Effectiveness of Written Dietary Advice for Improving Blood Lipids in Primary Care Adults—A Pragmatic Randomized Controlled Trial (MYDICLIN)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Randomization, Allocation Concealment, and Blinding
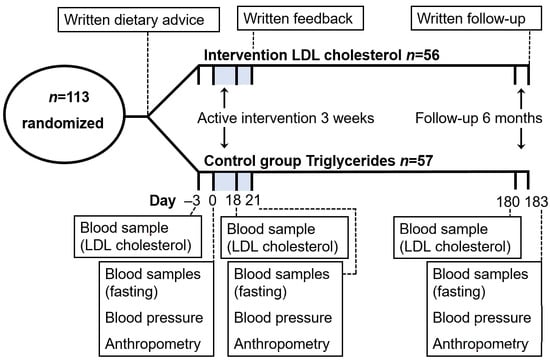
2.3. Dietary Intervention and Investigations
2.4. Clinical Trial Registry and Ethics
2.5. Statistical Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef]
- Ference, B.A. Mendelian randomization studies: Using naturally randomized genetic data to fill evidence gaps. Curr. Opin. Lipidol. 2015, 26, 566–571. [Google Scholar] [CrossRef]
- Holmes, M.V.; Asselbergs, F.W.; Palmer, T.M.; Drenos, F.; Lanktree, M.B.; Nelson, C.P.; Dale, C.E.; Padmanabhan, S.; Finan, C.; Swerdlow, D.I.; et al. Mendelian randomization of blood lipids for coronary heart disease. Eur. Heart J. 2015, 36, 539–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoeneck, M.; Iggman, D. The effects of foods on LDL cholesterol levels: A systematic review of the accumulated evidence from systematic reviews and meta-analyses of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1325–1338. [Google Scholar] [CrossRef]
- Kendall, C.W.; Jenkins, D.J. A dietary portfolio: Maximal reduction of low-density lipoprotein cholesterol with diet. Curr. Atheroscler. Rep. 2004, 6, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Rees, K.; Dyakova, M.; Wilson, N.; Ward, K.; Thorogood, M.; Brunner, E. Dietary advice for reducing cardiovascular risk. Cochrane Database Syst. Rev. 2013, 3, CD002128. [Google Scholar]
- US Preventive Services Task Force; Krist, A.H..; Davidson, K.W.; Mangione, C.M.; Barry, M.J.; Cabana, M.; Caughey, A.B.; Donahue, K.; Doubeni, C.A.; Epling, J.W., Jr.; et al. Behavioral Counseling Interventions to Promote a Healthy Diet and Physical Activity for Cardiovascular Disease Prevention in Adults with Cardiovascular Risk Factors: US Preventive Services Task Force Recommendation Statement. JAMA 2020, 324, 2069–2075. [Google Scholar] [CrossRef] [PubMed]
- Dudum, R.; Juraschek, S.P.; Appel, L.J. Dose-dependent effects of lifestyle interventions on blood lipid levels: Results from the PREMIER trial. Patient Educ. Couns. 2019, 102, 1882–1891. [Google Scholar] [CrossRef]
- Kulick, D.; Langer, R.D.; Ashley, J.M.; Gans, K.M.; Schlauch, K.; Feller, C. Live well: A practical and effective low-intensity dietary counseling intervention for use in primary care patients with dyslipidemia–A randomized controlled pilot trial. BMC Fam. Pract. 2013, 14, 59. [Google Scholar] [CrossRef] [Green Version]
- Thompson, R.L.; Summerbell, C.D.; Hooper, L.; Higgins, J.P.; Little, P.S.; Talbot, D.; Ebrahim, S. Dietary advice given by a dietitian versus other health professional or self-help resources to reduce blood cholesterol. Cochrane Database Syst. Rev. 2003, 3, CD001366. [Google Scholar] [CrossRef] [PubMed]
- Teasdale, N.; Elhussein, A.; Butcher, F.; Piernas, C.; Cowburn, G.; Hartmann-Boyce, J.; Saksena, R.; Scarborough, P. Systematic review and meta-analysis of remotely delivered interventions using self-monitoring or tailored feedback to change dietary behavior. Am. J. Clin. Nutr. 2018, 107, 247–256. [Google Scholar] [CrossRef] [PubMed]
