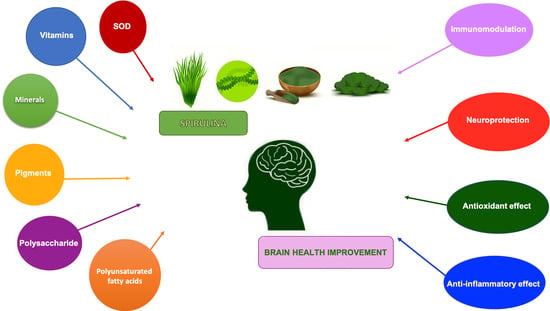
Beneficial Effects of Spirulina Consumption on Brain Health
Abstract
:1. Introduction
2. Role of Spirulina in the Brain
Effects of Spirulina on Glial Cells Activation
3. Beneficial Effects of Spirulina in Neurodegenerative Diseases
3.1. Spirulina in PD
3.2. Spirulina in AD
3.3. Spirulina in MS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kulshreshtha, A.; Zacharia, A.J.; Jarouliya, U.; Bhadauriya, P.; Prasad, G.B.K.S.; Bisen, P.S. Spirulina in health care management. Curr. Pharm. Biotechnol. 2008, 9, 400–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinha, S.; Patro, N.; Patro, I.K. Maternal Protein Malnutrition: Current and Future Perspectives of Spirulina Supplementation in Neuroprotection. Front. Neurosci. 2018, 12, 966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Romero, D.; Izquierdo-Vega, J.A.; Morales-González, J.A.; Madrigal-Bujaidar, E.; Chamorro-Cevallos, G.; Sánchez-Gutiérrez, M.; Betanzos-Cabrera, G.; Alvarez-Gonzalez, I.; Morales-González, Á.; Madrigal-Santillán, E. Evidence of Some Natural Products with Antigenotoxic Effects. Part 2: Plants, Vegetables, and Natural Resin. Nutrients 2018, 10, 1954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez-Salmeán, G.; Fabila-Castillo, L.; Chamorro-Cevallos, G. Nutritional and toxicological aspects of Spirulina (Arthrospira). Nutr. Hosp. 2015, 32, 34–40. [Google Scholar] [PubMed]
- Costa, J.A.V.; Freitas, B.C.B.; Rosa, G.M.; Moraes, L.; Morais, M.G.; Mitchell, B.G. Operational and economic aspects of Spirulina-based biorefinery. Bioresour. Technol. 2019, 292, 121946. [Google Scholar] [CrossRef] [PubMed]
- Marles, R.J.; Barrett, M.L.; Barnes, J.; Chavez, M.L.; Gardiner, P.; Ko, R.; Mahady, G.B.; Dog, T.L.; Sarma, N.D.; Giancaspro, G.I.; et al. United States pharmacopeia safety evaluation of spirulina. Crit. Rev. Food Sci. Nutr. 2011, 51, 593–604. [Google Scholar] [CrossRef]
- Hutadilok-Towatana, N.; Reanmongkol, W.; Panichayupakaranant, P. Evaluation of the toxicity of Arthrospira (Spirulina) platensis extract. J. Appl. Phycol. 2010, 22, 599–605. [Google Scholar] [CrossRef]
- Lafarga, T.; Fernández-Sevilla, J.M.; González-López, C.; Acién-Fernández, F.G. Spirulina for the food and functional food industries. Food Res. Int. 2020, 137, 109356. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, L.; Miron, A.; Klímová, B.; Wan, D.; Kuča, K. The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: An overview. Arch. Toxicol. 2016, 90, 1817–1840. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, Y.; Zhang, R.; Cai, T.; Cai, Y. Medical Application of Spirulina platensis Derived C-Phycocyanin. Evid. Based Complement. Alternat. Med. 2016, 2016, 7803846. [Google Scholar]
- Finkel, Z.V.; Follows, M.J.; Liefer, J.D.; Brown, C.M.; Benner, I.; Irwin, A.J. Phylogenetic Diversity in the Macromolecular Composition of Microalgae. PLoS ONE 2016, 11, e0155977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ljubic, A.; Safafar, H.; Holdt, S.L.; Jacobsen, C. Biomass composition of Arthrospira platensis during cultivation on industrial process water and harvesting. J. Appl. Phycol. 2018, 30, 943–954. [Google Scholar] [CrossRef] [Green Version]
- Madrigal-Santillán, E.; Madrigal-Bujaidar, E.; Álvarez-González, I.; Sumaya-Martínez, M.T.; Gutiérrez-Salinas, J.; Bautista, M.; Morales-González, Á.; García-Luna y González-Rubio, M.; Aguilar-Faisal, J.L.; Morales-González, J.A. Review of natural products with hepatoprotective effects. World J. Gastroenterol. 2014, 20, 14787–14804. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, M. Microalgae Nutraceuticals. Foods 2016, 5, 54. [Google Scholar] [CrossRef]
- Deng, R.; Chow, T.-J. Hypolipidemic, antioxidant, and antiinflammatory activities of microalgae Spirulina. Cardiovasc. Ther. 2010, 28, e33–e45. [Google Scholar] [CrossRef] [Green Version]
- Finamore, A.; Palmery, M.; Bensehaila, S.; Peluso, I. Antioxidant, Immunomodulating, and Microbial-Modulating Activities of the Sustainable and Ecofriendly Spirulina. Oxid. Med. Cell. Longev. 2017, 2017, 3247528. [Google Scholar] [CrossRef] [Green Version]
- Qureshi, M.A.; Garlich, J.D.; Kidd, M.T. Dietary Spirulina platensis enhances humoral and cell-mediated immune functions in chickens. Immunopharmacol. Immunotoxicol. 1996, 18, 465–476. [Google Scholar] [CrossRef]
- Reboreda-Hernandez, O.A.; Juarez-Serrano, A.L.; Garcia-Luna, I.; Rivero-Ramirez, N.L.; Ortiz-Butron, R.; Nogueda-Torres, B.; Gonzalez-Rodriguez, N. Arthrospira maxima Paradoxical Effect on Trypanosoma cruzi Infection. Iran. J. Parasitol. 2020, 15, 223–232. [Google Scholar] [CrossRef]
- Al-Batshan, H.A.; Al-Mufarrej, S.I.; Al-Homaidan, A.A.; Qureshi, M.A. Enhancement of chicken macrophage phagocytic function and nitrite production by dietary Spirulina platensis. Immunopharmacol. Immunotoxicol. 2001, 23, 281–289. [Google Scholar] [CrossRef]
- Løbner, M.; Walsted, A.; Larsen, R.; Bendtzen, K.; Nielsen, C.H. Enhancement of human adaptive immune responses by administration of a high-molecular-weight polysaccharide extract from the cyanobacterium Arthrospira platensis. J. Med. Food. 2008, 11, 313–322. [Google Scholar] [CrossRef] [Green Version]
- Trushina, E.N.; Gladkikh, O.; Gadzhieva, Z.M.; Mustafina, O.K.; Pozdniakov, A.L. The influence of Spirulina and Selen-Spirulina on some indexes of rat’s immune status. Vopr. Pitan. 2007, 76, 21–25. [Google Scholar] [PubMed]
