p > 0.05) |
| Ågren et al. [34] | Finland | Rheumatoid arthritis (29) | M/F | VG: 49.0; NVD: 53.0 | VG (16) vs. NVD (13) | 3 months (parallel) | TC, LDL-C, HDL-C, TGs | TC: −0.94; LDL-C: −0.74; HDL-C: −0.16; TGs: −0.11 | p < 0.001 for TC and LDL-C; p > 0.05 (ns) for HDL-C and TGs |
| Barnard et al. [26] | USA | Healthy pre-menopausal women (35) | F | All: 36.1 | LFVG vs. usual diet + placebo pill | 5 menstrual cycles for each arm (crossover) | TC, LDL-C, HDL-C, VLDL-C, TGs | TC: −0.54; LDL-C: −0.3; HDL-C: −0.2; VLDL-C: +0.08; TGs: +0.18 | p < 0.001 for all but TGs (p < 0.01) |
| Barnard et al. [29] | USA | T2DM (99) | M/F | LFVG: 56.7; ADA: 54.6 | LFVG (49) vs. ADA-recommended diet (50) | 22 weeks (parallel) | TC, non-HDL-C, LDL-C, HDL-C, VLDL-C, TGs | ITT analysis: TC: −0.09; non-HDL-C: −0.05; LDL-C: −0.03; HDL-C: −0.05; VLDL-C: +0.03; TGs: −0.04; Medication-change-adjusted analysis: TC: −0.38; non-HDL-C: −0.29; LDL-C: −0.31; HDL-C: −0.08; VLDL-C: +0.01; TGs: +0.01 | ns (p > 0.05) difference between groups for all outcomes in ITT analysis; significantly lower TC (p = 0.01), non-HDL-C (p = 0.05) and LDL-C (p = 0.02) in analyses adjusted for medication changes. |
| Barnard et al. [30] | USA | T2DM (99) | M/F | LFVG: 56.7; ADA: 54.6 | LFVG (49) vs. ADA-recommended diet (50) | 74 weeks (parallel) | TC, non-HDL-C, LDL-C, HDL-C, VLDL-C, TGs | ITT analysis: TC: −0.18; non-HDL-C: −0.21; LDL-C: −0.11; HDL-C: +0.01; VLDL-C: −0.02; TGs: −0.29; Medication-change-adjusted analysis: TC: −0.35; non-HDL-C: −0.35; LDL-C: −0.26; HDL-C: −0.01; VLDL-C: −0.05; TGs: −0.32 | ns (p > 0.05) difference between groups for all outcomes in ITT analysis; significantly lower TC (p = 0.01), non-HDL-C (p = 0.02) and LDL-C (p = 0.03) in analyses adjusted for medication changes. |
| Barnard et al. [31] | USA | T2DM (45) | M/F | LFVG: 61.0; portion-controlled: 61.0 | LFVG (21) vs. portion-controlled group (24) | 20 weeks (parallel) | TC, LDL-C, HDL-C, TGs | TC: + 0.21; LDL-C: +0.02; HDL-C: +0.03; TGs: +0.52 | ns (p > 0.05) difference between groups for all outcomes |
| Barnard et al. [28] | USA | Overweight (62) | M/F | LFVG: 58.3; MD: 56.6 | LFVG (30) vs. MD (32) | 36 weeks: 16 weeks × 2 (crossover) with a 4-week washout in between | TC, LDL-C, HDL-C, TGs, VLDL-C | TC: −0.29; LDL-C: −0.28; HDL-C: −0.11; TGs: +0.23; VLDL-C: +0.11 | Treatment effect: p = 0.04 for TC and LDL-C; p = 0.009 for HDL-C; p = 0.01 for TGs and VLDL-C |
| Burke et al. [41] | USA | Overweight/obese (176) | M/F | LOV-D: 45.4; STD-D: 43.3 | LOV-D (90) vs. STD-D (96) | 18 months: 12-month intervention, 6-month maintenance phase (parallel) | TC, TGs | Changes given in %: STD-D baseline to 18 months (preference group yes/no): TC: −1.4/+2.5; TGs: +1.0/−6.7; LOV-D (preference group yes/no): TC: +1.0/−0.1; TGs: +8.6/−5.5 | ns (p > 0.05) difference between groups for all outcomes |
| Cooper et al. [44] | USA | Healthy (15) | M/F | All: 28.0 | LOV vs. typical USA diet | 6 weeks: 3 weeks × 2 (crossover) | TC, LDL-C, HDL-C, TGs | TC: −0.52; LDL-C: −0.41; HDL-C: −0.10; TGs: −0.02 | p < 0.05 for TC; p < 0.025 for LDL-C; ns (p > 0.05) for other outcomes |
| Djekic et al. [46] | Sweden | Overweight (31) | M/F | LOV: 67.0; NVD: 68.0 | Isocaloric LOV (16) vs. NVD (15) [both adhering to Nordic Recommendations] | 12 weeks: 4 weeks × 2 (crossover) with a 4-week washout in between | TC, LDL-C, HDL-C, TGs | TC: −0.13; LDL-C: −0.10; HDL-C: −0.03; TGs: +0.06 | p = 0.01 for TC, p = 0.02 for LDL-C; ns (p > 0.05) for all other outcomes |
| Elkan et al. [35] | Sweden | Rheumatoid arthritis (66) | M/F | VG: 50.0; NVD: 50.8 | VG gluten-free (38) vs. NVD (28) | 12 months (parallel) | TC, LDL-C, HDL-C, TGs | TC: −1.2; LDL-C: −1.1; HDL-C: 0.0; TGs: 0.0 | p < 0.001 for LDL-C; no significance test reported for difference between diet groups for all other outcomes |
| Ferdowsian et al. [27] | USA | Overweight and T2DM (113) | M/F | 21 to 65 | LFVG (68) vs. usual-diet control (45) | 22 weeks (parallel) | TC, LDL-C, HDL-C, TGs | TC: −0.21; LDL-C: −0.08; HDL-C: −0.10; TGs: −0.20 | p = 0.002 for HDL-C; ns (p > 0.05) for all other outcomes |
| Gardner et al. [49] | USA | Healthy and overweight (120) | M/F | LFLOV: M: 48.0 & F: 48.0; LFD: M: 49.0 & F: 46.0 | LFLOV (59) vs. Eucaloric LFD (61) | 4 weeks (parallel) | TC, LDL-C, HDL-C, TGs | TC: −0.22, LDL-C: −0.18, HDL-C: −0.04; TGs: −0.01 | Lower TC (p = 0.01) and LDL-C (p = 0.02); ns differences for HDL-C and TGs |
| Gonciulea and Sellmeyer [47] | USA | Overweight and pre-menopausal (173) | F | APD: 62.7; DPD: 64.5; NSPD: 62.2; SPD: 64.6 | Energy- and protein-matched APD vs. DPD vs. NSPD vs. SPD | 6 weeks (parallel) | TC, LDL-C, HDL-C, TGs | SPD vs. APD: TC: −0.56; LDL-C: −0.43; HDL-C: −0.14; TGs: +0.06; SPD vs. DPD: TC: −0.77; LDL-C: −0.69; HDL-C: −0.16; TGs: +0.14; NSPD vs. APD: TC: −0.35; LDL-C: −0.26; HDL-C: −0.09; TGs: +0.05; NSPD vs. DPD: TC: −0.56; LDL-C: −0.49; HDL-C: −0.11; TGs: +0.13 | SPD vs. APD: p < 0.001 for TC and LDL-C, p = 0.008 for HDL-C; SPD vs. DPD: p < 0.001 for TC and LDL-C, p = 0.003 for HDL-C; NSPD vs. APD: p = 0.02 for TC, p = 0.04 for HDL-C; NSPD vs. DPD: p = 0.003 for TC, p = 0.005 for LDL-C, p = 0.05 for HDL-C; all other results ns (p > 0.05) |
| Hall et al. [37] | USA | Overweight (20) | M/F | All: 29.9 | LFPBD vs. ABKD | 4 weeks: 2 weeks × 2 (crossover) | TC, LDL-C, HDL-C, TGs | TC: −1.11; LDL-C: −0.72; HDL-C: −0.25; TGs: +0.34 | p < 0.001 for all |
| Hunt et al. [45] | USA | Healthy (21) | F | All: 33.2 | LOV vs. NVD | 8 weeks: 4 weeks × 2 (crossover) | TC, LDL-C, HDL-C, TGs | TC: −0.37; LDL-C: −0.25; HDL-C: −0.14; TGs: +0.06 | p = 0.001 for TC and LDL-C; p = 0.05 for HDL-C; p > 0.05 (ns) for TGs |
| Jenkins et al. [38] | Canada | Hyperlipidaemic (34) | M/F | All: 58.4 | Statin vs. Portfolio Diet vs. low saturated fat control diet | 3 × 1 month (crossover) intervention periods with a 2-to-6-week washout period between | TC, LDL-C, HDL-C, TGs | TC: −1.12; LDL-C: −0.99; HDL-C: +0.04; TGs: −0.38 | p < 0.005 for TC and LDL-C; ns (p > 0.05) for HDL-C and TGs; results were non-significantly different for all included outcomes |
| Jenkins et al. [39] | Canada | Overweight and hyperlipidaemia (44) | M/F | LCPBD: 56.1; LFLOV: 57.8 | LCPBD (22) vs. LFLOV (22) | 1-month parallel, metabolically controlled study | TC, LDL-C, HDL-C, TGs | LCPBD: TC: −1.34; LDL-C: −0.96; HDL-C: −0.05; TGs: −0.86; LFLOV: TC: −0.83; LDL-C: −0.57; HDL-C: −0.08; TGs: −0.45 | LCPBD had significantly lower TC (p = 0.001), LDL-C (p = 0.002), and TGs (p = 0.02) vs. LFLOV; ns (p > 0.05) changes in HDL-C between groups |
| Jenkins et al. [40] | Canada | Overweight and hyperlipidaemia (39) | M/F | LCPBD: 57.6; LFLOV: 55.3 | LCPBD (20) vs. LFLOV (19) | 6 months (parallel) | TC, LDL-C, HDL-C, TGs | LCPBD: TC: −0.66; LDL-C: −0.47; HDL-C: +0.04; TGs: −0.73; LFLOV: TC: −0.26; LDL-C: 0.00; HDL-C: −0.01; TGs: −0.45 | LCPBD had significantly lower TC (p < 0.001), LDL-C (p < 0.001), and TGs (p = 0.005) vs. LFLOV; ns (p > 0.05) changes in HDL-C between groups |
| Kahleova et al. [23] | USA | Overweight (222) | M/F | LFVG: 53.0; Control: 57.0 | LFVG (117) vs. usual diet control (105) | 16 weeks (parallel) | TC, LDL-C, HDL-C, TGs | TC: −0.6; LDL-C: −0.5; HDL-C: −0.01; TGs +0.20 | p < 0.001 for TC and LDL-C; p = 0.02 for TGs; ns difference for HDL-C |
| Ling et al. [36] | Finland | Healthy (18) | M/F | VG: 48.0; NVD: 37.5 | Uncooked VG (including fermented foods) vs. mixed NVD | 4 weeks (parallel) | TC, LDL-C, HDL-C, TGs | TC: −0.77; LDL-C: −0.74; HDL-C: −0.09; TGs: −0.31 | No significance tests were conducted between groups. The VG diet significantly lowered TC (p < 0.001), LDL-C (p < 0.001), HDL-C (p < 0.01), and TGs (p < 0.05) vs. baseline values. |
| Mishra et al. [24] | USA | Overweight and T2DM (291) | M/F | LFVG: 44.3; Control: 46.1 | LFVG (142) vs. usual-diet control (149) | 18 weeks (parallel) | TC, LDL-C, HDL-C, TGs | TC: −0.21; LDL-C: −0.19; HDL-C: −0.07; TGs: +0.13 | p < 0.01 for TC, LDL-C, and HDL-C; p < 0.05 for TGs |
| Nicholson et al. [32] | USA | T2DM (11) | M/F | LFVG: 51; Conventional LFD: 60 | LFVG (7) vs. conventional LFD (4) | 12 weeks (parallel) | TC, HDL-C, TGs | TC: 0.00; HDL-C: −0.18; TGs: +0.19 | p < 0.05 for HDL-C, ns (p > 0.05) for TC and TGs |
| Shah et al. [33] | USA | Coronary artery disease (100) | M/F | VG: 63.0; AHA: 59.5 | VG (50) vs. AHA-recommended diet (50) | 8 weeks (parallel) | TC, non-HDL-C; LDL-C, HDL-C, TGs | TC: −0.13; non-HDL-C: 0.00; LDL-C: −0.21; TGs: +0.11 | ns (p > 0.0015) differences between groups for all outcomes using linear regression analysis (Bonferroni correction applied) |
| Sofi et al. [43] | Italy | Overweight/obese with elevated TC or LDL-C or TGs or glucose (118) | M/F | LCLOV: 49.5; LCMD: 52.0 | Isocaloric hypocaloric LCLOV vs. LCMD | 6 months: 3 months × 2 (crossover) | TC, LDL-C, HDL-C, TGs | TC: −0.14; LDL-C: −0.24 mmol/L; HDL-C: −0.03; TGs: +0.14 | p ≤ 0.01 for LDL-C and TGs; ns (p > 0.05) for other outcomes |