- El-Alti, L.; Sandman, L.; Munthe, C. Person Centered Care and Personalized Medicine: Irreconcilable Opposites or Potential Companions. Health Care Anal. 2019, 27, 45–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkins, L.; Michie, S. Designing interventions to change eating behaviours. Proc. Nutr. Soc. 2015, 74, 164–170. [Google Scholar] [CrossRef]
- Baranowski, T. 24-hour recall and diet record methods. In Nutritional Epidemiology, 3rd ed.; Willett, W., Ed.; Oxford University Press: New York, NY, USA, 2013; pp. 49–69. [Google Scholar]
- Schectman, G.; Sasse, E. Variability of lipid measurements: Relevance for the clinician. Clin. Chem. 1993, 39, 1495–1503. [Google Scholar] [CrossRef]
- Bjermo, H.; Iggman, D.; Kullberg, J.; Dahlman, I.; Johansson, L.; Persson, L.; Berglund, J.; Pulkki, K.; Basu, S.; Uusitupa, M.; et al. Effects of n-6 PUFAs compared with SFAs on liver fat, lipoproteins, and inflammation in abdominal obesity: A randomized controlled trial. Am. J. Clin. Nutr. 2012, 95, 1003–1012. [Google Scholar] [CrossRef] [Green Version]
- Iggman, D.; Rosqvist, F.; Larsson, A.; Arnlöv, J.; Beckman, L.; Rudling, M.; Risérus, U. Role of dietary fats in modulating cardiometabolic risk during moderate weight gain: A randomized double-blind overfeeding trial (LIPOGAIN study). J. Am. Heart Assoc. 2014, 3, e001095. [Google Scholar] [CrossRef] [Green Version]
- Baron, J.A.; Gleason, R.; Crowe, B.; Mann, J.I. Preliminary trial of the effect of general practice based nutritional advice. Br. J. Gen. Pract. 1990, 40, 137–141. [Google Scholar]
- Anderson, J.W.; Garrity, T.F.; Wood, C.L.; Whitis, S.E.; Smith, B.M.; Oeltgen, P.R. Prospective, randomized, controlled comparison of the effects of low-fat and low-fat plus high-fiber diets on serum lipid concentrations. Am. J. Clin. Nutr. 1992, 56, 887–894. [Google Scholar] [CrossRef]
- Hellénius, M.L.; de Faire, U.; Berglund, B.; Hamsten, A.; Krakau, I. Diet and exercise are equally effective in reducing risk for cardiovascular disease. Results of a randomized controlled study in men with slightly to moderately raised cardiovascular risk factors. Atherosclerosis 1993, 103, 81–91. [Google Scholar] [CrossRef]
- Neil, H.A.; Roe, L.; Godlee, R.J.; Moore, J.W.; Clark, G.M.; Brown, J.; Thorogood, M.; Stratton, I.M.; Lancaster, T.; Mant, D.; et al. Randomised trial of lipid lowering dietary advice in general practice: The effects on serum lipids, lipoproteins, and antioxidants. BMJ 1995, 310, 569–573. [Google Scholar] [CrossRef] [Green Version]
- Keyserling, T.C.; Ammerman, A.S.; Davis, C.E.; Mok, M.C.; Garrett, J.; Simpson, R., Jr. A randomized controlled trial of a physician-directed treatment program for low-income patients with high blood cholesterol: The Southeast Cholesterol Project. Arch. Fam. Med. 1997, 6, 135–145. [Google Scholar] [CrossRef]
- Brekke, H.K.; Lenner, R.A.; Taskinen, M.R.; Månsson, J.E.; Funahashi, T.; Matsuzawa, Y.; Jansson, P.A. Lifestyle modification improves risk factors in type 2 diabetes relatives. Diabetes Res. Clin. Pract. 2005, 68, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Graziani, C.; Diamond, J.J. Cholesterol-lowering effect of the Food for Heart Nutrition Education Program. J. Am. Diet. Assoc. 2004, 104, 1868–1872. [Google Scholar] [CrossRef] [PubMed]
- Djuric, Z.; Ren, J.; Mekhovich, O.; Venkatranamoorthy, R.; Heilbrun, L.K. Effects of high fruit-vegetable and/or low-fat intervention on plasma micronutrient levels. J. Am. Coll. Nutr. 2006, 25, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Little, P.; Kelly, J.; Barnett, J.; Dorward, M.; Margetts, B.; Warm, D. Randomised controlled factorial trial of dietary advice for patients with a single high blood pressure reading in primary care. BMJ 2004, 328, 1054. [Google Scholar] [CrossRef] [Green Version]