- Reddy, C.M.; Bhat, V.B.; Kiranmai, G.; Reddy, M.N.; Reddanna, P.; Madyastha, K.M. Selective inhibition of cyclooxygenase-2 by C-phycocyanin, a biliprotein from Spirulina platensis. Biochem. Biophys. Res. Commun. 2000, 277, 599–603. [Google Scholar] [CrossRef] [Green Version]
- Abdelkhalek, N.K.M.; Ghazy, E.W.; Abdel-Daim, M.M. Pharmacodynamic interaction of Spirulina platensis and deltamethrin in freshwater fish Nile tilapia, Oreochromis niloticus: Impact on lipid peroxidation and oxidative stress. Environ. Sci. Pollut. Res. 2015, 22, 3023–3031. [Google Scholar] [CrossRef] [PubMed]
- Gemma, C.; Mesches, M.H.; Sepesi, B.; Choo, K.; Holmes, D.B.; Bickford, P.C. Diets enriched in foods with high antioxidant activity reverse age-induced decreases in cerebellar β-adrenergic function and increases in proinflammatory cytokines. J. Neurosci. 2002, 22, 6114–6120. [Google Scholar] [CrossRef]
- Sagara, T.; Nishibori, N.; Kishibuchi, R.; Itoh, M.; Morita, K. Non-protein components of Arthrospira (Spirulina) platensis protect PC12 cells against iron-evoked neurotoxic injury. J. Appl. Phycol. 2015, 27, 849–855. [Google Scholar] [CrossRef]
- Padyana, A.K.; Bhat, V.B.; Madyastha, K.M.; Rajashankar, K.R.; Ramakumar, S. Crystal structure of a light-harvesting protein C-phycocyanin from Spirulina platensis. Biochem. Biophys. Res. Commun. 2001, 282, 893–898. [Google Scholar] [CrossRef]
- McCarty, M.F.; Barroso-Aranda, J.; Contreras, F. Oral phycocyanobilin may diminish the pathogenicity of activated brain microglia in neurodegenerative disorders. Med. Hypotheses 2010, 74, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Minic, S.L.; Stanic-Vucinic, D.; Mihailovic, J.; Krstic, M.; Nikolic, M.R.; Velickovic, T.C. Digestion by pepsin releases biologically active chromopeptides from C-phycocyanin, a blue-colored biliprotein of microalga Spirulina. J. Proteom. 2016, 147, 132–139. [Google Scholar] [CrossRef] [Green Version]
- Minic, S.; Stanic-Vucinic, D.; Radomirovic, M.; Radibratovic, M.; Milcic, M.; Nikolic, M.; Velickovic, T.C. Characterization and effects of binding of food-derived bioactive phycocyanobilin to bovine serum albumin. Food Chem. 2018, 239, 1090–1099. [Google Scholar] [CrossRef] [Green Version]
- McCarty, M.F. Clinical potential of Spirulina as a source of phycocyanobilin. J. Med. Food 2007, 10, 566–570. [Google Scholar] [CrossRef]
- Riss, J.; Décordé, K.; Sutra, T.; Delage, M.; Baccou, J.-C.; Jouy, N.; Brune, J.-P.; Oréal, H.; Cristol, J.-P.; Rouanet, J.-M. Phycobiliprotein C-phycocyanin from Spirulina platensis is powerfully responsible for reducing oxidative stress and NADPH oxidase expression induced by an atherogenic diet in hamsters. J. Agric. Food Chem. 2007, 55, 7962–7967. [Google Scholar] [CrossRef] [PubMed]
- Cherdkiatikul, T.; Suwanwong, Y. Production of the α and β Subunits of Spirulina Allophycocyanin and C-Phycocyanin in Escherichia coli: A Comparative Study of Their Antioxidant Activities. J. Biomol. Screen 2014, 19, 959–965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vo, T.S.; Ryu, B.; Kim, S.K. Purification of novel anti-inflammatory peptides from enzymatic hydrolysate of the edible microalgal Spirulina maxima. J. Funct. Foods 2013, 5, 1336–1346. [Google Scholar] [CrossRef]
- Shimamatsu, H. A pond for edible Spirulina production and its hydraulic studies. Hydrobiologia 1987, 151, 83–89. [Google Scholar] [CrossRef]
- Bai, S.-K.; Lee, S.-J.; Na, H.-J.; Ha, K.-S.; Han, J.-A.; Lee, H.; Kwon, Y.-G.; Chung, C.-K.; Kim, Y.-M. β-Carotene inhibits inflammatory gene expression in lipopolysaccharide-stimulated macrophages by suppressing redox-based NF-kappaB activation. Exp. Mol. Med. 2005, 37, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Katsuura, S.; Imamura, T.; Bando, N.; Yamanishi, R. β-Carotene and β-cryptoxanthin but not lutein evoke redox and immune changes in RAW264 murine macrophages. Mol. Nutr. Food Res. 2009, 53, 1396–1405. [Google Scholar] [CrossRef]
- Yogianti, F.; Kunisada, M.; Nakano, E.; Ono, R.; Sakumi, K.; Oka, S.; Nakabeppu, Y.; Nishigori, C. Inhibitory effects of dietary Spirulina platensis on UVB-induced skin inflammatory responses and carcinogenesis. J. Investig. Dermatol. 2014, 134, 2610–2619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.M.; Lee, J.Y.; Im, A.-R.; Chae, S. Phycocyanin Protects Against UVB-induced Apoptosis Through the PKC α/βII-Nrf-2/HO-1 Dependent Pathway in Human Primary Skin Cells. Molecules 2018, 23, 478. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.; Varadharaj, S.; Ganesan, L.P.; Shobha, J.C.; Naidu, M.U.; Parinandi, N.L.; Tridandapani, S.; Kutala, V.K.; Kuppusamy, P. C-phycocyanin protects against ischemia-reperfusion injury of heart through involvement of p38 MAPK and ERK signaling. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H2136–H2145. [Google Scholar] [CrossRef] [Green Version]
- Patil, J.; Matte, A.; Mallard, C.; Sandberg, M. Spirulina diet to lactating mothers protects the antioxidant system and reduces inflammation in post-natal brain after systemic inflammation. Nutr. Neurosci. 2018, 21, 59–69. [Google Scholar] [CrossRef]
- Patro, N.; Sharma, A.; Kariaya, K.; Patro, I. Spirulina platensis protects neurons via suppression of glial activation and peripheral sensitization leading to restoration of motor function in collagen-induced arthritic rats. Indian J. Exp. Biol. 2011, 49, 739–748. [Google Scholar] [PubMed]