| Soroka et al. [48] | Israel | Chronic renal failure (9) | M/F | 30 to 85 | Soya-based vegetarian low-protein diet vs. animal-based low-protein diet | 12 months: 6 months × 2 (crossover) | TC, LDL-C, HDL-C, TGs | TC: −0.03; LDL-C: −0.10; HDL-C: −0.07; TGs: +0.56 | ns (p > 0.05) for all comparisons |
| Wright et al. [25] | New Zealand | Overweight/obese with comorbidities (49) | M/F | All: 56.0 | LFVG (25) vs. control (normal GP care; 24) | 6 months (parallel) | TC, LDL-C, HDL-C, TGs | TC: −0.5; LDL-C: −0.4; HDL-C: −0.2; TGs: +0.2; Excluding dropouts: LFVG vs. control for TC: −0.56 | p = 0.001 for HDL-C; ns (p > 0.05) for all other differences in outcomes; p = 0.05 for differences in TC excluding dropouts |
3.2. Cohort Studies of Plant-Based Diets and the Lipid Profile
3.3. Randomised Controlled Trials of Plant-Based Diets and the Lipoprotein Profile
3.3.1. Vegan Dietary Interventions and the Lipoprotein Profile
3.3.2. Vegetarian Dietary Interventions and Apolipoprotein B Concentrations
3.3.3. Summary of Vegan and Vegetarian Dietary Interventions and the Lipoprotein Profile
3.4. Randomised Controlled Trials of Plant-Based Diets and the Inflammatory Profile
3.4.1. Vegan Dietary Interventions and the Inflammatory Profile
3.4.2. Vegetarian Dietary Interventions and the Inflammatory Profile
3.4.3. Summary of Vegan and Vegetarian Dietary Interventions and the Inflammatory Profile
| Reference | Country | Population (n) | Sex | Age (Years) | Intervention (n) | Study Length (Design) | Outcomes | * Results | Significance |
|---|---|---|---|---|---|---|---|---|---|
| Acharya et al. [42] | USA | Overweight/obese (143) | M/F | LOV-D: 45.2; STD-D: 43.5 | LOV-D (64) vs. STD-D (79) | 6 months (parallel) | Adiponectin (µg/mL) | Changes from baseline (%): LOV-D: +9.4; STD-D: +7.2 (difference: +2.2) | ns (p = 0.45) difference between groups |
| Barnard et al. [30] | USA | T2DM (99) | M/F | LFVG: 56.7; ADA: 54.6 | LFVG (49) vs. ADA-recommended diet (50) | 74 weeks (parallel) | CRP (mg/L) | ITT analysis: −5.0 | ns (p = 0.65) difference between groups |
| Dinu et al. [54] | Italy | Healthy (118) | M/F | LOV: 50.5; MD: 52 | LOV (54) vs. MD (53) | 3 months | Leptin (ng/mL), adiponectin (µg/mL), LAR, resistin (ng/mL) | LOV: leptin: −0.58, adiponectin: +0.49, LAR: −0.12, resistin: −0.12; MD: leptin: −1.35 (difference: +0.77), adiponectin: +0.45 (difference: +0.04), LAR: −0.17 (difference +0.05), resistin: +0.04 (difference: −0.16) | ns (p > 0.05) difference between groups for all outcomes |
| Djekic et al. [46] | Sweden | Overweight (31) | M/F | LOV: 67.0; NVD: 68.0 | Isocaloric LOV (16) vs. NVD (15) [both adhering to Nordic Recommendations] | 12 weeks: 4 weeks × 2 (crossover) with a 4-week washout in between | hsCRP (mg/L) | Difference: −0.09 | ns (p = 0.6) difference between groups |
| Elkan et al. [35] | Sweden | Rheumatoid arthritis (66) | M/F | VG: 50.0; NVD: 50.8 | VG gluten-free (38) vs. NVD (28) | 12 months (parallel) | CRP (mg/L) | VG: −8; NVD: −10 (difference: +2) | no significance test reported for difference between diet groups |
| Hall et al. [37] | USA | Overweight (20) | M/F | All: 29.9 | LFPBD vs. ABKD | 4 weeks: 2 weeks × 2 (crossover) | hsCRP (mg/L) | LFPBD: −0.9; ABKD: 0 (difference: −0.9) | p = 0.003 |
| Jenkins et al. [39] | Canada | Overweight with hyperlipidaemia (44) | M/F | LCPBD: 56.1; LFLOV: 57.8 | LCPBD (22) vs. LFLOV (22) | 1-month parallel, metabolically controlled study | hsCRP (mg/L) | LCPBD: −0.89; LFLOV: −0.69 (difference: −0.2) | ns (p = 0.66) difference between groups |
| Jenkins et al. [40] | Canada | Overweight with hyperlipidaemia (39) | M/F | LCPBD: 57.6; LFLOV: 55.3 | LCPBD (20) vs. LFLOV (19) | 6 months (parallel) | hsCRP (mg/dL) | LCPBD: −0.4; LFLOV: −0.2 (difference: −0.2) | ns (p = 0.082) difference between groups |
| Lederer et al. [52] | Germany | Healthy (53) | M/F | VG: 33.2; OD: 29.9 | VG (26) vs. meat-rich diet (27) | 4 weeks (w/ pre-treatment controlled mixed diet for 1 week) | Leukocytes (thousands/μL), monocytes (thousands/μL), hsCRP (mg/dL), lymphocytes (thousands/μL) | VG: hsCRP: −0.2, leukocytes: −0.6, lymphocytes: −35.7, monocytes: −0.03; Meat-rich diet: CRP: +0.2 (difference: −0.04), leukocytes: 0 (difference: −0.06), lymphocytes: +0.8 (difference: −35.78), monocytes: +0.03 (difference: −0.06) | Leukocytes (p = 0.003), monocytes (p = 0.032); ns (p > 0.05) differences for all other outcomes |
| Shah et al. [33] | USA | Coronary artery disease (100) | M/F | VG: 63.0; AHA: 59.5 | VG (50) vs. AHA-recommended diet (50) | 8 weeks (parallel) | hsCRP (mg/L), WBC count (K/μL), NLR | Adjusted β for VG vs. AHA-recommended diet (as reference): hsCRP: 0.67, WBC count: 1.06, NLR: 1.20 | hsCRP (p = 0.02); ns (p > 0.05) differences for all other outcomes |
| Sofi et al. [43] | Italy | Overweight or obesity with elevated TC or LDL-C or TGs or glucose (118) | M/F | LCLOV: 49.5; LCMD: 52.0 | Isocaloric hypocaloric LCLOV vs. LCMD | 6 months: 3 months × 2 (crossover) | WBC count (× 10³/mm³), IL-6 (pg/mL), TNF-α (pg/mL) | LOV: WBC count: +0.16, IL-6: +0.07, TNF-α: +0.45; MD: WBC count: −0.09 (difference: +0.25), IL-6: −0.09 (difference: +0.16), TNF-α: −0.34 (difference: +0.79) | ns (p > 0.05) differences for all outcomes |
| Wells et al. [53] | USA | Healthy (21) | M | 59 to 78 | LOV (10) vs beef-containing diet (11) | 12 weeks (w/ pre-treatment vegetarian diet for 2 weeks) | WBC count (10⁹/L) | LOV: −0.2; Beef-containing diet: +0.5 (difference: −0.7) | no significance test reported for difference between diet groups |
3.5. Plant-Based Diet Indices and Lipid and Inflammatory Profiles
3.5.1. Prospective Cohort Studies of Plant-Based Diet Indices and the Lipid Profile
3.5.2. Cross-Sectional Studies of Plant-Based Diet Indices and the Lipid Profile
3.5.3. Summary of Studies Investigating Plant-Based Diet Indices and the Lipid Profile
| Reference | Country | Population (n) | Sex | Age (Years) | Intervention (n) | Outcome(s) | Results | Significance |
|---|---|---|---|---|---|---|---|---|
| Alvarez-Alvarez et al. [62] | Spain | Overweight/obese with metabolic syndrome (6,874) | M/F | 64 to 65 | PVG | LDL-C, HDL-C, TGs | Regression β coefficient for pro-vegetarian diet index (mmol/L): LDL-C: −0.724 (−1.622, 0.173); HDL-C: −0.039 (−0.328, 0.249); TGs: 1.120 (−0.860, 3.101) | p > 0.05 for all |
| Amini et al. [55] | Iran | Healthy (178) | M/F | 67.0 | Adherence to PDI, hPDI, uPDI | TC, LDL-C, HDL-C, TGs | T3 vs. T1 for hPDI (HDL-C): +0.11 mmol/L; for uPDI (HDL-C): +0.09 mmol/L | p = 0.02 for both; ns differences in all other outcomes |
| González-Ortiz et al. [61] | Sweden | Chronic kidney disease patients (418) | M | 71.0 | Adherence to PDI | Hyperlipidaemia (TC > 5.2, TGs > 1.71 or treatment with lipid-lowering medications) | No significant difference across quintiles of PDI adherence in rates of hyperlipidaemia | p-trend = 0.82 |
| Oncina-Cánovas et al. [63] | Spain | Overweight/obese with metabolic syndrome (6,439) | M/F | 64.5 to 65.7 | Adherence to gPVG, hPVG, uPVG | HDL-C, TGs | Q5 vs. Q1 of gPVG: β: +0.07 (0.00, 0.14) for HDL-C; uPVG: β = +0.08 (0.02, 0.13) for TGs and = −0.11 (−0.18, −0.04) for HDL-C. Per 5-unit increment in uPVG: β: −0.02 (−0.04, −0.01) for HDL-C and +0.02 (0.01, 0.03) for TGs. Non-significant associations between the hPVG and outcomes of interest | p = 0.046 for gPVG; p = 0.003 (TGs) and p = 0.001 (HDL-C) for uPVG (Q5 vs. Q1) |
| Weston et al. [60] | Jackson Heart Study (USA) | Healthy (3,635) | M/F | 51.9 to 55.5 | Adherence to PDI, hPDI, uPDI (tertiles) | TC | T3 vs. T1 for PDI: +0.2; for hPDI: +0.02; for uPDI = −0.02 | p-trend for PDI = 0.001; p-trend for hPDI = 0.133; p-trend for uPDI = 0.551 |
3.5.4. Cross-Sectional Studies of Plant-Based Diet Indices and the Inflammatory Profile
3.5.5. Summary of Studies Investigating Plant-Based Diet Indices and the Inflammatory Profile
| Reference | Country | Population (n) | Sex | Age (Years) | Intervention (n) | Outcome(s) | Results | Significance |
|---|---|---|---|---|---|---|---|---|
| Baden et al. [64] | USA | Healthy (831) | F | 45.0 | Adherence to overall PDI, hPDI, uPDI | Adiponectin (ng/mL), Leptin (ng/mL), hsCRP (mg/L), IL-6 (pg/mL) | Per 10-point increase in: PDI: adiponectin +1.1%, leptin: −1.7%, hsCRP: −7.5%, IL-6: +5.5%; hPDI: adiponectin +3.0%, leptin −7.2%, hsCRP −13.6%, IL-6 −0.7%; uPDI: adiponectin −1.6%, leptin +4.4%, hsCRP +3.3%, IL-6 +1.1% | PDI: IL-6 (p = 0.05); hPDI: adiponectin (p = 0.025), leptin (p < 0.001), hsCRP (p = 0.001); uPDI: leptin (p = 0.037); ns (p > 0.05) differences for all other outcomes |
| Gonzalez-Ortiz et al. [61] | Sweden | Chronic kidney disease patients (418) | M | 71.0 | Adherence to PDI | CRP (mg/L), IL-6 (ng/L) | Per unit increase (β) in PDI: CRP: −0.02 (−0.04 to −0.002), IL-6: −0.17 (- 0.33 to −0.001) | CRP (p = 0.03) and IL-6 (p = 0.04) |
