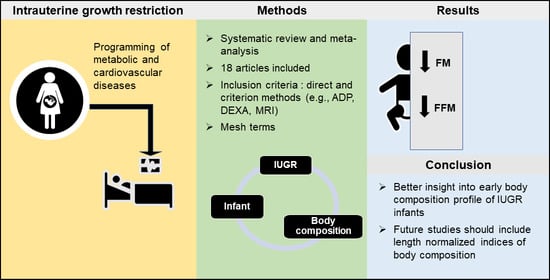
Body Composition of Infants Born with Intrauterine Growth Restriction: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria, Information Resources, and Search Strategy
2.3. Data Extraction and Items
2.4. Assessment of Quality
2.5. Data Synthesis
3. Results
3.1. Study Selection
3.2. Study Description and Results
3.3. Study Characteristics
3.4. Settings
3.5. Method of Assessment
3.6. Measurements
3.7. Participant Characteristics
3.8. Types of Studies
3.9. Meta-Analysis (IUGR vs. Normal Intrauterine Growth)
3.9.1. Fat Free Mass (in Grams)
Neonatal Period
From 6 Weeks to 6 Months of Age
3.9.2. Fat Mass (in Grams)
Neonatal Period
From 6 Weeks to 6 Months of Age
3.10. Meta-Analysis (SGA vs. AGA)
3.10.1. Fat Free Mass (in Grams)
3.10.2. Fat Mass (in Grams)
At Term Age
3.11. Quality of Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| %FM | Percentage fat mass |
| %FFM | Percentage fat-free mass |
| ADP | Air displacement plethysmography |
| DEXA | Dual-energy X-ray absorptiometry |
| FM | Fat mass |
| FMI | Fat mass index |
| FFM | Fat-free mass |
| IUGR | Intrauterine growth restriction |
| MRI | Magnetic resonance imaging |
| SGA | Small for gestational age |
References
- Calkins, K.; Devaskar, S.U. Fetal origins of adult disease. Curr. Probl. Pediatr. Adolesc. Health Care 2011, 41, 158–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faraci, M.; Renda, E.; Monte, S.; Di Prima, F.A.F.; Valenti, O.; De Domenico, R.; Giorgio, E.; Hyseni, E. Fetal growth restriction: Current perspectives. J. Prenat. Med. 2011, 5, 31–33. [Google Scholar] [PubMed]
- Beune, I.M.; Bloomfield, F.H.; Ganzevoort, W.; Embleton, N.D.; Rozance, P.J.; van Wassenaer-Leemhuis, A.G.; Wynia, K.; Gordijn, S.J. Consensus based definition of growth restriction in the newborn. J. Pediatr. 2018, 196, 71–76.e1. [Google Scholar] [CrossRef]
- Goldenberg, R.L.; Cliver, S.P. Small for gestational age and intrauterine growth restriction: Definitions and standards. Clin. Obstet. Gynecol. 1997, 40, 704–714. [Google Scholar] [CrossRef]
- Okada, T.; Takahashi, S.; Nagano, N.; Yoshikawa, K.; Usukura, Y.; Hosono, S. Early postnatal alteration of body composition in preterm and small-for-gestational-age infants: Implications of catch-up fat. Pediatr. Res. 2015, 77, 136–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Priante, E.; Verlato, G.; Giordano, G.; Stocchero, M.; Visentin, S.; Mardegan, V.; Baraldi, E. Intrauterine growth restriction: New insight from the metabolomic approach. Metabolites 2019, 9, 267. [Google Scholar] [CrossRef] [Green Version]
- de Fluiter, K.S.; van Beijsterveldt, I.A.L.P.; Breij, L.M.; Acton, D.; Hokken-Koelega, A.C.S. Association between fat mass in early life and later fat mass trajectories. JAMA Pediatr. 2020, 174, 1141–1148. [Google Scholar] [CrossRef]
- Goossens, G.H. The metabolic phenotype in obesity: Fat mass, body fat distribution, and adipose tissue function. Obes. Facts 2017, 10, 207–215. [Google Scholar] [CrossRef]
- Rolland-Cachera, M.F.; Péneau, S. Growth trajectories associated with adult obesity. World Rev. Nutr. Diet. 2013, 106, 127–134. [Google Scholar] [CrossRef]
- Roggero, P.; Giannì, M.L.; Liotto, N.; Taroni, F.; Morniroli, D.; Mosca, F. Small for gestational age preterm infants: Nutritional strategies and quality of growth after discharge. J. Matern. Fetal Neonatal Med. 2011, 24 (Suppl. 1), 144–146. [Google Scholar] [CrossRef]
- Tudehope, D.; Vento, M.; Bhutta, Z.; Pachi, P. Nutritional requirements and feeding recommendations for small for gestational age infants. J. Pediatr. 2013, 162, S81–S89. [Google Scholar] [CrossRef] [PubMed]