- Tocher, D.R.; Betancor, M.B.; Sprague, M.; Olsen, R.E.; Napier, J.A. Omega-3 Long-Chain Polyunsaturated Fatty Acids, EPA and DHA: Bridging the Gap between Supply and Demand. Nutrients 2019, 11, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capelli, B.; Cysewski, G.R. Potential health benefits of spirulina microalgae. Nutrafoods 2010, 9, 19–26. [Google Scholar] [CrossRef]
- Cianciulli, A.; Calvello, R.; Porro, C.; Trotta, T.; Panaro, M.A. Understanding the role of SOCS signaling in neurodegenerative diseases: Current and emerging concepts. Cytokine Growth Factor Rev. 2017, 37, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Bickford, P.C.; Gould, T.; Briederick, L.; Chadman, K.; Pollock, A.; Young, D.; Shukitt-Hale, B.; Joseph, J. Antioxidant-rich diets improve cerebellar physiology and motor learning in aged rats. Brain Res. 2000, 866, 211–217. [Google Scholar] [CrossRef]
- Joseph, J.A.; Shukitt-Hale, B.; Denisova, N.A.; Bielinski, D.; Martin, A.; McEwen, J.J.; Bickford, P.C. Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplementation. J. Neurosci. 1999, 19, 8114–8121. [Google Scholar] [CrossRef]
- Wang, Y.; Chang, C.-F.; Chou, J.; Chen, H.-L.; Deng, X.; Harvey, B.K.; Cadet, J.L.; Bickford, P.C. Dietary supplementation with blueberries, spinach, or spirulina reduces ischemic brain damage. Exp. Neurol. 2005, 193, 75–84. [Google Scholar] [CrossRef]
- Abdullahi, D.; Annuar, A.A.; Sanusi, J. Improved spinal cord gray matter morphology induced by Spirulina platensis following spinal cord injury in rat models. Ultrastruct. Pathol. 2020, 44, 359–371. [Google Scholar] [CrossRef]
- Double, K.L.; Gerlach, M.; Youdim, M.B.; Riederer, P. Impaired iron homeostasis in Parkinson’s disease. J. Neural. Transm. Suppl. 2000, 6, 37–58. [Google Scholar]
- Hirsch, E.C.; Faucheux, B.A. Iron metabolism and Parkinson’s disease. Mov. Disord. 1998, 13, 39–45. [Google Scholar] [PubMed]
- Cornett, C.R.; Markesbery, W.R.; Ehmann, W.D. Imbalances of trace elements related to oxidative damage in Alzheimer’s disease brain. Neurotoxicology 1998, 19, 339–345. [Google Scholar] [PubMed]
- Smith, M.A.; Wehr, K.; Harris, P.L.; Siedlak, S.L.; Connor, J.R.; Perry, G. Abnormal localization of iron regulatory protein in Alzheimer’s disease. Brain Res. 1998, 788, 232–236. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, W.; Qu, S.; Pan, T.; Wang, X.; Le, W. Neuroprotection by iron chelator against proteasome inhibitor-induced nigral degeneration. Biochem. Biophys. Res. Commun. 2005, 333, 544–549. [Google Scholar] [CrossRef]
- Zheng, H.; Weiner, L.M.; Bar-Am, O.; Epsztejn, S.; Cabantchik, Z.I.; Warshawsky, A.; Youdim, M.B.H.; Fridkin, M. Design, synthesis, and evaluation of novel bifunctional iron-chelators as potential agents for neuroprotection in Alzheimer’s, Parkinson’s, and other neurodegenerative diseases. Bioorg. Med. Chem. 2005, 13, 773–783. [Google Scholar] [CrossRef]
- Opara, E.C. Oxidative stress. Dis. Mon. 2006, 52, 183–198. [Google Scholar] [CrossRef]
- Bermejo-Bescós, P.; Piñero-Estrada, E.; del Fresno, A.M.V. Neuroprotection by Spirulina platensis protean extract and phycocyanin against iron-induced toxicity in SH-SY5Y neuroblastoma cells. Toxicol. In Vitro 2008, 22, 1496–1502. [Google Scholar] [CrossRef]
- Healy, S.D.; Rowe, C. A critique of comparative studies of brain size. Proc. Biol. Sci. 2007, 274, 453–464. [Google Scholar] [CrossRef] [Green Version]
- Coviello, C.; Keunen, K.; Kersbergen, K.J.; Groenendaal, F.; Leemans, A.; Peels, B.; Isgum, I.; Viergever, M.A.; de Vries, L.S.; Buonocore, G.; et al. Effects of early nutrition and growth on brain volumes, white matter microstructure, and neurodevelopmental outcome in preterm newborns. Pediatr. Res. 2018, 83, 102–110. [Google Scholar] [CrossRef] [Green Version]
- Steenweg-de Graaff, J.; Tiemeier, H.; Steegers-Theunissen, R.P.M.; Hofman, A.; Jaddoe, V.W.V.; Verhulst, F.C.; Roza, S.J. Maternal dietary patterns during pregnancy and child internalising and externalising problems. The Generation R Study. Clin. Nutr. 2014, 33, 115–121. [Google Scholar] [CrossRef] [Green Version]
- Takeda, A.; Suzuki, M.; Tempaku, M.; Ohashi, K.; Tamano, H. Influx of extracellular Zn(2+) into the hippocampal CA1 neurons is required for cognitive performance via long-term potentiation. Neuroscience 2015, 304, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Castro, L.A.; Padilla-Gómez, E.; Parga-Martínez, N.J.; Castro-Rodríguez, D.C.; Quirarte, G.L.; Díaz-Cintra, S.; Nathanielsz, P.W.; Zambrano, E. Hippocampal mechanisms in impaired spatial learning and memory in male offspring of rats fed a low-protein isocaloric diet in pregnancy and/or lactation. Hippocampus 2018, 28, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Patro, N.; Patro, I.K. Amelioration of neurobehavioral and cognitive abilities of F1 progeny following dietary supplementation with Spirulina to protein malnourished mothers. Brain Behav. Immun. 2020, 85, 69–87. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Patro, N.; Tiwari, P.K.; Patro, I.K. Maternal Spirulina supplementation during pregnancy and lactation partially prevents oxidative stress, glial activation and neuronal damage in protein malnourished F1 progeny. Neurochem. Int. 2020, 141, 104877. [Google Scholar] [CrossRef] [PubMed]