| Pourreza et al. [65] | Iran | Overweight or obese (390) | F | 18 to 48 | Adherence to PDI, hPDI, uPDI | hsCRP (mg/L) | Per unit increase (β) in PDI: −0.01 (−0.12 to 0.10); hPDI: −0.06 (−0.15 to 0.03); uPDI: 0.07 (−0.01 to 0.17) | ns (p > 0.05) differences for all outcomes |
4. Discussion
4.1. Plant-Based Dietary Patterns and Cardiovascular Disease Risk
4.1.1. Vegans and Vegetarian Diets and Cardiovascular Disease Risk
4.1.2. Plant-Based Indices and Cardiovascular Disease
4.2. Plant Food Groups as Mediators of Plant-Based Dietary Pattern Associations with Cardiovascular Disease Risk
4.2.1. Plant Food Group Associations with Cardiovascular Disease Risk
4.2.2. Plant Food Group Effects on Lipid, Lipoprotein, and Inflammatory Biomarkers of Cardiovascular Disease
4.3. Mechanisms Underpinning Plant-Based Dietary Pattern Associations with Reduced Cardiovascular Risk
4.3.1. Lipid and Lipoprotein Profiles and Atherosclerotic Cardiovascular Disease
4.3.2. Inflammation and Atherosclerotic Cardiovascular Disease
4.4. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Gan, Z.H.; Cheong, H.C.; Tu, Y.K.; Kuo, P.H. Association between Plant-Based Dietary Patterns and Risk of Cardiovascular Disease: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Nutrients 2021, 13, 3952. [Google Scholar] [CrossRef] [PubMed]
- Rees, K.; Al-Khudairy, L.; Takeda, A.; Stranges, S. Vegan dietary pattern for the primary and secondary prevention of cardiovascular diseases. Cochrane Database Syst. Rev. 2021, 2, Cd013501. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT-Lancet Commission on Healthy Diets from Sustainable Food Systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef] [PubMed]
- Chai, B.C.; van der Voort, J.R.; Grofelnik, K.; Eliasdottir, H.G.; Klöss, I.; Perez-Cueto, F.J.A. Which Diet Has the Least Environmental Impact on Our Planet? A Systematic Review of Vegan, Vegetarian and Omnivorous Diets. Sustainability 2019, 11, 4110. [Google Scholar] [CrossRef] [Green Version]
- Hayek, M.; Harwatt, H.; Ripple, W.; Mueller, N. The carbon opportunity cost of animal-sourced food production on land. Nat. Sustain. 2021, 4, 21–24. [Google Scholar] [CrossRef]
- Poore, J.; Nemecek, T. Reducing food’s environmental impacts through producers and consumers. Science 2018, 360, 987–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. J. Prev. Cardiol. 2022, 29, 5–115. [Google Scholar] [CrossRef]
- Lichtenstein, A.H.; Appel, L.J.; Vadiveloo, M.; Hu, F.B.; Kris-Etherton, P.M.; Rebholz, C.M.; Sacks, F.M.; Thorndike, A.N.; Van Horn, L.; Wylie-Rosett, J. 2021 Dietary Guidance to Improve Cardiovascular Health: A Scientific Statement from the American Heart Association. Circulation 2021, 144, e472–e487. [Google Scholar] [CrossRef]
- Belardo, D.; Michos, E.D.; Blankstein, R.; Blumenthal, R.S.; Ferdinand, K.C.; Hall, K.; Klatt, K.; Natajaran, P.; Ostfeld, R.J.; Reddy, K.; et al. Practical, Evidence-Based Approaches to Nutritional Modifications to Reduce Atherosclerotic Cardiovascular Disease: An American Society for Preventive Cardiology Clinical Practice Statement. Am. J. Prev. Cardiol. 2022, 10, 100323. [Google Scholar] [CrossRef]
- Hedayatnia, M.; Asadi, Z.; Zare-Feyzabadi, R.; Yaghooti-Khorasani, M.; Ghazizadeh, H.; Ghaffarian-Zirak, R.; Nosrati-Tirkani, A.; Mohammadi-Bajgiran, M.; Rohban, M.; Sadabadi, F.; et al. Dyslipidemia and cardiovascular disease risk among the MASHAD study population. Lipids Health Dis. 2020, 19, 42. [Google Scholar] [CrossRef]
- Yusuf, S.; Hawken, S.; Ounpuu, S.; Dans, T.; Avezum, A.; Lanas, F.; McQueen, M.; Budaj, A.; Pais, P.; Varigos, J.; et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet 2004, 364, 937–952. [Google Scholar] [CrossRef] [PubMed]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behbodikhah, J.; Ahmed, S.; Elyasi, A.; Kasselman, L.J.; De Leon, J.; Glass, A.D.; Reiss, A.B. Apolipoprotein B and Cardiovascular Disease: Biomarker and Potential Therapeutic Target. Metabolites 2021, 11, 690. [Google Scholar] [CrossRef] [PubMed]
- Blake, G.J.; Otvos, J.D.; Rifai, N.; Ridker, P.M. Low-density lipoprotein particle concentration and size as determined by nuclear magnetic resonance spectroscopy as predictors of cardiovascular disease in women. Circulation 2002, 106, 1930–1937. [Google Scholar] [CrossRef]
- Rizzo, M.; Pernice, V.; Frasheri, A.; Berneis, K. Atherogenic lipoprotein phenotype and LDL size and subclasses in patients with peripheral arterial disease. Atherosclerosis 2008, 197, 237–241. [Google Scholar] [CrossRef]
- Garvey, W.T.; Kwon, S.; Zheng, D.; Shaughnessy, S.; Wallace, P.; Hutto, A.; Pugh, K.; Jenkins, A.J.; Klein, R.L.; Liao, Y. Effects of insulin resistance and type 2 diabetes on lipoprotein subclass particle size and concentration determined by nuclear magnetic resonance. Diabetes 2003, 52, 453–462. [Google Scholar] [CrossRef] [Green Version]
- Arsenault, B.J.; Lemieux, I.; Després, J.P.; Gagnon, P.; Wareham, N.J.; Stroes, E.S.; Kastelein, J.J.; Khaw, K.T.; Boekholdt, S.M. HDL particle size and the risk of coronary heart disease in apparently healthy men and women: The EPIC-Norfolk prospective population study. Atherosclerosis 2009, 206, 276–281. [Google Scholar] [CrossRef]
- Shah, P.K.; Lecis, D. Inflammation in atherosclerotic cardiovascular disease. F1000Research 2019, 8, F1000. [Google Scholar] [CrossRef] [Green Version]
- Lusis, A.J. Atherosclerosis. Nature 2000, 407, 233–241. [Google Scholar] [CrossRef]
- Ain, Q.U.; Sarfraz, M.; Prasesti, G.K.; Dewi, T.I.; Kurniati, N.F. Confounders in Identification and Analysis of Inflammatory Biomarkers in Cardiovascular Diseases. Biomolecules 2021, 11, 1464. [Google Scholar] [CrossRef]
- Sabatine, M.S.; Morrow, D.A.; Jablonski, K.A.; Rice, M.M.; Warnica, J.W.; Domanski, M.J.; Hsia, J.; Gersh, B.J.; Rifai, N.; Ridker, P.M.; et al. Prognostic significance of the Centers for Disease Control/American Heart Association high-sensitivity C-reactive protein cut points for cardiovascular and other outcomes in patients with stable coronary artery disease. Circulation 2007, 115, 1528–1536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koenig, W.; Löwel, H.; Baumert, J.; Meisinger, C. C-reactive protein modulates risk prediction based on the Framingham Score: Implications for future risk assessment: Results from a large cohort study in southern Germany. Circulation 2004, 109, 1349–1353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahleova, H.; Petersen, K.F.; Shulman, G.I.; Alwarith, J.; Rembert, E.; Tura, A.; Hill, M.; Holubkov, R.; Barnard, N.D. Effect of a Low-Fat Vegan Diet on Body Weight, Insulin Sensitivity, Postprandial Metabolism and Intramyocellular and Hepatocellular Lipid Levels in Overweight Adults: A Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e2025454. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Xu, J.; Agarwal, U.; Gonzales, J.; Levin, S.; Barnard, N.D. A multicenter randomized controlled trial of a plant-based nutrition program to reduce body weight and cardiovascular risk in the corporate setting: The GEICO study. Eur. J. Clin. Nutr. 2013, 67, 718–724. [Google Scholar] [CrossRef] [Green Version]
- Wright, N.; Wilson, L.; Smith, M.; Duncan, B.; McHugh, P. The BROAD study: A randomised controlled trial using a whole food plant-based diet in the community for obesity, ischaemic heart disease or diabetes. Nutr. Diabetes 2017, 7, e256. [Google Scholar] [CrossRef] [Green Version]
- Barnard, N.D.; Scialli, A.R.; Bertron, P.; Hurlock, D.; Edmonds, K.; Talev, L. Effectiveness of a low-fat vegetarian diet in altering serum lipids in healthy premenopausal women. Am. J. Cardiol. 2000, 85, 969–972. [Google Scholar] [CrossRef]
- Ferdowsian, H.R.; Barnard, N.D.; Hoover, V.J.; Katcher, H.I.; Levin, S.M.; Green, A.A.; Cohen, J.L. A multicomponent intervention reduces body weight and cardiovascular risk at a GEICO corporate site. Am. J. Health Promot. 2010, 24, 384–387. [Google Scholar] [CrossRef]
- Barnard, N.D.; Alwarith, J.; Rembert, E.; Brandon, L.; Nguyen, M.; Goergen, A.; Horne, T.; do Nascimento, G.F.; Lakkadi, K.; Tura, A.; et al. A Mediterranean Diet and Low-Fat Vegan Diet to Improve Body Weight and Cardiometabolic Risk Factors: A Randomized, Cross-Over Trial. J. Am. Coll. Nutr. 2022, 41, 127–139. [Google Scholar] [CrossRef]
- Barnard, N.D.; Cohen, J.; Jenkins, D.J.; Turner-McGrievy, G.; Gloede, L.; Jaster, B.; Seidl, K.; Green, A.A.; Talpers, S. A low-fat vegan diet improves glycemic control and cardiovascular risk factors in a randomized clinical trial in individuals with type 2 diabetes. Diabetes Care 2006, 29, 1777–1783. [Google Scholar] [CrossRef] [Green Version]
- Barnard, N.D.; Cohen, J.; Jenkins, D.J.; Turner-McGrievy, G.; Gloede, L.; Green, A.; Ferdowsian, H. A low-fat vegan diet and a conventional diabetes diet in the treatment of type 2 diabetes: A randomized, controlled, 74-wk clinical trial. Am. J. Clin. Nutr. 2009, 89, 1588s–1596s. [Google Scholar] [CrossRef] [Green Version]