- Moy, T.F.; Yanek, L.R.; Raqueño, J.V.; Bezirdjian, P.J.; Blumenthal, R.S.; Wilder, L.B.; Becker, D.M. Dietary Counseling for High Blood Cholesterol in Families at Risk of Coronary Disease. Prev. Cardiol. 2001, 4, 158–164. [Google Scholar] [CrossRef]
- Ammerman, A.S.; Keyserling, T.C.; Atwood, J.R.; Hosking, J.D.; Zayed, H.; Krasny, C. A randomized controlled trial of a public health nurse directed treatment program for rural patients with high blood cholesterol. Prev. Med. 2003, 36, 340–351. [Google Scholar] [CrossRef]
- Anderssen, S.A.; Carroll, S.; Urdal, P.; Holme, I. Combined diet and exercise intervention reverses the metabolic syndrome in middle-aged males: Results from the Oslo Diet and Exercise Study. Scand. J. Med. Sci. Sports 2007, 17, 687–695. [Google Scholar] [CrossRef]
- Blumenthal, J.A.; Babyak, M.A.; Sherwood, A.; Craighead, L.; Lin, P.H.; Johnson, J.; Watkins, L.L.; Wang, J.T.; Kuhn, C.; Feinglos, M.; et al. Effects of the dietary approaches to stop hypertension diet alone and in combination with exercise and caloric restriction on insulin sensitivity and lipids. Hypertension 2010, 55, 1199–1205. [Google Scholar] [CrossRef] [Green Version]
- Piernas, C.; Aveyard, P.; Lee, C.; Tsiountsioura, M.; Noreik, M.; Astbury, N.M.; Oke, J.; Madigan, C.; Jebb, S.A. Evaluation of an intervention to provide brief support and personalized feedback on food shopping to reduce saturated fat intake (PC-SHOP): A randomized controlled trial. PLoS Med. 2020, 17, e1003385. [Google Scholar] [CrossRef]
- Lord, S.P.; Sheng, E.; Imel, Z.E.; Baer, J.; Atkins, D.C. More than reflections: Empathy in motivational interviewing includes language style synchrony between therapist and client. Behav. Ther. 2015, 46, 296–303. [Google Scholar] [CrossRef] [Green Version]
- Bóveda-Fontán, J.; Barragán-Brun, N.; Campiñez-Navarro, M.; Pérula-de Torres, L.Á.; Bosch-Fontcuberta, J.M.; Martín-Álvarez, R.; Arbonies-Ortiz, J.C.; Novo-Rodríguez, J.M.; Criado-Larumbe, M.; Fernández-García, J.A.; et al. Effectiveness of motivational interviewing in patients with dyslipidemia: A randomized cluster trial. BMC Fam. Pract. 2015, 16, 151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundh, A.; Lexchin, J.; Mintzes, B.; Schroll, J.B.; Bero, L. Industry sponsorship and research outcome. Cochrane Database Syst. Rev. 2017, 2, MR000033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, C.; Lundh, A.; Rasmussen, K.; Hróbjartsson, A. Financial conflicts of interest in systematic reviews: Associations with results, conclusions, and methodological quality. Cochrane Database Syst. Rev. 2019, 8, MR000047. [Google Scholar] [CrossRef] [PubMed]
- Carlisle, J.B. Data fabrication and other reasons for non-random sampling in 5087 randomised, controlled trials in anaesthetic and general medical journals. Anaesthesia 2017, 72, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Riksmaten–vuxna 2010–11. Livsmedels- och Näringsintag Bland Vuxna i Sverige; Livsmedelsverket: Uppsala, Sweden, 2012; Available online: https://www.livsmedelsverket.se/globalassets/publikationsdatabas/rapporter/2011/riksmaten_2010_20111.pdf (accessed on 28 October 2021).