- Pavón-Fuentes, N.; Marín-Prida, J.; Llópiz-Arzuaga, A.; Falcón-Cama, V.; Campos-Mojena, R.; Cervantes-Llanos, M.; Piniella-Matamoros, B.; Pentón-Arias, E.; Pentón-Rol, G. Phycocyanobilin reduces brain injury after endothelin-1- induced focal cerebral ischaemia. Clin. Exp. Pharmacol. Physiol. 2020, 47, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhang, L.; Hui, X.; Sun, X.; Yang, J.; Wang, J.; Wu, H.; Wang, X.; Zheng, Z.; Che, F.; et al. Selenium-containing protein from selenium-enriched Spirulina platensis antagonizes oxygen glucose deprivation-induced neurotoxicity by inhibiting ROS-mediated oxidative damage through regulating mPTP opening. Pharm. Biol. 2021, 59, 629–638. [Google Scholar] [CrossRef]
- Koh, E.-J.; Seo, Y.-J.; Choi, J.; Lee, H.Y.; Kang, D.-H.; Kim, K.-J.; Lee, B.-Y. Spirulina maxima Extract Prevents Neurotoxicity via Promoting Activation of BDNF/CREB Signaling Pathways in Neuronal Cells and Mice. Molecules 2017, 22, 1363. [Google Scholar] [CrossRef]
- Koh, E.-J.; Kim, K.-J.; Choi, J.; Kang, D.-H.; Lee, B.-Y. Spirulina maxima extract prevents cell death through BDNF activation against amyloid β 1-42 (Aβ 1-42) induced neurotoxicity in PC12 cells. Neurosci. Lett. 2018, 673, 33–38. [Google Scholar] [CrossRef]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms underlying inflammation in neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef] [Green Version]
- Spencer, J.P.E.; Vafeiadou, K.; Williams, R.J.; Vauzour, D. Neuroinflammation: Modulation by flavonoids and mechanisms of action. Mol. Asp. Med. 2012, 33, 83–97. [Google Scholar] [CrossRef]
- Schain, M.; Kreisl, W.C. Neuroinflammation in Neurodegenerative Disorders-a Review. Curr. Neurol. Neurosci. Rep. 2017, 17, 25. [Google Scholar] [CrossRef] [PubMed]
- Matejuk, A.; Ransohoff, R.M. Crosstalk Between Astrocytes and Microglia: An Overview. Front. Immunol. 2020, 11, 1416. [Google Scholar] [CrossRef]
- Napoli, I.; Neumann, H. Microglial clearance function in health and disease. Neuroscience 2009, 158, 1030–1038. [Google Scholar] [CrossRef]
- Diaz-Aparicio, I.; Beccari, S.; Abiega, O.; Sierra, A. Clearing the corpses: Regulatory mechanisms, novel tools, and therapeutic potential of harnessing microglial phagocytosis in the diseased brain. Neural Regen. Res. 2016, 11, 1533–1539. [Google Scholar] [PubMed]
- Mosser, C.-A.; Baptista, S.; Arnoux, I.; Audinat, E. Microglia in CNS development: Shaping the brain for the future. Prog. Neurobiol. 2017, 150, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Kumar, H.; Lim, H.-W.; More, S.V.; Kim, B.-W.; Koppula, S.; Kim, I.S.; Choi, D.-K. The role of free radicals in the aging brain and Parkinson’s Disease: Convergence and parallelism. Int. J. Mol. Sci. 2012, 13, 10478–10504. [Google Scholar] [CrossRef] [Green Version]
- Bolós, M.; Perea, J.R.; Avila, J. Alzheimer’s disease as an inflammatory disease. Biomol. Concepts 2017, 8, 37–43. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging. 2018, 13, 757–772. [Google Scholar] [CrossRef] [Green Version]
- Skaper, S.D.; Facci, L.; Zusso, M.; Giusti, P. Corrigendum: An Inflammation-Centric View of Neurological Disease: Beyond the Neuron. Front. Cell Neurosci. 2020, 13, 578. [Google Scholar] [CrossRef] [Green Version]
- Piovan, A.; Battaglia, J.; Filippini, R.; Dalla Costa, V.; Facci, L.; Argentini, C.; Pagetta, A.; Giusti, P.; Zusso, M. Pre- and Early Post-treatment With Arthrospira platensis (Spirulina) Extract Impedes Lipopolysaccharide-triggered Neuroinflammation in Microglia. Front. Pharmacol. 2021, 12, 724993. [Google Scholar] [CrossRef]
- Mitra, S.; Siddiqui, W.A.; Khandelwal, S. C-Phycocyanin protects against acute tributyltin chloride neurotoxicity by modulating glial cell activity along with its anti-oxidant and anti-inflammatory property: A comparative efficacy evaluation with N-acetyl cysteine in adult rat brain. Chem. Biol. Interact. 2015, 238, 138–150. [Google Scholar] [CrossRef]
- McCarty, M.F. NADPH Oxidase Activity in Cerebral Arterioles Is a Key Mediator of Cerebral Small Vessel Disease-Implications for Prevention. Healthcare 2015, 3, 233–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rimbau, V.; Camins, A.; Romay, C.; González, R.; Pallàs, M. Protective effects of C-phycocyanin against kainic acid-induced neuronal damage in rat hippocampus. Neurosci. Lett. 1999, 276, 75–78. [Google Scholar] [CrossRef]
- Pérez-Juárez, A.; Chamorro, G.; Alva-Sánchez, C.; Paniagua-Castro, N.; Pacheco-Rosado, J. Neuroprotective effect of Arthrospira (Spirulina) platensis against kainic acid-neuronal death. Pharm. Biol. 2016, 54, 1408–1412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piniella-Matamoros, B.; Marin-Prida, J.; Penton-Rol, G. Nutraceutical and therapeutic potential of Phycocyanobilin for treating Alzheimer’s disease. J. Biosci. 2021, 46, 42. [Google Scholar] [CrossRef]
- Pabon, M.M.; Jernberg, J.N.; Morganti, J.; Contreras, J.; Hudson, C.E.; Klein, R.L.; Bickford, P.C. A spirulina-enhanced diet provides neuroprotection in an α-synuclein model of Parkinson’s disease. PLoS ONE 2012, 7, e45256. [Google Scholar] [CrossRef] [Green Version]
- Tobón-Velasco, J.C.; Palafox-Sánchez, V.; Mendieta, L.; García, E.; Santamaría, A.; Chamorro-Cevallos, G.; Limón, I.D. Anti-oxidant effect of Spirulina (Arthrospira) maxima in a neurotoxic model caused by 6-OHDA in the rat striatum. J. Neural. Transm. 2013, 120, 1179–1189. [Google Scholar] [CrossRef]