- Barnard, N.D.; Levin, S.M.; Gloede, L.; Flores, R. Turning the Waiting Room into a Classroom: Weekly Classes Using a Vegan or a Portion-Controlled Eating Plan Improve Diabetes Control in a Randomized Translational Study. J. Acad. Nutr. Diet. 2018, 118, 1072–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicholson, A.S.; Sklar, M.; Barnard, N.D.; Gore, S.; Sullivan, R.; Browning, S. Toward improved management of NIDDM: A randomized, controlled, pilot intervention using a lowfat, vegetarian diet. Prev. Med. 1999, 29, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.; Newman, J.D.; Woolf, K.; Ganguzza, L.; Guo, Y.; Allen, N.; Zhong, J.; Fisher, E.A.; Slater, J. Anti-Inflammatory Effects of a Vegan Diet Versus the American Heart Association-Recommended Diet in Coronary Artery Disease Trial. Am. Heart J. 2018, 7, e011367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agren, J.J.; Tvrzicka, E.; Nenonen, M.T.; Helve, T.; Hänninen, O. Divergent changes in serum sterols during a strict uncooked vegan diet in patients with rheumatoid arthritis. Br. J. Nutr. 2001, 85, 137–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elkan, A.C.; Sjöberg, B.; Kolsrud, B.; Ringertz, B.; Hafström, I.; Frostegård, J. Gluten-free vegan diet induces decreased LDL and oxidized LDL levels and raised atheroprotective natural antibodies against phosphorylcholine in patients with rheumatoid arthritis: A randomized study. Arthritis Res. Ther. 2008, 10, R34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ling, W.H.; Laitinen, M.; Hänninen, O. Shifting from conventional diet to an uncooked vegan diet reversibly alters serum lipid and apolipoprotein levels. Nutr. Res. 1992, 12, 1431–1440. [Google Scholar] [CrossRef]
- Hall, K.D.; Guo, J.; Courville, A.B.; Boring, J.; Brychta, R.; Chen, K.Y.; Darcey, V.; Forde, C.G.; Gharib, A.M.; Gallagher, I.; et al. Effect of a plant-based, low-fat diet versus an animal-based, ketogenic diet on ad libitum energy intake. Nat. Med. 2021, 27, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.; Kendall, C.W.; Marchie, A.; Faulkner, D.A.; Wong, J.M.; de Souza, R.; Emam, A.; Parker, T.L.; Vidgen, E.; Lapsley, K.G.; et al. Effects of a dietary portfolio of cholesterol-lowering foods vs lovastatin on serum lipids and C-reactive protein. Jama 2003, 290, 502–510. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, D.J.; Wong, J.M.; Kendall, C.W.; Esfahani, A.; Ng, V.W.; Leong, T.C.; Faulkner, D.A.; Vidgen, E.; Greaves, K.A.; Paul, G.; et al. The effect of a plant-based low-carbohydrate (“Eco-Atkins”) diet on body weight and blood lipid concentrations in hyperlipidemic subjects. Arch. Intern. Med. 2009, 169, 1046–1054. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, D.J.; Wong, J.M.; Kendall, C.W.; Esfahani, A.; Ng, V.W.; Leong, T.C.; Faulkner, D.A.; Vidgen, E.; Paul, G.; Mukherjea, R.; et al. Effect of a 6-month vegan low-carbohydrate (‘Eco-Atkins’) diet on cardiovascular risk factors and body weight in hyperlipidaemic adults: A randomised controlled trial. BMJ Open 2014, 4, e003505. [Google Scholar] [CrossRef]
- Burke, L.E.; Hudson, A.G.; Warziski, M.T.; Styn, M.A.; Music, E.; Elci, O.U.; Sereika, S.M. Effects of a vegetarian diet and treatment preference on biochemical and dietary variables in overweight and obese adults: A randomized clinical trial. Am. J. Clin. Nutr. 2007, 86, 588–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acharya, S.D.; Brooks, M.M.; Evans, R.W.; Linkov, F.; Burke, L.E. Weight loss is more important than the diet type in improving adiponectin levels among overweight/obese adults. J. Am. Coll. Nutr. 2013, 32, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Dinu, M.; Pagliai, G.; Cesari, F.; Gori, A.M.; Sereni, A.; Becatti, M.; Fiorillo, C.; Marcucci, R.; Casini, A. Low-Calorie Vegetarian Versus Mediterranean Diets for Reducing Body Weight and Improving Cardiovascular Risk Profile: CARDIVEG Study (Cardiovascular Prevention with Vegetarian Diet). Circulation 2018, 137, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.S.; Goldberg, R.B.; Trevisan, M.; Tsong, Y.; Liu, K.; Stamler, J.; Rubenstein, A.; Scanu, A.M. The selective lipid-lowering effect of vegetarianism on low density lipoproteins in a cross-over experiment. Atherosclerosis 1982, 44, 293–305. [Google Scholar] [CrossRef]
- Hunt, J.R.; Matthys, L.A.; Johnson, L.K. Zinc absorption, mineral balance and blood lipids in women consuming controlled lactoovovegetarian and omnivorous diets for 8 week. Am. J. Clin. Nutr. 1998, 67, 421–430. [Google Scholar] [CrossRef] [Green Version]
- Djekic, D.; Shi, L.; Brolin, H.; Carlsson, F.; Särnqvist, C.; Savolainen, O.; Cao, Y.; Bäckhed, F.; Tremaroli, V.; Landberg, R.; et al. Effects of a Vegetarian Diet on Cardiometabolic Risk Factors, Gut Microbiota and Plasma Metabolome in Subjects with Ischemic Heart Disease: A Randomized, Crossover Study. J. Am. Heart Assoc. 2020, 9, e016518. [Google Scholar] [CrossRef]
- Gonciulea, A.R.; Sellmeyer, D.E. The effect of dietary protein source on serum lipids: Secondary data analysis from a randomized clinical trial. J. Clin. Lipidol. 2017, 11, 46–54. [Google Scholar] [CrossRef]
- Soroka, N.; Silverberg, D.S.; Greemland, M.; Birk, Y.; Blum, M.; Peer, G.; Iaina, A. Comparison of a vegetable-based (soya) and an animal-based low-protein diet in predialysis chronic renal failure patients. Nephron 1998, 79, 173–180. [Google Scholar] [CrossRef]
- Gardner, C.D.; Coulston, A.; Chatterjee, L.; Rigby, A.; Spiller, G.; Farquhar, J.W. The effect of a plant-based diet on plasma lipids in hypercholesterolemic adults: A randomized trial. Ann. Intern. Med. 2005, 142, 725–733. [Google Scholar] [CrossRef]
- Shang, P.; Shu, Z.; Wang, Y.; Li, N.; Du, S.; Sun, F.; Xia, Y.; Zhan, S. Veganism does not reduce the risk of the metabolic syndrome in a Taiwanese cohort. Asia Pac. J. Clin. Nutr. 2011, 20, 404–410. [Google Scholar]
- Chiu, Y.F.; Hsu, C.C.; Chiu, T.H.; Lee, C.Y.; Liu, T.T.; Tsao, C.K.; Chuang, S.C.; Hsiung, C.A. Cross-sectional and longitudinal comparisons of metabolic profiles between vegetarian and non-vegetarian subjects: A matched cohort study. Br. J. Nutr. 2015, 114, 1313–1320. [Google Scholar] [CrossRef] [PubMed]
- Lederer, A.K.; Maul-Pavicic, A.; Hannibal, L.; Hettich, M.; Steinborn, C.; Gründemann, C.; Zimmermann-Klemd, A.M.; Müller, A.; Sehnert, B.; Salzer, U.; et al. Vegan diet reduces neutrophils, monocytes and platelets related to branched-chain amino acids—A randomized, controlled trial. Clin. Nutr. 2020, 39, 3241–3250. [Google Scholar] [CrossRef]
- Wells, A.M.; Haub, M.D.; Fluckey, J.; Williams, D.K.; Chernoff, R.; Campbell, W.W. Comparisons of vegetarian and beef-containing diets on hematological indexes and iron stores during a period of resistive training in older men. J. Am. Diet. Assoc. 2003, 103, 594–601. [Google Scholar] [CrossRef] [Green Version]
- Dinu, M.; Colombini, B.; Pagliai, G.; Cesari, F.; Gori, A.; Giusti, B.; Marcucci, R.; Sofi, F. Effects of a dietary intervention with Mediterranean and vegetarian diets on hormones that influence energy balance: Results from the CARDIVEG study. Int. J. Food Sci. Nutr. 2020, 71, 362–369. [Google Scholar] [CrossRef]
- Amini, M.R.; Shahinfar, H.; Djafari, F.; Sheikhhossein, F.; Naghshi, S.; Djafarian, K.; Clark, C.C.; Shab-Bidar, S. The association between plant-based diet indices and metabolic syndrome in Iranian older adults. Nutr. Health 2021, 27, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Satija, A.; Bhupathiraju, S.N.; Rimm, E.B.; Spiegelman, D.; Chiuve, S.E.; Borgi, L.; Willett, W.C.; Manson, J.E.; Sun, Q.; Hu, F.B. Plant-Based Dietary Patterns and Incidence of Type 2 Diabetes in US Men and Women: Results from Three Prospective Cohort Studies. PLoS Med. 2016, 13, e1002039. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Lee, K.; Rebholz, C.M.; Kim, J. Plant-based diets and incident metabolic syndrome: Results from a South Korean prospective cohort study. PLoS Med. 2020, 17, e1003371. [Google Scholar] [CrossRef]
- Lee, K.; Kim, H.; Rebholz, C.M.; Kim, J. Association between Different Types of Plant-Based Diets and Risk of Dyslipidemia: A Prospective Cohort Study. Nutrients 2021, 13, 220. [Google Scholar] [CrossRef]
- Zhu, R.; Fogelholm, M.; Poppitt, S.D.; Silvestre, M.P.; Møller, G.; Huttunen-Lenz, M.; Stratton, G.; Sundvall, J.; Råman, L.; Jalo, E.; et al. Adherence to a Plant-Based Diet and Consumption of Specific Plant Foods-Associations with 3-Year Weight-Loss Maintenance and Cardiometabolic Risk Factors: A Secondary Analysis of the PREVIEW Intervention Study. Nutrients 2021, 13, 3916. [Google Scholar] [CrossRef] [PubMed]
- Weston, L.J.; Kim, H.; Talegawkar, S.A.; Tucker, K.L.; Correa, A.; Rebholz, C.M. Plant-based diets and incident cardiovascular disease and all-cause mortality in African Americans: A cohort study. PLoS Med. 2022, 19, e1003863. [Google Scholar] [CrossRef]