- Nordic Council of Ministers. Nordic Nutrition Recommendations 2012: Integrating Nutrition and Physical Activity, 5th ed.; Nordic Council of Ministers: Copenhagen, Denmark, 2014; Available online: https://0-doi-org.brum.beds.ac.uk/10.6027/Nord2014-002 (accessed on 28 October 2021).






| All (n = 113) a | Intervention Group (n = 56) | Control Group (n = 57) | p | |
|---|---|---|---|---|
| Age (years) | 66.1 (57.5–71.9) | 66.0 (52.1–73.4) | 66.1 (61.0–71.6) | 0.45 |
| Sex: female, male, (n, %) | 78 (69%), 35 (31%) | 38 (68%), 18 (32%) | 40 (70%), 17 (30%) | 0.84 |
| Diabetes, n (%) | 7 (6%) | 3 (5%) | 4 (7%) | 1.00 |
| Impaired fasting glucose (n, %) | 9 (8%) | 4 (7%) | 5 (9%) | 1.00 |
| Body weight (kg) | 77.7 (69.1–87.2) | 77.9 (68.0–85.2) | 77.0 (69.8–88.0) | 0.70 |
| BMI (kg/m2) | 27.6 ± 4.5 | 27.4 ± 4.6 | 27.7 ± 4.4 | 0.72 |
| Waist circumference (cm) | 93.8 ± 12.1 | 93.3 ± 12.4 | 94.4 ± 11.9 | 0.64 |
| SBP (mmHg) | 139.7 ± 21.1 | 137.4 ± 20.4 | 142.0 ± 21.7 | 0.25 |
| DBP (mmHg) | 80.9 ± 10.2 | 79.9 ± 9.8 | 81.9 ± 10.5 | 0.31 |
| LDL cholesterol (mmol/L) | 3.94 ± 0.94 | 4.09 ± 0.96 | 3.79 ± 0.91 | 0.09 |
| Total cholesterol (mmol/L) | 5.68 ± 0.98 | 5.78 ± 1.03 | 5.57 ± 0.91 | 0.25 |
| HDL cholesterol (mmol/L) | 1.58 ± 0.36 | 1.58 ± 0.35 | 1.58 ± 0.38 | 0.96 |
| Apolipoprotein B (g/L) | 1.01 ± 0.22 | 1.03 ± 0.21 | 0.99 ± 0.22 | 0.26 |
| Apolipoprotein AI (g/L) | 1.43 ± 0.19 | 1.43 ± 0.20 | 1.43 ± 0.18 | 0.87 |
| Apolipoprotein B: AI ratio | 0.72 ± 0.18 | 0.73 ± 0.17 | 0.70 ± 0.18 | 0.38 |
| Triglycerides (mmol/L) | 1.1 (0.8–1.5) | 1.15 (0.8–1.5) | 1.1 (0.7–1.5) | 0.53 |
| Glucose b (mmol/L) | 5.5 (5.1–5.7) | 5.3 (5.0–5.7) | 5.4 (5.2–5.7) | 0.55 |
| HbA1c (mmol/mol) | 38 (36–41) | 38 (35–41) | 38 (37–41) | 0.58 |
| Intervention Group (n = 56) | Control Group (n = 56) | p | Intervention Group (n = 56) | Control Group (n = 56) | p | |
|---|---|---|---|---|---|---|
| 3 Weeks | 6 Months | |||||
| Body weight (kg) | −0.36 ± 0.67 | −0.81 ± 1.08 | 0.007 | 0.04 ± 2.22 | −0.84 ± 2.57 | 0.06 |
| BMI (kg/m2) | −0.13 ± 0.23 | −0.28 ± 0.38 | 0.01 | 0.02 ± 0.79 | −0.29 ± 0.87 | 0.051 |
| Waist circumference (cm) | −2.16 ± 3.58 | −1.90 ± 3.08 | 0.65 | −3.75 ± 5.06 | −4.44 ± 4.19 | 0.44 |
| SBP (mmHg) | −4.59 ± 12.0 | −5.05 ± 12.7 | 0.71 | −3.16 ± 13.7 | −3.37 ± 14.5 | 0.58 |