- Chamorro, G.; Pérez-Albiter, M.; Serrano-García, N.; Mares-Sámano, J.J.; Rojas, P. Spirulina maxima pretreatment partially protects against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity. Nutr. Neurosci. 2006, 9, 207–212. [Google Scholar] [CrossRef]
- Zhang, F.; Lu, J.; Zhang, J.-G.; Xie, J.-X. Protective effects of a polysaccharide from Spirulina platensis on dopaminergic neu-rons in an MPTP-induced Parkinson’s disease model in C57BL/6J mice. Neural Regen Res. 2015, 10, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Koh, E.-J.; Kim, K.-J.; Song, J.-H.; Choi, J.; Lee, H.Y.; Kang, D.-H.; Heo, H.J.; Lee, B.-Y. Spirulina maxima Extract Ameliorates Learning and Memory Impairments via Inhibiting GSK-3β Phosphorylation Induced by Intracerebroventricular Injection of Amyloid-β 1-42 in Mice. Int. J. Mol. Sci. 2017, 18, 2401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marín-Prida, J.; Pavón-Fuentes, N.; Llópiz-Arzuaga, A.; Fernández-Massó, J.R.; Delgado-Roche, L.; Mendoza-Marí, Y.; San-tana, S.P.; Cruz-Ramírez, A.; Valenzuela-Silva, C.; Nazábal-Gálvez, M.; et al. Phycocyanobilin promotes PC12 cell survival and modulates immune and inflammatory genes and oxidative stress markers in acute cerebral hypoperfusion in rats. Toxicol. Appl. Pharmacol. 2013, 272, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, M.; Perumal, Y.; Bansal, S.; Arora, S.; Chopra, K. Phycocyanin alleviates ICV-STZ induced cognitive and molecular deficits via PI3-Kinase dependent pathway. Food Chem. Toxicol. 2020, 145, 111684. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-Llanos, M.; Lagumersindez-Denis, N.; Marín-Prida, J.; Pavón-Fuentes, N.; Falcon-Cama, V.; Piniella-Matamoros, B.; Camacho-Rodríguez, H.; Fernández-Massó, J.R.; Valenzuela-Silva, C.; Raíces-Cruz, I.; et al. Beneficial effects of oral ad-ministration of C-Phycocyanin and Phycocyanobilin in rodent models of experimental autoimmune encephalomyelitis. Life Sci. 2018, 194, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Pentón-Rol, G.; Marín-Prida, J.; Pardo-Andreu, G.; Martínez-Sánchez, G.; Acosta-Medina, E.F.; Valdivia-Acosta, A.; Lagumersindez-Denis, N.; Rodríguez-Jiménez, E.; Llópiz-Arzuaga, A.; López-Saura, P.A.; et al. C-Phycocyanin is neuropro-tective against global cerebral ischemia/reperfusion injury in gerbils. Brain Res. Bull. 2011, 86, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Elsonbaty, S.M.; Ismail, A.F.M. Nicotine encourages oxidative stress and impairment of rats’ brain mitigated by Spirulina platensis lipopolysaccharides and low-dose ionizing radiation. Arch. Biochem. Biophys. 2020, 689, 108382. [Google Scholar] [CrossRef] [PubMed]
- Min, S.K.; Park, J.S.; Luo, L.; Kwon, Y.S.; Lee, H.C.; Shim, H.J.; Kim, I.-D.; Lee, J.-K.; Shin, H.S. Assessment of C-phycocyanin effect on astrocytes-mediated neuroprotection against oxidative brain injury using 2D and 3D astrocyte tissue model. Sci. Rep. 2015, 5, 14418. [Google Scholar] [CrossRef] [Green Version]
- Hefti, F.; Hartikka, J.; Knusel, B. Function of neurotrophic factors in the adult and aging brain and their possible use in the treatment of neurodegenerative diseases. Neurobiol. Aging 1989, 10, 515–533. [Google Scholar] [CrossRef]
- Chen, A.; Xiong, L.-J.; Tong, Y.; Mao, M. The neuroprotective roles of BDNF in hypoxic ischemic brain injury. Biomed. Rep. 2013, 1, 167–176. [Google Scholar] [CrossRef] [Green Version]
- Pentón-Rol, G.; Marín-Prida, J.; Falcón-Cama, V. C-Phycocyanin and Phycocyanobilin as Remyelination Therapies for Enhancing Recovery in Multiple Sclerosis and Ischemic Stroke: A Preclinical Perspective. Behav. Sci. 2018, 8, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachstetter, A.D.; Jernberg, J.; Schlunk, A.; Vila, J.L.; Hudson, C.; Cole, M.J.; Shytle, R.D.; Tan, J.; Sanberg, P.R.; Sanberg, C.D.; et al. Spirulina promotes stem cell genesis and protects against LPS induced declines in neural stem cell proliferation. PLoS ONE 2010, 5, e10496. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, P.; Park, H.; Baumann, M.; Dunlop, J.; Frydman, J.; Kopito, R.; McCampbell, A.; Leblanc, G.; Venkateswaran, A.; Nurmi, A.; et al. Protein misfolding in neurodegenerative diseases: Implications and strategies. Transl. Neurodegener. 2017, 6, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elbaz, A.; Carcaillon, L.; Kab, S.; Moisan, F. Epidemiology of Parkinson’s disease. Rev. Neurol. 2016, 172, 14–26. [Google Scholar] [CrossRef]
- Balestrino, R.; Schapira, A.H.V. Parkinson disease. Eur. J. Neurol. 2020, 27, 27–42. [Google Scholar] [CrossRef]
- Ascherio, A.; Schwarzschild, M.A. The epidemiology of Parkinson’s disease: Risk factors and prevention. Lancet Neurol. 2016, 15, 1257–1272. [Google Scholar] [CrossRef]
- Deng, H.; Wang, P.; Jankovic, J. The genetics of Parkinson disease. Ageing Res. Rev. 2018, 42, 72–85. [Google Scholar] [CrossRef]
- Bras, J.M.; Singleton, A. Genetic susceptibility in Parkinson’s disease. Biochim. Biophys. Acta 2009, 1792, 597–603. [Google Scholar] [CrossRef] [Green Version]
- Sveinbjornsdottir, S. The clinical symptoms of Parkinson’s disease. J. Neurochem. 2016, 139, 318–324. [Google Scholar] [CrossRef] [Green Version]
- Chaudhuri, K.R.; Healy, D.G.; Schapira, A.H.V. Non-motor symptoms of Parkinson’s disease: Diagnosis and management. Lancet Neurol. 2006, 5, 235–245. [Google Scholar] [CrossRef]