- González-Ortiz, A.; Xu, H.; Avesani, C.M.; Lindholm, B.; Cederholm, T.; Risérus, U.; Ärnlöv, J.; Espinosa-Cuevas, A.; Carrero, J.J. Plant-based diets, insulin sensitivity and inflammation in elderly men with chronic kidney disease. J. Nephrol. 2020, 33, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Alvarez, I.; Toledo, E.; Lecea, O.; Salas-Salvadó, J.; Corella, D.; Buil-Cosiales, P.; Zomeño, M.D.; Vioque, J.; Martinez, J.A.; Konieczna, J.; et al. Adherence to a priori dietary indexes and baseline prevalence of cardiovascular risk factors in the PREDIMED-Plus randomised trial. Eur. J. Nutr. 2020, 59, 1219–1232. [Google Scholar] [CrossRef] [PubMed]
- Oncina-Cánovas, A.; Vioque, J.; González-Palacios, S.; Martínez-González, M.; Salas-Salvadó, J.; Corella, D.; Zomeño, D.; Martínez, J.A.; Alonso-Gómez, Á.M.; Wärnberg, J.; et al. Pro-vegetarian food patterns and cardiometabolic risk in the PREDIMED-Plus study: A cross-sectional baseline analysis. Eur. J. Nutr. 2022, 61, 357–372. [Google Scholar] [CrossRef] [PubMed]
- Baden, M.Y.; Satija, A.; Hu, F.B.; Huang, T. Change in Plant-Based Diet Quality Is Associated with Changes in Plasma Adiposity-Associated Biomarker Concentrations in Women. J. Nutr. 2019, 149, 676–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pourreza, S.; Khademi, Z.; Mirzababaei, A.; Yekaninejad, M.S.; Sadeghniiat-Haghighi, K.; Naghshi, S.; Mirzaei, K. Association of plant-based diet index with inflammatory markers and sleep quality in overweight and obese female adults: A cross-sectional study. Int. J. Clin. Pract. 2021, 75, e14429. [Google Scholar] [CrossRef] [PubMed]
- Orlich, M.J.; Singh, P.N.; Sabaté, J.; Jaceldo-Siegl, K.; Fan, J.; Knutsen, S.; Beeson, W.L.; Fraser, G.E. Vegetarian dietary patterns and mortality in Adventist Health Study 2. JAMA Intern. Med. 2013, 173, 1230–1238. [Google Scholar] [CrossRef]
- Key, T.J.; Fraser, G.E.; Thorogood, M.; Appleby, P.N.; Beral, V.; Reeves, G.; Burr, M.L.; Chang-Claude, J.; Frentzel-Beyme, R.; Kuzma, J.W.; et al. Mortality in vegetarians and nonvegetarians: Detailed findings from a collaborative analysis of 5 prospective studies. Am. J. Clin. Nutr. 1999, 70, 516s–524s. [Google Scholar] [CrossRef] [Green Version]
- Crowe, F.L.; Appleby, P.N.; Travis, R.C.; Key, T.J. Risk of hospitalization or death from ischemic heart disease among British vegetarians and nonvegetarians: Results from the EPIC-Oxford cohort study. Am. J. Clin. Nutr. 2013, 97, 597–603. [Google Scholar] [CrossRef] [Green Version]
- Chiu, T.H.T.; Chang, H.R.; Wang, L.Y.; Chang, C.C.; Lin, M.N.; Lin, C.L. Vegetarian diet and incidence of total, ischemic and hemorrhagic stroke in 2 cohorts in Taiwan. Neurology 2020, 94, e1112–e1121. [Google Scholar] [CrossRef] [Green Version]
- Baden, M.Y.; Liu, G.; Satija, A.; Li, Y.; Sun, Q.; Fung, T.T.; Rimm, E.B.; Willett, W.C.; Hu, F.B.; Bhupathiraju, S.N. Changes in Plant-Based Diet Quality and Total and Cause-Specific Mortality. Circulation 2019, 140, 979–991. [Google Scholar] [CrossRef]
- Satija, A.; Bhupathiraju, S.N.; Spiegelman, D.; Chiuve, S.E.; Manson, J.E.; Willett, W.; Rexrode, K.M.; Rimm, E.B.; Hu, F.B. Healthful and Unhealthful Plant-Based Diets and the Risk of Coronary Heart Disease in US Adults. J. Am. Coll. Cardiol. 2017, 70, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Shan, Z.; Li, Y.; Baden, M.Y.; Bhupathiraju, S.N.; Wang, D.D.; Sun, Q.; Rexrode, K.M.; Rimm, E.B.; Qi, L.; Willett, W.C.; et al. Association between Healthy Eating Patterns and Risk of Cardiovascular Disease. JAMA Intern. Med. 2020, 180, 1090–1100. [Google Scholar] [CrossRef] [PubMed]
- Heianza, Y.; Zhou, T.; Sun, D.; Hu, F.B.; Manson, J.E.; Qi, L. Genetic susceptibility, plant-based dietary patterns and risk of cardiovascular disease. Am. J. Clin. Nutr. 2020, 112, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Bhupathiraju, S.N.; Sawicki, C.M.; Goon, S.; Gujral, U.P.; Hu, F.B.; Kandula, N.R.; Kanaya, A.M. A healthy plant-based diet is favorably associated with cardiometabolic risk factors among participants of South Asian ancestry. Am. J. Clin. Nutr. 2022, 116, 1078–1090. [Google Scholar] [CrossRef] [PubMed]
- Qian, F.; Liu, G.; Hu, F.B.; Bhupathiraju, S.N.; Sun, Q. Association Between Plant-Based Dietary Patterns and Risk of Type 2 Diabetes: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2019, 179, 1335–1344. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Song, X.Y.; Wu, J.; Chen, G.C.; Neelakantan, N.; van Dam, R.M.; Feng, L.; Yuan, J.M.; Pan, A.; Koh, W.P. Association between Dietary Patterns in Midlife and Healthy Ageing in Chinese Adults: The Singapore Chinese Health Study. J. Am. Med. Dir. Assoc. 2021, 22, 1279–1286. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- de Lorgeril, M.; Salen, P.; Martin, J.L.; Monjaud, I.; Delaye, J.; Mamelle, N. Mediterranean diet, traditional risk factors and the rate of cardiovascular complications after myocardial infarction: Final report of the Lyon Diet Heart Study. Circulation 1999, 99, 779–785. [Google Scholar] [CrossRef]
- Morze, J.; Danielewicz, A.; Hoffmann, G.; Schwingshackl, L. Diet Quality as Assessed by the Healthy Eating Index, Alternate Healthy Eating Index, Dietary Approaches to Stop Hypertension Score and Health Outcomes: A Second Update of a Systematic Review and Meta-Analysis of Cohort Studies. J. Acad. Nutr. Diet. 2020, 120, 1998–2031.e15. [Google Scholar] [CrossRef]
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-a systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef] [Green Version]
- Du, H.; Li, L.; Bennett, D.; Yang, L.; Guo, Y.; Key, T.J.; Bian, Z.; Chen, Y.; Walters, R.G.; Millwood, I.Y.; et al. Fresh fruit consumption and all-cause and cause-specific mortality: Findings from the China Kadoorie Biobank. Int. J. Epidemiol. 2017, 46, 1444–1455. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Ku, P.W.; Chou, P. Lifestyles and Mortality in Taiwan: An 11-Year Follow-Up Study. Asia Pac. J. Public Health 2017, 29, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Whole grain consumption and risk of cardiovascular disease, cancer and all cause and cause specific mortality: Systematic review and dose-response meta-analysis of prospective studies. BMJ 2016, 353, i2716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aune, D.; Norat, T.; Romundstad, P.; Vatten, L.J. Whole grain and refined grain consumption and the risk of type 2 diabetes: A systematic review and dose-response meta-analysis of cohort studies. Eur. J. Epidemiol. 2013, 28, 845–858. [Google Scholar] [CrossRef]
- Zong, G.; Gao, A.; Hu, F.B.; Sun, Q. Whole Grain Intake and Mortality from All Causes, Cardiovascular Disease and Cancer: A Meta-Analysis of Prospective Cohort Studies. Circulation 2016, 133, 2370–2380. [Google Scholar] [CrossRef] [Green Version]
- Song, M.; Fung, T.T.; Hu, F.B.; Willett, W.C.; Longo, V.D.; Chan, A.T.; Giovannucci, E.L. Association of Animal and Plant Protein Intake with All-Cause and Cause-Specific Mortality. JAMA Intern. Med. 2016, 176, 1453–1463. [Google Scholar] [CrossRef]
- Afshin, A.; Micha, R.; Khatibzadeh, S.; Mozaffarian, D. Consumption of nuts and legumes and risk of incident ischemic heart disease, stroke and diabetes: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2014, 100, 278–288. [Google Scholar] [CrossRef] [Green Version]
- Aune, D. Plant Foods, Antioxidant Biomarkers and the Risk of Cardiovascular Disease, Cancer and Mortality: A Review of the Evidence. Adv. Nutr. 2019, 10, S404–S421. [Google Scholar] [CrossRef] [Green Version]
- Marventano, S.; Izquierdo Pulido, M.; Sánchez-González, C.; Godos, J.; Speciani, A.; Galvano, F.; Grosso, G. Legume consumption and CVD risk: A systematic review and meta-analysis. Public Health Nutr. 2017, 20, 245–254. [Google Scholar] [CrossRef] [Green Version]
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Nut consumption and risk of cardiovascular disease, total cancer, all-cause and cause-specific mortality: A systematic review and dose-response meta-analysis of prospective studies. BMC Med. 2016, 14, 207. [Google Scholar] [CrossRef] [Green Version]
- Hooper, L.; Al-Khudairy, L.; Abdelhamid, A.S.; Rees, K.; Brainard, J.S.; Brown, T.J.; Ajabnoor, S.M.; O’Brien, A.T.; Winstanley, L.E.; Donaldson, D.H.; et al. Omega-6 fats for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2018, 7, Cd011094. [Google Scholar] [CrossRef] [PubMed]
- Dayton, S.; Pearce, M.L. Diet and atherosclerosis. Lancet 1970, 1, 473–474. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Guasch-Ferré, M.; Li, Y.; Hu, F.B. Dietary intake and biomarkers of linoleic acid and mortality: Systematic review and meta-analysis of prospective cohort studies. Am. J. Clin. Nutr. 2020, 112, 150–167. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Li, Y.; Chiuve, S.E.; Stampfer, M.J.; Manson, J.E.; Rimm, E.B.; Willett, W.C.; Hu, F.B. Association of Specific Dietary Fats with Total and Cause-Specific Mortality. JAMA Intern. Med. 2016, 176, 1134–1145. [Google Scholar] [CrossRef]