| DBP (mmHg) | −1.96 ± 6.92 | −1.65 ± 6.74 | 0.47 | −0.39 ± 7.87 | −0.96 ± 7.1 | 0.99 |
| LDL cholesterol (mmol/L) | −0.21 ± 0.38 | −0.11 ± 0.34 a | 0.45 | −0.046 ± 0.47 | 0.020 ± 0.41 a | 0.70 |
| Total cholesterol (mmol/L) | −0.19 ± 0.46 | −0.14 ± 0.44 | 0.87 | 0.034 ± 0.49 | 0.079 ± 0.47 | 0.89 |
| HDL cholesterol (mmol/L) | −0.009 ± 0.13 | −0.005 ± 0.13 | 0.87 | −0.054 ± 0.15 | −0.014 ± 0.12 | 0.10 |
| Apolipoprotein B (g/L) | −0.040 ± 0.09 | −0.024 ± 0.09 | 0.84 | −0.019 ± 0.10 | −0.019 ± 0.09 | 0.54 |
| Apolipoprotein AI (g/L) | −0.003 ± 0.10 | −0.022 ± 0.10 | 0.27 | 0.036 ± 0.12 | 0.035 ± 0.09 | 0.93 |
| Apolipoprotein B:AI ratio | −0.028 ± 0.07 | −0.005 ± 0.07 | 0.18 | −0.031 ± 0.09 | −0.032 ± 0.06 | 0.43 |
| Triglycerides (mmol/L) | 0.002 ± 0.41 | −0.030 ± 0.29 | 0.59 | 0.052 ± 0.43 | 0.044 ± 0.40 | 0.87 |
| Glucose (mmol/L) | −0.007 ± 0.58 | −0.12 ± 0.44 | 0.26 | 0.096 ± 0.75 | −0.058 ± 0.47 | 0.17 |
| HbA1c (mmol/mol) | −0.57 ± 1.49 | −0.32 ± 1.39 | 0.28 | −0.70 ± 1.81 | −0.58 ± 1.79 | 0.80 |
| Change in Intervention Group Responders b | ||||||
| 3 Weeks | 6 Months | |||||
| LDL cholesterol (mmol/L) | −0.67 ± 0.27 | 5 × 10−7 c | −0.31 ± 0.38 | 0.01 c | ||
| Intervention Group—LDL Cholesterol (n = 56) | |||||
|---|---|---|---|---|---|
| 3 Weeks | 6 Months | ||||
| Dietary Advice | Adherence (Maximum 21 Days per Advice), Median (IQR) | Beta (95% CI) in mmol/L per Daily Dietary Change | p | Beta (95% CI) in mmol/L per Daily Dietary Change | p |
| Total no. of reported daily dietary changes (out of maximum 273) | 56 (32.75–92) | −0.002 (−0.004, 0.000) | 0.051 | −0.002 (−0.004, 0.001) | 0.26 |
| Oils (replacing solid fats) | 0.5 (0–7.5) | −0.003 (−0.019, 0.012) | 0.69 | 0.000 (−0.019, 0.019) | 0.98 |
| Nuts and seeds | 4 (0–11) | 0.002 (−0.012, 0.017) | 0.73 | −0.004 (−0.022, 0.013) | 0.61 |
| Low−fat dairy products | 0.5 (0–15) | −0.008 (−0.021, 0.006) | 0.25 | −0.016 (−0.032, −0.000) | 0.048 |
| Foods with added plant sterols | 0 (0–0) | −0.027 (−0.047, −0.007) | 0.009 | −0.016 (−0.042, 0.010) | 0.22 |
| Pulses/soy (replacing meat and eggs) | 0 (0–3.75) | −0.014 (−0.043, 0.015) | 0.32 | −0.007 (−0.042, 0.029) | 0.71 |
| Probiotics, e.g., A-fil b and yogurt | 5.5 (0–15) | −0.006 (−0.019, 0.008) | 0.41 | −0.001 (−0.018, 0.016) | 0.92 |