- Kaur, R.; Mehan, S.; Singh, S. Understanding multifactorial architecture of Parkinson’s disease: Pathophysiology to management. Neurol. Sci. 2019, 40, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Teravskis, P.J.; Covelo, A.; Miller, E.C.; Singh, B.; Martell-Martínez, H.A.; Benneyworth, M.A.; Gallardo, C.; Oxnard, B.R.; Araque, A.; Lee, M.K.; et al. A53T Mutant α-Synuclein Induces Tau-Dependent Postsynaptic Impairment Independently of Neurodegenerative Changes. J. Neurosci. 2018, 38, 9754–9767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wakabayashi, K.; Tanji, K.; Odagiri, S.; Miki, Y.; Mori, F.; Takahashi, H. The Lewy body in Parkinson’s disease and related neurodegenerative disorders. Mol. Neurobiol. 2013, 47, 495–508. [Google Scholar] [CrossRef]
- Reale, M.; Greig, N.H.; Kamal, M.A. Peripheral chemo-cytokine profiles in Alzheimer’s and Parkinson’s diseases. Mini Rev. Med. Chem. 2009, 9, 1229–1241. [Google Scholar] [CrossRef] [PubMed]
- Reale, M.; Iarlori, C.; Thomas, A.; Gambi, D.; Perfetti, B.; Di Nicola, M.; Onofrj, M. Peripheral cytokines profile in Parkinson’s disease. Brain Behav. Immun. 2009, 23, 55–63. [Google Scholar] [CrossRef]
- Rojanathammanee, L.; Murphy, E.J.; Combs, C.K. Expression of mutant α-synuclein modulates microglial phenotype in vitro. J. Neuroinflamm. 2011, 8, 44. [Google Scholar] [CrossRef] [Green Version]
- Hou, L.; Bao, X.; Zang, C.; Yang, H.; Sun, F.; Che, Y.; Wu, X.; Li, S.; Zhang, D.; Wang, Q. Integrin CD11b mediates α-synuclein-induced activation of NADPH oxidase through a Rho-dependent pathway. Redox Biol. 2018, 14, 600–608. [Google Scholar] [CrossRef]
- Liu, Y.; Jovcevski, B.; Pukala, T.L. C-Phycocyanin from Spirulina Inhibits α-Synuclein and Amyloid-β Fibril Formation but Not Amorphous Aggregation. J. Nat. Prod. 2019, 82, 66–73. [Google Scholar] [CrossRef]
- Huang, Z.; Guo, B.J.; Wong, R.N.S.; Jiang, Y. Characterization and antioxidant activity of selenium-containing phycocyanin isolated from Spirulina platensis. Food Chem. 2007, 100, 1137–1143. [Google Scholar] [CrossRef]
- Thaakur, S.R.; Jyothi, B. Effect of spirulina maxima on the haloperidol induced tardive dyskinesia and oxidative stress in rats. J. Neural. Transm. 2007, 114, 1217–1225. [Google Scholar] [CrossRef]
- McGeer, P.L.; McGeer, E.G. The α-synuclein burden hypothesis of Parkinson disease and its relationship to Alzheimer disease. Exp. Neurol. 2008, 212, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Godoy, M.C.P.; Tarelli, R.; Ferrari, C.C.; Sarchi, M.I.; Pitossi, F.J. Central and systemic IL-1 exacerbates neurodegeneration and motor symptoms in a model of Parkinson’s disease. Brain 2008, 131, 1880–1894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zujovic, V.; Benavides, J.; Vigé, X.; Carter, C.; Taupin, V. Fractalkine modulates TNF-αsecretion and neurotoxicity induced by microglial activation. Glia 2000, 29, 305–315. [Google Scholar] [CrossRef]
- Zujovic, V.; Schussler, N.; Jourdain, D.; Duverger, D.; Taupin, V. In vivo neutralization of endogenous brain fractalkine increases hippocampal TNF-α and 8-isoprostane production induced by intracerebroventricular injection of LPS. J. Neuroimmunol. 2001, 115, 135–143. [Google Scholar] [CrossRef]
- Strömberg, I.; Gemma, C.; Vila, J.; Bickford, P.C. Blueberry- and spirulina-enriched diets enhance striatal dopamine recovery and induce a rapid, transient microglia activation after injury of the rat nigrostriatal dopamine system. Exp. Neurol. 2005, 196, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Pabon, M.M.; Bachstetter, A.D.; Hudson, C.E.; Gemma, C.; Bickford, P.C. CX3CL1 reduces neurotoxicity and microglial activation in a rat model of Parkinson’s disease. J. Neuroinflamm. 2011, 8, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Christian, P.K.; Panchal, K.; Guruprasad, B.R.; Tiwari, A.K. Supplementation of Spirulina (Arthrospira platensis) Improves Lifespan and Locomotor Activity in Paraquat-Sensitive DJ-1β Δ93 Flies, a Parkinson’s Disease Model in Drosophila melanogaster. J. Diet. Suppl. 2017, 14, 573–588. [Google Scholar] [CrossRef] [PubMed]
- Pan, R.-Y.; Ma, J.; Kong, X.-X.; Wang, X.-F.; Li, S.-S.; Qi, X.-L.; Yan, Y.-H.; Cheng, J.; Liu, Q.; Jin, W.; et al. Sodium rutin ameliorates Alzheimer’s disease-like pathology by enhancing microglial amyloid-β clearance. Sci. Adv. 2019, 5, eaau6328. [Google Scholar] [CrossRef] [Green Version]
- De la Monte, S.M. Contributions of brain insulin resistance and deficiency in amyloid-related neurodegeneration in Alzheimer’s disease. Drugs 2012, 72, 49–66. [Google Scholar] [CrossRef]
- Magalingam, K.B.; Radhakrishnan, A.; Ping, N.S.; Haleagrahara, N. Current Concepts of Neurodegenerative Mechanisms in Alzheimer’s Disease. Biomed. Res. Int. 2018, 2018, 3740461. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.Y.; Kim, H.V.; Jo, S.; Lee, C.J.; Choi, S.Y.; Kim, D.J.; Kim, Y. Corrigendum: EPPS rescues hippocampus-dependent cognitive deficits in APP/PS1 mice by disaggregation of amyloid-β oligomers and plaques. Nat. Commun. 2016, 7, 10755. [Google Scholar] [CrossRef] [Green Version]
- Ono, K. Alzheimer’s disease as oligomeropathy. Neurochem. Int. 2018, 119, 57–70. [Google Scholar] [CrossRef]
- Borutaite, V.; Morkuniene, R.; Valincius, G. β-amyloid oligomers: Recent developments. Biomol. Concepts 2011, 2, 211–222. [Google Scholar] [CrossRef]