- Gunness, P.; Gidley, M.J. Mechanisms underlying the cholesterol-lowering properties of soluble dietary fibre polysaccharides. Food Funct. 2010, 1, 149–155. [Google Scholar] [CrossRef]
- Hollænder, P.L.; Ross, A.B.; Kristensen, M. Whole-grain and blood lipid changes in apparently healthy adults: A systematic review and meta-analysis of randomized controlled studies. Am. J. Clin. Nutr. 2015, 102, 556–572. [Google Scholar] [CrossRef] [Green Version]
- Jiao, J.; Xu, J.Y.; Zhang, W.; Han, S.; Qin, L.Q. Effect of dietary fiber on circulating C-reactive protein in overweight and obese adults: A meta-analysis of randomized controlled trials. Int. J. Food Sci. Nutr. 2015, 66, 114–119. [Google Scholar] [CrossRef]
- Mensink, R. Effects of Saturated Fatty Acids on Serum Lipids and Lipoproteins: A Systematic Review and Regression Analysis; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Del Gobbo, L.C.; Falk, M.C.; Feldman, R.; Lewis, K.; Mozaffarian, D. Effects of tree nuts on blood lipids, apolipoproteins and blood pressure: Systematic review, meta-analysis and dose-response of 61 controlled intervention trials. Am. J. Clin. Nutr. 2015, 102, 1347–1356. [Google Scholar] [CrossRef] [Green Version]
- Sabaté, J.; Oda, K.; Ros, E. Nut consumption and blood lipid levels: A pooled analysis of 25 intervention trials. Arch. Intern. Med. 2010, 170, 821–827. [Google Scholar] [CrossRef] [Green Version]
- Kris-Etherton, P.M.; Yu-Poth, S.; Sabaté, J.; Ratcliffe, H.E.; Zhao, G.; Etherton, T.D. Nuts and their bioactive constituents: Effects on serum lipids and other factors that affect disease risk. Am. J. Clin. Nutr. 1999, 70, 504s–511s. [Google Scholar] [CrossRef] [Green Version]
- Kozłowska-Wojciechowska, M.; Jastrzebska, M.; Naruszewicz, M.; Foltyńska, A. Impact of margarine enriched with plant sterols on blood lipids, platelet function and fibrinogen level in young men. Metabolism 2003, 52, 1373–1378. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Hu, F.B.; Ros, E.; Sabaté, J. The role of tree nuts and peanuts in the prevention of coronary heart disease: Multiple potential mechanisms. J. Nutr. 2008, 138, 1746s–1751s. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, G.; Etherton, T.D.; Martin, K.R.; Gillies, P.J.; West, S.G.; Kris-Etherton, P.M. Dietary alpha-linolenic acid inhibits proinflammatory cytokine production by peripheral blood mononuclear cells in hypercholesterolemic subjects. Am. J. Clin. Nutr. 2007, 85, 385–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazidi, M.; Rezaie, P.; Ferns, G.A.; Gao, H.K. Impact of different types of tree nut, peanut and soy nut consumption on serum C-reactive protein (CRP): A systematic review and meta-analysis of randomized controlled clinical trials. Medicine 2016, 95, e5165. [Google Scholar] [CrossRef]
- Li, S.S.; Blanco Mejia, S.; Lytvyn, L.; Stewart, S.E.; Viguiliouk, E.; Ha, V.; de Souza, R.J.; Leiter, L.A.; Kendall, C.W.C.; Jenkins, D.J.A.; et al. Effect of Plant Protein on Blood Lipids: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2017, 6, e006659. [Google Scholar] [CrossRef] [Green Version]
- Blanco Mejia, S.; Messina, M.; Li, S.S.; Viguiliouk, E.; Chiavaroli, L.; Khan, T.A.; Srichaikul, K.; Mirrahimi, A.; Sievenpiper, J.L.; Kris-Etherton, P.; et al. A Meta-Analysis of 46 Studies Identified by the FDA Demonstrates that Soy Protein Decreases Circulating LDL and Total Cholesterol Concentrations in Adults. J. Nutr. 2019, 149, 968–981. [Google Scholar] [CrossRef]
- Salehi-Abargouei, A.; Saraf-Bank, S.; Bellissimo, N.; Azadbakht, L. Effects of non-soy legume consumption on C-reactive protein: A systematic review and meta-analysis. Nutrition 2015, 31, 631–639. [Google Scholar] [CrossRef]
- Chiavaroli, L.; Nishi, S.K.; Khan, T.A.; Braunstein, C.R.; Glenn, A.J.; Mejia, S.B.; Rahelić, D.; Kahleová, H.; Salas-Salvadó, J.; Jenkins, D.J.A.; et al. Portfolio Dietary Pattern and Cardiovascular Disease: A Systematic Review and Meta-analysis of Controlled Trials. Prog. Cardiovasc. Dis. 2018, 61, 43–53. [Google Scholar] [CrossRef]
- Sniderman, A.D.; Thanassoulis, G.; Glavinovic, T.; Navar, A.M.; Pencina, M.; Catapano, A.; Ference, B.A. Apolipoprotein B Particles and Cardiovascular Disease: A Narrative Review. JAMA Cardiol. 2019, 4, 1287–1295. [Google Scholar] [CrossRef]
- Sniderman, A.D.; Islam, S.; Yusuf, S.; McQueen, M.J. Discordance analysis of apolipoprotein B and non-high density lipoprotein cholesterol as markers of cardiovascular risk in the INTERHEART study. Atherosclerosis 2012, 225, 444–449. [Google Scholar] [CrossRef]
- Pencina, M.J.; D’Agostino, R.B.; Zdrojewski, T.; Williams, K.; Thanassoulis, G.; Furberg, C.D.; Peterson, E.D.; Vasan, R.S.; Sniderman, A.D. Apolipoprotein B improves risk assessment of future coronary heart disease in the Framingham Heart Study beyond LDL-C and non-HDL-C. Eur. J. Prev. Cardiol. 2015, 22, 1321–1327. [Google Scholar] [CrossRef] [PubMed]
- Lawler, P.R.; Akinkuolie, A.O.; Ridker, P.M.; Sniderman, A.D.; Buring, J.E.; Glynn, R.J.; Chasman, D.I.; Mora, S. Discordance between Circulating Atherogenic Cholesterol Mass and Lipoprotein Particle Concentration in Relation to Future Coronary Events in Women. Clin. Chem. 2017, 63, 870–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkins, J.T.; Li, R.C.; Sniderman, A.; Chan, C.; Lloyd-Jones, D.M. Discordance between Apolipoprotein B and LDL-Cholesterol in Young Adults Predicts Coronary Artery Calcification: The CARDIA Study. J. Am. Coll. Cardiol. 2016, 67, 193–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borén, J.; Williams, K.J. The central role of arterial retention of cholesterol-rich apolipoprotein-B-containing lipoproteins in the pathogenesis of atherosclerosis: A triumph of simplicity. Curr. Opin. Lipidol. 2016, 27, 473–483. [Google Scholar] [CrossRef]
- Borén, J.; Chapman, M.J.; Krauss, R.M.; Packard, C.J.; Bentzon, J.F.; Binder, C.J.; Daemen, M.J.; Demer, L.L.; Hegele, R.A.; Nicholls, S.J.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: Pathophysiological, genetic and therapeutic insights: A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2020, 41, 2313–2330. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.; Cannon, C.P.; Murphy, S.A.; Qin, J.; Ray, K.K.; Braunwald, E. Impact of triglyceride levels beyond low-density lipoprotein cholesterol after acute coronary syndrome in the PROVE IT-TIMI 22 trial. J. Am. Coll. Cardiol. 2008, 51, 724–730. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, G.G.; Abt, M.; Bao, W.; DeMicco, D.; Kallend, D.; Miller, M.; Mundl, H.; Olsson, A.G. Fasting triglycerides predict recurrent ischemic events in patients with acute coronary syndrome treated with statins. J. Am. Coll. Cardiol. 2015, 65, 2267–2275. [Google Scholar] [CrossRef] [Green Version]
- Marston, N.A.; Giugliano, R.P.; Melloni, G.E.M.; Park, J.G.; Morrill, V.; Blazing, M.A.; Ference, B.; Stein, E.; Stroes, E.S.; Braunwald, E.; et al. Association of Apolipoprotein B-Containing Lipoproteins and Risk of Myocardial Infarction in Individuals with and Without Atherosclerosis: Distinguishing Between Particle Concentration, Type and Content. JAMA Cardiol. 2022, 7, 250–256. [Google Scholar] [CrossRef]
- Ference, B.A.; Kastelein, J.J.P.; Ray, K.K.; Ginsberg, H.N.; Chapman, M.J.; Packard, C.J.; Laufs, U.; Oliver-Williams, C.; Wood, A.M.; Butterworth, A.S.; et al. Association of Triglyceride-Lowering LPL Variants and LDL-C-Lowering LDLR Variants with Risk of Coronary Heart Disease. JAMA 2019, 321, 364–373. [Google Scholar] [CrossRef] [Green Version]
- Balling, M.; Afzal, S.; Varbo, A.; Langsted, A.; Davey Smith, G.; Nordestgaard, B.G. VLDL Cholesterol Accounts for One-Half of the Risk of Myocardial Infarction Associated with apoB-Containing Lipoproteins. J. Am. Coll. Cardiol. 2020, 76, 2725–2735. [Google Scholar] [CrossRef]
- Phillips, C.M.; Perry, I.J. Lipoprotein particle subclass profiles among metabolically healthy and unhealthy obese and non-obese adults: Does size matter? Atherosclerosis 2015, 242, 399–406. [Google Scholar] [CrossRef]