| Oats, barley, and rye | 3 (0–15.75) | −0.006 (−0.020, 0.007) | 0.32 | 0.004 (−0.012, 0.021) | 0.58 |
| Fruits, berries, and vegetables (including avocado and tomato) | 5.5 (0–14.75) | −0.002 (−0.015, 0.012) | 0.79 | 0.001 (−0.016, 0.017) | 0.91 |
| Filtered coffee (replacing unfiltered) | 0 (0–6.5) | −0.012 (−0.025, 0.001) | 0.08 | −0.015 (−0.031, 0.000) | 0.06 |
| Tea (preferably green) | 3 (0–10.75) | −0.011 (−0.026, 0.004) | 0.13 | 0.001 (−0.018, 0.019) | 0.95 |
| Turmeric | 0.5 (0–9.5) | −0.019 (−0.034, −0.005) | 0.01 | −0.012 (−0.030, 0.007) | 0.21 |
| Garlic | 0.5 (0–5) | −0.005 (−0.027, 0.017) | 0.68 | −0.012 (−0.039, 0.015) | 0.36 |
| Dark chocolate | 1.5 (0–6.5) | −0.009 (−0.028, 0.010) | 0.36 | −0.006 (−0.029, 0.018) | 0.64 |
| Control Group—Triglycerides (n = 57) | |||||
| 3 Weeks | 6 Months | ||||
| Dietary Advice | Adherence (Maximum 21 Days per Advice), Median (IQR) | Beta (95% CI) in mmol/L per Daily Dietary Change | p | Beta (95% CI) in mmol/L per Daily Dietary Change | p |
| Total no. of reported daily dietary changes (out of maximum 63) | 14 (7–25) | −0.005 (−0.012, 0.002) | 0.15 | −0.003 (−0.013, 0.006) | 0.48 |
| Less alcohol | 0 (0–2) | −0.007 (−0.031, 0.016) | 0.53 | −0.013 (−0.046, 0.019) | 0.42 |
| Less carbohydrates | 9 (3–14.5) | −0.014 (−0.024, −0.003) | 0.01 | −0.008 (−0.023, 0.008) | 0.33 |
| Fatty fish | 4 (0–8) | 0.004 (−0.012, 0.020) | 0.59 | 0.003 (−0.019, 0.025) | 0.76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rydell, A.; Hellsten, M.; Lindow, M.; Iggman, D. Effectiveness of Written Dietary Advice for Improving Blood Lipids in Primary Care Adults—A Pragmatic Randomized Controlled Trial (MYDICLIN). Nutrients 2022, 14, 1022. https://0-doi-org.brum.beds.ac.uk/10.3390/nu14051022
Rydell A, Hellsten M, Lindow M, Iggman D. Effectiveness of Written Dietary Advice for Improving Blood Lipids in Primary Care Adults—A Pragmatic Randomized Controlled Trial (MYDICLIN). Nutrients. 2022; 14(5):1022. https://0-doi-org.brum.beds.ac.uk/10.3390/nu14051022
Chicago/Turabian StyleRydell, Andreas, Mikael Hellsten, Martin Lindow, and David Iggman. 2022. "Effectiveness of Written Dietary Advice for Improving Blood Lipids in Primary Care Adults—A Pragmatic Randomized Controlled Trial (MYDICLIN)" Nutrients 14, no. 5: 1022. https://0-doi-org.brum.beds.ac.uk/10.3390/nu14051022