- Guo, J.-P.; Yu, S.; McGeer, P.L. Simple in vitro assays to identify amyloid-βaggregation blockers for Alzheimer’s disease therapy. J. Alzheimer’s Dis. 2010, 19, 1359–1370. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, H.J.; Lee, K.W. Naturally occurring phytochemicals for the prevention of Alzheimer’s disease. J. Neurochem. 2010, 112, 1415–1430. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.-C.; Jing, P. Molecular Interaction of Protein-Pigment C-Phycocyanin with Bovine Serum Albumin in a Gomphosis Structure Inhibiting Amyloid Formation. Int. J. Mol. Sci. 2020, 21, 8207. [Google Scholar] [CrossRef]
- Singh, N.K.; Hasan, S.S.; Kumar, J.; Raj, I.; Pathan, A.A.; Parmar, A.; Shakil, S.; Gourinath, S.; Madamwar, D. Crystal structure and interaction of phycocyanin with β-secretase: A putative therapy for Alzheimer’s disease. CNS Neurol. Disord. Drug Targets 2014, 13, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Yan, R. A Close Look at BACE1 Inhibitors for Alzheimer’s Disease Treatment. CNS Drugs 2019, 33, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Ozben, T.; Ozben, S. Neuro-inflammation and anti-inflammatory treatment options for Alzheimer’s disease. Clin. Biochem. 2019, 72, 87–89. [Google Scholar] [CrossRef] [PubMed]
- Ugolini, F.; Lana, D.; Nardiello, P.; Nosi, D.; Pantano, D.; Casamenti, F.; Giovannini, M.G. Different Patterns of Neurodegeneration and Glia Activation in CA1 and CA3 Hippocampal Regions of TgCRND8 Mice. Front. Aging Neurosci. 2018, 10, 372. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Gan, L.; Yan, S.; Yan, Y.; Huang, W. Effect of C-phycocyanin on HDAC3 and miRNA-335 in Alzheimer’s disease. Transl. Neurosci. 2020, 11, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.X.; Park, Y.-K.; Lee, J.-Y. Anti-Inflammatory Effects of Spirulina platensis Extract via the Modulation of Histone Deacetylases. Nutrients 2016, 8, 381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.; Wang, S.; Yu, L.; Jin, J.; Ye, X.; Liu, Y.; Xu, Y. HDAC3 negatively regulates spatial memory in a mouse model of Alzheimer’s disease. Aging Cell. 2017, 16, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Janczura, K.J.; Volmar, C.-H.; Sartor, G.C.; Rao, S.J.; Ricciardi, N.R.; Lambert, G.; Brothers, S.P.; Wahlestedt, C. Inhibition of HDAC3 reverses Alzheimer’s disease-related pathologies in vitro and in the 3xTg-AD mouse model. Proc. Natl. Acad. Sci. USA 2018, 115, E11148–E11157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, D.G.; Lue, L.-F.; Tang, T.M.; Adler, C.H.; Caviness, J.N.; Sabbagh, M.N.; Serrano, G.E.; Sue, L.I.; Beach, T.G. Changes in CD200 and intercellular adhesion molecule-1 (ICAM-1) levels in brains of Lewy body disorder cases are associated with amounts of Alzheimer’s pathology not α-synuclein pathology. Neurobiol. Aging 2017, 54, 175–186. [Google Scholar] [CrossRef]
- Frohman, E.M.; Frohman, T.C.; Gupta, S.; de Fougerolles, A.; van den Noort, S. Expression of intercellular adhesion molecule 1 (ICAM-1) in Alzheimer’s disease. J. Neurol. Sci. 1991, 106, 105–111. [Google Scholar] [CrossRef]
- Guha, S.; Paidi, R.K.; Goswami, S.; Saha, P.; Biswas, S.C. ICAM-1 protects neurons against Amyloid-β and improves cognitive behaviors in 5xFAD mice by inhibiting NF-Κb. Brain Behav. Immun. 2021, 100, 194–210. [Google Scholar] [CrossRef]
- De Paola, M.; Buanne, P.; Biordi, L.; Bertini, R.; Ghezzi, P.; Mennini, T. Chemokine MIP-2/CXCL2, acting on CXCR2, induces motor neuron death in primary cultures. Neuroimmunomodulation 2007, 14, 310–316. [Google Scholar] [CrossRef]
- Nylander, A.; Hafler, D.A. Multiple sclerosis. J. Clin. Investig. 2012, 122, 1180–1188. [Google Scholar] [CrossRef]
- Sospedra, M.; Martin, R. Immunology of multiple sclerosis. Annu. Rev. Immunol. 2005, 23, 683–747. [Google Scholar] [CrossRef] [Green Version]
- Correale, J.; Marrodan, M.; Ysrraelit, M.C. Mechanisms of Neurodegeneration and Axonal Dysfunction in Progressive Multiple Sclerosis. Biomedicines 2019, 7, 14. [Google Scholar] [CrossRef] [Green Version]
- Friese, M.A.; Schattling, B.; Fugger, L. Mechanisms of neurodegeneration and axonal dysfunction in multiple sclerosis. Nat. Rev. Neurol. 2014, 10, 225–238. [Google Scholar] [CrossRef]
- Pentón-Rol, G.; Martínez-Sánchez, G.; Cervantes-Llanos, M.; Lagumersindez-Denis, N.; Acosta-Medina, E.F.; Falcón-Cama, V.; Alonso-Ramírez, R.; Valenzuela-Silva, C.; Rodríguez-Jiménez, E.; Llópiz-Arzuaga, A.; et al. C-Phycocyanin ameliorates experimental autoimmune encephalomyelitis and induces regulatory T cells. Int. Immunopharmacol. 2011, 11, 29–38. [Google Scholar] [CrossRef]
- Martin, R.; McFarland, H.F. Immunological aspects of experimental allergic encephalomyelitis and multiple sclerosis. Crit. Rev. Clin. Lab. Sci. 1995, 32, 121–182. [Google Scholar] [CrossRef]
- Constantinescu, C.S.; Farooqi, N.; O’Brien, K.; Gran, B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br. J. Pharmacol. 2011, 164, 1079–1106. [Google Scholar] [CrossRef]
- Bittner, S.; Afzali, A.M.; Wiendl, H.; Meuth, S.G. Myelin oligodendrocyte glycoprotein (MOG35-55) induced experimental autoimmune encephalomyelitis (EAE) in C57BL/6 mice. J. Vis. Exp. 2014, 15, 51275. [Google Scholar]
- Khoury, S.J.; Hancock, W.W.; Weiner, H.L. Oral tolerance to myelin basic protein and natural recovery from experimental autoimmune encephalomyelitis are associated with downregulation of inflammatory cytokines and differential upregulation of transforming growth factor β, interleukin 4, and prostaglandin E expression in the brain. J. Exp. Med. 1992, 176, 1355–1364. [Google Scholar] [PubMed]