- Frazier-Wood, A.C.; Garvey, W.T.; Dall, T.; Honigberg, R.; Pourfarzib, R. Opportunities for using lipoprotein subclass profile by nuclear magnetic resonance spectroscopy in assessing insulin resistance and diabetes prediction. Metab. Syndr. Relat. Disord. 2012, 10, 244–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musunuru, K.; Orho-Melander, M.; Caulfield, M.P.; Li, S.; Salameh, W.A.; Reitz, R.E.; Berglund, G.; Hedblad, B.; Engström, G.; Williams, P.T.; et al. Ion mobility analysis of lipoprotein subfractions identifies three independent axes of cardiovascular risk. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1975–1980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoogeveen, R.C.; Gaubatz, J.W.; Sun, W.; Dodge, R.C.; Crosby, J.R.; Jiang, J.; Couper, D.; Virani, S.S.; Kathiresan, S.; Boerwinkle, E.; et al. Small dense low-density lipoprotein-cholesterol concentrations predict risk for coronary heart disease: The Atherosclerosis Risk In Communities (ARIC) study. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1069–1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, M.Y.; Steffen, B.T.; Guan, W.; McClelland, R.L.; Warnick, R.; McConnell, J.; Hoefner, D.M.; Remaley, A.T. New automated assay of small dense low-density lipoprotein cholesterol identifies risk of coronary heart disease: The Multi-ethnic Study of Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 196–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mora, S.; Caulfield, M.P.; Wohlgemuth, J.; Chen, Z.; Superko, H.R.; Rowland, C.M.; Glynn, R.J.; Ridker, P.M.; Krauss, R.M. Atherogenic Lipoprotein Subfractions Determined by Ion Mobility and First Cardiovascular Events After Random Allocation to High-Intensity Statin or Placebo: The Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) Trial. Circulation 2015, 132, 2220–2229. [Google Scholar] [CrossRef] [Green Version]
- Lamarche, B.; St-Pierre, A.C.; Ruel, I.L.; Cantin, B.; Dagenais, G.R.; Després, J.P. A prospective, population-based study of low density lipoprotein particle size as a risk factor for ischemic heart disease in men. Can. J. Cardiol. 2001, 17, 859–865. [Google Scholar]
- Freedman, D.S.; Otvos, J.D.; Jeyarajah, E.J.; Barboriak, J.J.; Anderson, A.J.; Walker, J.A. Relation of lipoprotein subclasses as measured by proton nuclear magnetic resonance spectroscopy to coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 1046–1053. [Google Scholar] [CrossRef] [Green Version]
- Carmena, R.; Duriez, P.; Fruchart, J.C. Atherogenic lipoprotein particles in atherosclerosis. Circulation 2004, 109, III-2–III-7. [Google Scholar] [CrossRef] [Green Version]
- Cromwell, W.C.; Otvos, J.D. Low-density lipoprotein particle number and risk for cardiovascular disease. Curr. Atheroscler. Rep. 2004, 6, 381–387. [Google Scholar] [CrossRef]
- Mora, S.; Szklo, M.; Otvos, J.D.; Greenland, P.; Psaty, B.M.; Goff, D.C., Jr.; O’Leary, D.H.; Saad, M.F.; Tsai, M.Y.; Sharrett, A.R. LDL particle subclasses, LDL particle size and carotid atherosclerosis in the Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis 2007, 192, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Ip, S.; Lichtenstein, A.H.; Chung, M.; Lau, J.; Balk, E.M. Systematic review: Association of low-density lipoprotein subfractions with cardiovascular outcomes. Ann. Intern. Med. 2009, 150, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Morze, J.; Melloni, G.E.; Rynkiewicz, A.; Gruchala, M.; Guasch-Ferre, M.; Ruff, C.T.; Hu, F.B.; Sabatine, M.S.; Marston, N.A. The relative importance of particle count, type and size of ApoB-containing lipoproteins in risk of myocardial infarction. Eur. Heart J. 2022, 43, 2295. [Google Scholar] [CrossRef]
- Toth, P.P.; Barter, P.J.; Rosenson, R.S.; Boden, W.E.; Chapman, M.J.; Cuchel, M.; D’Agostino, R.B.; Davidson, M.H.; Davidson, W.S.; Heinecke, J.W.; et al. High-density lipoproteins: A consensus statement from the National Lipid Association. J. Clin. Lipidol. 2013, 7, 484–525. [Google Scholar] [CrossRef]
- Mahdy Ali, K.; Wonnerth, A.; Huber, K.; Wojta, J. Cardiovascular disease risk reduction by raising HDL cholesterol--current therapies and future opportunities. Br. J. Pharmacol. 2012, 167, 1177–1194. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, G.G.; Olsson, A.G.; Abt, M.; Ballantyne, C.M.; Barter, P.J.; Brumm, J.; Chaitman, B.R.; Holme, I.M.; Kallend, D.; Leiter, L.A.; et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N. Engl. J. Med. 2012, 367, 2089–2099. [Google Scholar] [CrossRef] [Green Version]
- Lincoff, A.M.; Nicholls, S.J.; Riesmeyer, J.S.; Barter, P.J.; Brewer, H.B.; Fox, K.A.A.; Gibson, C.M.; Granger, C.; Menon, V.; Montalescot, G.; et al. Evacetrapib and Cardiovascular Outcomes in High-Risk Vascular Disease. N. Engl. J. Med. 2017, 376, 1933–1942. [Google Scholar] [CrossRef]
- von Eckardstein, A.; Nordestgaard, B.G.; Remaley, A.T.; Catapano, A.L. High-density lipoprotein revisited: Biological functions and clinical relevance. Eur. Heart J. 2022. [Google Scholar] [CrossRef]
- Rohatgi, A.; Khera, A.; Berry, J.D.; Givens, E.G.; Ayers, C.R.; Wedin, K.E.; Neeland, I.J.; Yuhanna, I.S.; Rader, D.R.; de Lemos, J.A.; et al. HDL cholesterol efflux capacity and incident cardiovascular events. N. Engl. J. Med. 2014, 371, 2383–2393. [Google Scholar] [CrossRef] [Green Version]
- Libby, P. Inflammation and cardiovascular disease mechanisms. Am. J. Clin. Nutr. 2006, 83, 456S–460S. [Google Scholar] [CrossRef] [Green Version]
- Sampson, U.K.; Fazio, S.; Linton, M.F. Residual cardiovascular risk despite optimal LDL cholesterol reduction with statins: The evidence, etiology and therapeutic challenges. Curr. Atheroscler. Rep. 2012, 14, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alfaddagh, A.; Martin, S.S.; Leucker, T.M.; Michos, E.D.; Blaha, M.J.; Lowenstein, C.J.; Jones, S.R.; Toth, P.P. Inflammation and cardiovascular disease: From mechanisms to therapeutics. Am. J. Prev. Cardiol. 2020, 4, 100130. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Cannon, C.P.; Morrow, D.; Rifai, N.; Rose, L.M.; McCabe, C.H.; Pfeffer, M.A.; Braunwald, E. C-reactive protein levels and outcomes after statin therapy. N. Engl. J. Med. 2005, 352, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Bohula, E.A.; Giugliano, R.P.; Cannon, C.P.; Zhou, J.; Murphy, S.A.; White, J.A.; Tershakovec, A.M.; Blazing, M.A.; Braunwald, E. Achievement of dual low-density lipoprotein cholesterol and high-sensitivity C-reactive protein targets more frequent with the addition of ezetimibe to simvastatin and associated with better outcomes in IMPROVE-IT. Circulation 2015, 132, 1224–1233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakynthinos, E.; Pappa, N. Inflammatory biomarkers in coronary artery disease. J. Cardiol. 2009, 53, 317–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Tardif, J.-C.; Kouz, S.; Waters, D.D.; Bertrand, O.F.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Ibrahim, R.; Gamra, H.; Kiwan, G.S.; et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N. Engl. J. Med. 2019, 381, 2497–2505. [Google Scholar] [CrossRef]
- Galkina, E.; Ley, K. Vascular Adhesion Molecules in Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2292–2301. [Google Scholar] [CrossRef]
- Ridker, P.M. From C-Reactive Protein to Interleukin-6 to Interleukin-1. Circ. Res. 2016, 118, 145–156. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Yang, X.; Bian, F.; Wu, P.; Xing, S.; Xu, G.; Li, W.; Chi, J.; Ouyang, C.; Zheng, T.; et al. TNF-α promotes early atherosclerosis by increasing transcytosis of LDL across endothelial cells: Crosstalk between NF-κB and PPAR-γ. J. Mol. Cell. Cardiol. 2014, 72, 85–94. [Google Scholar] [CrossRef]
- Hansson, G.K.; Libby, P.; Tabas, I. Inflammation and plaque vulnerability. J. Intern. Med. 2015, 278, 483–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Virmani, R.; Kolodgie, F.D.; Burke, A.P.; Farb, A.; Schwartz, S.M. Lessons from Sudden Coronary Death. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1262–1275. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J. Anatomic features in victims of sudden coronary death. Coronary artery pathology. Circulation 1992, 85, I19–I24. [Google Scholar] [PubMed]

| PICOS | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Population | Adults (>18 years old) | Children and adolescents (<18 years old) |
| Intervention/ Exposure | Plant-based diets (i.e., vegan/vegetarian diets) administered as an intervention, or plant-based dietary scores measured by food frequency questionnaire | Non-plant-based dietary patterns; unclear definitions or measurement of dietary exposures. |
| Comparison | For RCTs: control groups consisting of usual diet or another dietary pattern. For cohort studies: plant-based diet groups compared to non-vegetarian diet groups. For diet scores/indices, comparisons between quintiles or continuous measures (e.g., per 10-unit increment) were included | Comparisons where other exposures are included with diet vs. comparator, e.g., exercise, fasting, etc.; multifactorial interventions. |
| Outcomes | Lipid biomarkers: TC, LDL-C (directly measured LDL-C was included over calculated LDL-C where both were reported); HDL-C; Non-HDL-C; VLDL-C; TGs Lipoprotein biomarkers: VLDL-P concentrations; IDL-P concentrations; LDL-P concentrations; HDL-P concentrations; Non-HDL-P concentrations; apoB; Small, medium, large VLDL-P, LDL-P, HDL-P concentrations; Mean VLDL-P size; Mean LDL-P size; Mean HDL-P size Inflammatory biomarkers: C3; hsCRP/CRP; IL-6; TNF-α; Adiponectin; Leptin; LAR; Resistin; PAI-1; WBCs; Neutrophils; Lymphocytes; NLR; Monocytes; Basophils; Eosinophils; GlycA | All other outcomes Postprandial lipid outcomes Self-reported outcomes without objective measurements |
| Study type | RCTs (parallel, crossover, metabolic ward), prospective cohort studies, cross-sectional studies (only for studies using dietary scores/indices), systematic reviews and meta-analyses (of randomised controlled trials and/or prospective cohort studies) | Non-randomised trials, intervention trials without a control/comparator, reviews, case reports/series, editorials, commentaries, meeting abstracts, studies with legitimate expressions of concern |
| Search | Search Terms |
|---|---|
| #1: Population | Human adults aged 19+ (filter) |
| #2: Intervention/ Exposure | (“plant-based diet index” OR “plant-based diet” OR “plant-based dietary pattern” OR “plant-based diets” OR “plant-based diet scores” OR “plant-based dietary scores” OR “vegan” OR “vegan diet” OR “vegetarian” OR “vegetarian diet”) |
| #3: Study Types | (“cohort study” OR “follow-up” OR “randomized controlled trial” OR “randomised controlled trial” OR “RCT” OR “clinical trial” OR “meta-analysis” OR “cross-sectional” OR “case-control”) |
| #4: Outcomes | (“Lipids” OR “Plasma lipids” OR “Cholesterol” OR “Lipoproteins” OR “Subclasses” OR “Profiles” OR “Low-density lipoprotein” OR “LDL” OR “High-density lipoprotein” OR “HDL” OR “Triglycerides” OR “non-high-density lipoprotein” OR “non-HDL” OR “Small LDL” OR “Large LDL” OR “Intermediate-density lipoprotein” OR “IDL” OR “Very-low-density lipoprotein” OR “VLDL” OR “Apolipoprotein B” OR “apoB” OR “Inflammation” OR “inflammatory biomarkers” OR “complement component 3” OR “C3” OR “acute-phase response proteins” OR “APRPs” OR “high-sensitivity C-reactive protein” OR “hsCRP” OR “CRP” OR “C-reactive protein” OR “C reactive protein” OR “pro-inflammatory cytokines” OR “pro inflammatory cytokines” OR “interleukin 6” OR “IL6” OR “IL-6” OR “TNF-α” OR “TNFa” OR “TNF-alpha” OR “tumour necrosis factor alpha” OR “tumor necrosis factor alpha” OR “adipocytokines” OR “adiponectin” OR “leptin” OR “leptin-adiponectin ratio” OR “resistin” OR “PAI-1” OR “plasminogen activator inhibitor 1” OR “white blood cells” OR “white blood cell count” OR “leukocytes” OR “neutrophils” OR “lymphocytes” OR “neutrophil-lymphocyte ratio” OR “monocytes” OR “basophils” OR “eosinophils” OR “glycoprotein A” OR “GlycA”) |
| #5: Additional Filters | Language: English | PubMed: Title/Abstract | Scopus: Title/Abstract/Keywords |
| #6 | #1 AND #2 AND #3 AND #4 AND #5 |
| Reference | Cohort (Country) | Population (n) | Sex | Age (Years) | Exposure (n) | Follow-up | Outcomes | Results |
|---|---|---|---|---|---|---|---|---|
| Chiu et al. [51] | MJ Health Screening Database (Taiwan) | Healthy (5,734) | M/F | 48.9 for composite VD and NVD | LOV (624) vs. LV (173) vs. VG (159) vs. NVD (4778) | Median 2.12 years | High TC (≥ 5.17), high LDL-C (≥ 3.36), low HDL-C (< 1.03 [M]/1.29 [F]), high TGs (≥ 1.70) | Fully adjusted ORs (with 95% CIs): LOV vs. NVD: high TC: 0.99 (0.94, 1.03); high LDL-C: 1.01 (0.95, 1.06); low HDL-C: 1.08 (1.03, 1.12); high TGs: 1.04 (0.99, 1.09); VG vs. NVD: high TC: 0.97 (0.89, 1.06); high LDL-C: 0.92 (0.82, 1.04); low HDL-C: 1.04 (0.95, 1.14); high TGs: 1.00 (0.91, 1.10); LV vs. NVD: high TC: 1.05 (0.98, 1.13); high LDL-C: 1.01 (0.93, 1.10); low HDL-C: 0.99 (0.90, 1.10); high TGs: 1.02 (0.95, 1.11). p-values not reported. |
| Shang et al. [50] | MJ Health Screening Database (Taiwan) | Healthy (93,209) | M/F | NVD: 36.8; PV: 43.5; LOV: 37.9; VG: 44.1 | NVD (85,319) vs. PV (2461) vs. LOV (4313) vs. VG (1116) | Mean 3.75 years | Low HDL-C (< 1.03 [M]/1.29 [F]), high TGs (≥ 1.69) | Fully adjusted HRs (with 95% CIs): NVD vs. VG: low HDL-C: 0.72 (0.62, 0.84); high TGs: 0.86 (0.74, 1.09); PV vs. VG: low HDL-C: 0.70 (0.57, 0.84); high TGs: 0.85(0.71, 1.02); LOV vs. VG: low HDL-C: 0.98 (0.83, 1.17); high TGs: 0.92 (0.78, 1.09). p-values not reported. |
| Reference | Country | Population (n) | Sex | Age (Years) | Intervention | Study Length/ Design | Outcomes | * Results | Significance |
|---|---|---|---|---|---|---|---|---|---|
| Cooper et al. [44] | USA | Healthy (15) | M/F | All: 28.0 | LOV vs. typical USA diet | 6 weeks: 3 weeks × 2 (crossover) | apoB | −7.6 | p < 0.05 |
| Djekic et al. [46] | Sweden | Overweight (31) | M/F | LOV: 67.0; NVD: 68.0 | Isocaloric LOV (16) vs. NVD (15) [both adhering to Nordic Recommendations] | 12 weeks: 4 weeks × 2 (crossover) with a 4-week washout in between | apoB | −2.1 | ns (p > 0.05) |
| Hall et al. [37] | USA | Overweight (20) | M/F | All: 29.9 | LFPBD vs. ABKD | 4 weeks: 2 weeks × 2 (crossover) | VLDL-P, LDL-P, HDL-P, VLDL-P size, LDL-P size, HDL-P size, large LDL-P, medium LDL-P, small LDL-P, large HDL-P, medium HDL-P, small HDL-P, apoB | VLDL-P: +27.9; LDL-P: −443.0; HDL-P: −3.5; large LDL-P: +122.0; medium LDL-P: −176.0; small LDL-P: −438.0; large HDL-P: −1.0; medium HDL-P: +0.8; small HDL-P: −3.8; VLDL-P size: +1.6; LDL-P size: +0.1; HDL-P size: 0.0; apoB: −19.6 | p < 0.001 for all except large LDL-P (p = 0.002), medium LDL-P (p = 0.013), medium HDL-P (p = 0.05) and VLDL-P, LDL-P, and HDL-P size (all ns, or p > 0.05) |
| Hunt et al. [45] | USA | Healthy (21) | F | All: 33.2 | LOV vs. NVD | 8 weeks: 4 weeks × 2 (crossover) | apoB | −6 | p = 0.05 |
| Jenkins et al. [38] | Canada | Hyperlipidaemic (34) | M/F | All: 58.4 | Statin vs. Portfolio Diet vs. low-saturated-fat control diet | 3 × 1 month (crossover) intervention periods with a 2-to-6-week washout period between | apoB | −26 | p < 0.005; result for the Portfolio Diet vs. statin group was non-significantly different |
| Jenkins et al. [39] | Canada | Overweight and hyperlipidaemia (44) | M/F | LCPBD: 56.1; LFLOV: 57.8 | LCPBD (22) vs. LFLOV (22) | 1-month parallel, metabolically controlled study | apoB | LCPBD: −31; LFLOV diet: −19 | LCPBD had significantly lower apoB (p = 0.001) vs. LFLOV diet |
| Jenkins et al. [40] | Canada | Overweight and hyperlipidaemia (39) | M/F | LCPBD: 57.6; LFLOV: 55.3 | LCPBD (20) vs. LFLOV (19) | 6 months (parallel) | apoB | LCPBD: −22; LFLOV: −15 | LCPBD had significantly lower apoB (p < 0.001) vs. LFLOV diet |
| Ling et al. [36] | Finland | Healthy (18) | M/F | VG: 48.0; NVD: 37.5 | Uncooked VG (including fermented foods) vs. mixed NVD | 4 weeks (parallel) | apoB | −21 | No significance tests were conducted between groups. The VG diet significantly lowered apoB (p < 0.01) vs. baseline values |
| Shah et al. [33] | USA | CHD (100) | M/F | VG: 63.0; AHA: 59.5 | VG (50) vs. AHA-recommended diet (50) | 8 weeks (parallel) | LDL-P, HDL-P, large VLDL-P; small LDL-P; large HDL-P; VLDL-P size, LDL-P size; HDL-P size | LDL-P: −2; HDL-P: +3; large VLDL-P: 0; small LDL-P: +29; large HDL-P: −0.7; VLDL-P size: +1; LDL-P size: −0.1; HDL-P size: −0.1 | ns (p > 0.0015) differences between groups for all outcomes using linear regression analysis (Bonferroni correction applied) |
| Reference | Cohort (Country) | Population (n) | Sex | Age (Years) | Exposure (n) | Follow-up | Outcomes | Results |
|---|---|---|---|---|---|---|---|---|
| Kim et al. [57] | KoGES (Korea) | Healthy (5,646) | M/F | 49.0 to 52.4 | Adherence to PDI, hPDI, uPDI | Median of 8 years | Low HDL-C (<1.03 [M]/1.29 [F]), hypertriglyceridaemia (TGs > 1.70) | ns (p > 0.05) associations between PDI and hPDI and outcomes; HRs for Q5 vs. Q1 for uPDI: 1.25 (95% CI: 1.09, 1.43) for low HDL-C; 1.26 (95% CI: 1.08, 1.46) for hypertriglyceridaemia |
| Lee et al. [58] | KoGES (Korea) | Healthy (16,068) | M/F | 49.9 to 53.7 | Adherence to PDI, hPDI, uPDI | 14 years | Dyslipidaemia (One of the following: TGs ≥ 5.18; TC ≥ 6.12; HDL-C < 1.00; LDL-C ≥ 4.10; or use of any anti-dyslipidaemia medication) | Multivariable-adjusted HRs for highest vs. lowest quintiles for dyslipidaemia were 0.78 (95% CI: 0.69–0.88) for PDI, 0.63 (95% CI: 0.56–0.70) for hPDI, and 1.48 (95% CI: 1.30–1.69) for uPDI (p-trend < 0.001 for all) |
| Zhu et al. [59] | 8 European countries | Overweight/obese with pre-diabetes (710) | M/F | 57.0 | Novel plant-based diet score | 3 years | LDL-C | Fully adjusted result for longitudinal association with yearly changes in LDL-C: −0.03 (95% CI: −0.07, 0.001); ns (p = 0.057) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elliott, P.S.; Kharaty, S.S.; Phillips, C.M. Plant-Based Diets and Lipid, Lipoprotein, and Inflammatory Biomarkers of Cardiovascular Disease: A Review of Observational and Interventional Studies. Nutrients 2022, 14, 5371. https://0-doi-org.brum.beds.ac.uk/10.3390/nu14245371
Elliott PS, Kharaty SS, Phillips CM. Plant-Based Diets and Lipid, Lipoprotein, and Inflammatory Biomarkers of Cardiovascular Disease: A Review of Observational and Interventional Studies. Nutrients. 2022; 14(24):5371. https://0-doi-org.brum.beds.ac.uk/10.3390/nu14245371
Chicago/Turabian StyleElliott, Patrick S., Soraeya S. Kharaty, and Catherine M. Phillips. 2022. "Plant-Based Diets and Lipid, Lipoprotein, and Inflammatory Biomarkers of Cardiovascular Disease: A Review of Observational and Interventional Studies" Nutrients 14, no. 24: 5371. https://0-doi-org.brum.beds.ac.uk/10.3390/nu14245371