- Pentón-Rol, G.; Lagumersindez-Denis, N.; Muzio, L.; Bergami, A.; Furlan, R.; Fernández-Massó, J.R.; Nazabal-Galvez, M.; Llópiz-Arzuaga, A.; Herrera-Rolo, T.; Veliz-Rodriguez, T.; et al. Comparative Neuroregenerative Effects of C-Phycocyanin and IFN-β in a Model of Multiple Sclerosis in Mice. J. Neuroimmune Pharmacol. 2016, 11, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Carlson, N.G.; Bellamkonda, S.; Schmidt, L.; Redd, J.; Huecksteadt, T.; Weber, L.M.; Davis, E.; Wood, B.; Maruyama, T.; Rose, J.W. The role of the prostaglandin E2 receptors in vulnerability of oligodendrocyte precursor cells to death. J. Neuroinflamm. 2015, 12, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiow, L.R.; Favrais, G.; Schirmer, L.; Schang, A.-L.; Cipriani, S.; Andres, C.; Wright, J.N.; Nobuta, H.; Fleiss, B.; Gressens, P.; et al. Reactive astrocyte COX2-PGE2 production inhibits oligodendrocyte maturation in neonatal white matter injury. Glia 2017, 65, 2024–2037. [Google Scholar] [CrossRef] [PubMed]


| Component/Route of Administration | Animal Model | Summary of Results | Ref. |
|---|---|---|---|
| Spirulina/Orally | Adult male Sprague–Dawley rats with cerebral ischemia | Reduction of infarction area in the cerebral cortex | [48] |
| Increase in locomotor activity | |||
| Decline in TUNEL positive cells and caspase-3 activity | |||
| Spirulina/Orally | Protein malnourished Sprague Dawley female rats | Increased cerebral cortical thickness | [64] |
| Reduction in oxidative brain damage | |||
| Reduction in astrocyte and microglia activation | |||
| PCB/Intraperitoneally | Male Wistar rats with cerebral ischemia | Reduction in the area of cerebral infarction | [65] |
| Normalized expression of myelin basic protein and enzyme CNPase | |||
| Preserved vitality of cerebral cortex neurons | |||
| Spirulina maxima 70% ethanol extract (SM70EE)/Orally | Male ICR mice treated with scopolamine | Reduction in learning and memory deficits | [67] |
| C-PC/Intraperitoneally | Male Wistar rats treated with tributyltin chloride (TBTC) | Increased C-PC bioavailability in cerebral cortical tissue | [83] |
| Reduction in astrocyte and microglia activation | |||
| Reduction in oxidative stress and inflammation | |||
| C-PC/Orally | Male Sprague Dawley rats treated with kainic acid | Reduction in microglia and astroglia activation | [85] |
| Reduced incidence of neurobehavioral changes | |||
| Spirulina/Orally | Male SW mice treated with kainic acid | Reduction of neuron damage in CA3 hippocampal region | [86] |
| Spirulina/Orally | Male Fisher 344 rats treated with α-Syn in substantia nigra | Reduction in the number of activated microglial cell | [88] |
| Increased expression of the fractalkine receptor (CX3CR1) on microglia | |||
| Spirulina/Orally | Male Wistar rats treated with 6-OHDA | Reduction in oxidative stress | [89] |
| Preserved dopamine levels in the striatum | |||
| Normalized locomotor activity | |||
| Spirulina/Orally | male C-57 black mice treated with MPTP | Partial reduction of dopamine content in the striatum | [90] |
| Reduction in oxidative stress | |||
| Polysaccharide from Spirulina/Intraperitoneally | C57BL/6J mice treated with MPTP | Increased mRNA expression of dopamine transporter and tyrosine hydroxylase | [91] |
| Increased SOD and GPx activity | |||
| Spirulina maxima 70% ethanol extract (SM70EE)/Orally | Male ICR mice treated with Aβ1–42 | Reduced oxidative stress | [92] |
| Increased GSH, GPx1 and GR levels in the hippocampus | |||
| PCB/Intraperitoneally | Male Wistar rats subjected to permanent bilateral occlusion of the common carotid arteries | Modulated 190 genes associated to immunological and inflammatory processes | [93] |
| Phycocyanin/Intraperitoneally | Female Wistar rats treated with Streptozotocin | Reduction in neuroinflammation | [94] |
| Improved levels of BDNF, IGF-1, BCL-2 and ChAT | |||
| Improved gene expression of IRS-1, PI3-K, AKT | |||
| C-PC/Orally | Lewis rats with EAE | Restoration of motor function | [95] |
| Reduced oxidative damage | |||
| Preserved the integrity of myelin sheaths | |||
| PCB/Orally | C57BL/6 mice with EAE | [95] | |
| Improved clinical status of animals | |||
| Reduction in IL-6 and IFN-γ expression in the brain | |||
| C-PC/Orally or Intraperitoneally | Male Mongolian gerbils with global cerebral ischemia | Reduction in infarct volume | [96] |
| Decreased neuronal damage | |||
| Reduction in malondialdehyde (MDA), peroxidation potential (PP) and FRAP levels | |||
| S. platensis-LPS (S.LPS)/Intraperitoneally | Male albino Wistar rats treated with nicotine | Enhancement in antioxidant enzymes’ activities | [97] |
| Improved level of TNF-α, IL-17 and NF-κB in brain tissues | |||
| Prevention of Tau protein phosphorylation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trotta, T.; Porro, C.; Cianciulli, A.; Panaro, M.A. Beneficial Effects of Spirulina Consumption on Brain Health. Nutrients 2022, 14, 676. https://0-doi-org.brum.beds.ac.uk/10.3390/nu14030676
Trotta T, Porro C, Cianciulli A, Panaro MA. Beneficial Effects of Spirulina Consumption on Brain Health. Nutrients. 2022; 14(3):676. https://0-doi-org.brum.beds.ac.uk/10.3390/nu14030676
Chicago/Turabian StyleTrotta, Teresa, Chiara Porro, Antonia Cianciulli, and Maria Antonietta Panaro. 2022. "Beneficial Effects of Spirulina Consumption on Brain Health" Nutrients 14, no. 3: 676. https://0-doi-org.brum.beds.ac.uk/10.3390/nu14030676