- Giannì, M.L.; Roggero, P.; Liotto, N.; Taroni, F.; Polimeni, A.; Morlacchi, L.; Piemontese, P.; Consonni, D.; Mosca, F. Body composition in late preterm infants according to percentile at birth. Pediatr. Res. 2016, 79, 710–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van de Lagemaat, M.; Rotteveel, J.; Lafeber, H.; van Weissenbruch, M. Lean mass and fat mass accretion between term age and 6 months post-term in growth-restricted preterm infants. Eur. J. Clin. Nutr. 2014, 68, 1261–1263. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.J.; Wootton, S.A.; Leaf, A.A.; Jackson, A.A. Preterm birth and body composition at term equivalent age: A systematic review and meta-analysis. Pediatrics 2012, 130, e640–e649. [Google Scholar] [CrossRef] [Green Version]
- Victora, C.G.; Adair, L.; Fall, C.; Hallal, P.C.; Martorell, R.; Richter, L.; Sachdev, H.S.; Maternal and Child Undernutrition Study Group. Maternal and child undernutrition: Consequences for adult health and human capital. Lancet 2008, 371, 340–357. [Google Scholar] [CrossRef] [Green Version]
- Duren, D.L.; Sherwood, R.J.; Czerwinski, S.A.; Lee, M.; Choh, A.C.; Siervogel, R.M.; Cameron Chumlea, W. Body composition methods: Comparisons and interpretation. J. Diabetes Sci. Technol. 2008, 2, 1139–1146. [Google Scholar] [CrossRef] [Green Version]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [Green Version]
- Cochrane Effective Practice and Organisation of Care (EPOC). EPOC Resources for Review Authors. 2017. Available online: Epoc.cochrane.org/epoc-resources-review-authors (accessed on 28 November 2021).
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality if Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 26 January 2022).
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welch, V. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (Updated February 2021), 2nd ed.; John Wiley & Sons: Oxford, UK, 2021. [Google Scholar]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [Green Version]
- StataCorp. Stata Statistical Software: Release 16; StataCorp LLC.: College Station, TX, USA, 2019. [Google Scholar]
- Koo, W.; Walters, J.; Hockman, E. Body composition in neonates: Relationship between measured and derived anthropometry with dual-energy X-ray absorptiometry measurements. Pediatr. Res. 2004, 56, 694–700. [Google Scholar] [CrossRef] [Green Version]
- Demarini, S.; Koo, W.; Hockman, E. Bone, lean and fat mass of newborn twins versus singletons. Acta Paediatr. 2006, 95, 594–599. [Google Scholar] [CrossRef]
- Modi, N.; Thomas, E.; Harrington, T.; Uthaya, S.; Doré, C.; Bell, J. Determinants of adiposity during preweaning postnatal growth in appropriately grown and growth-restricted term infants. Pediatr. Res. 2006, 60, 345–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verkauskiene, R.; Beltrand, J.; Claris, O.; Chevenne, D.; Deghmoun, S.; Dorgeret, S.; Alison, M.; Gaucherand, P.; Sibony, O.; Lévy-Marchal, C. Impact of fetal growth restriction on body composition and hormonal status at birth in infants of small and appropriate weight for gestational age. Eur. J. Clin. Endocrinol. 2007, 157, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, L.; Sebastiani, G.; Lopez-Bermejo, A.; Díaz, M.; Gómez-Roig, M.; de Zegher, F. Gender specificity of body adiposity and circulating adiponectin, visfatin, insulin, and insulin growth factor-I at term birth: Relation to prenatal growth. J. Clin. Endocrinol. Metab. 2008, 93, 2774–2778. [Google Scholar] [CrossRef]
- Ibáñez, L.; Sebastiani, G.; Diaz, M.; Gómez-Roig, M.; Lopez-Bermejo, A.; de Zegher, F. Low body adiposity and high leptinemia in breast-fed infants born small-for-gestational-age. J. Pediatr. 2010, 156, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Moyer-Mileur, L.; Slater, H.; Thomson, J.; Mihalopoulos, N.; Byrne, J.; Varner, M. Newborn adiposity measured by plethysmography is not predicted by late gestation two-dimensional ultrasound measures of fetal growth. J. Nutr. 2009, 139, 1772–1778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Law, T.; Korte, J.; Katikaneni, L.; Wagner, C.; Ebeling, M.; Newman, R. Ultrasound assessment of intrauterine growth restriction: Relationship to neonatal body composition. Am. J. Obstet. Gynecol. 2011, 205, 255.e1–255.e6. [Google Scholar] [CrossRef]
- deZegher, F.; Sebastiani, G.; Diaz, M.; Sánchez-Infantes, D.; Lopez-Bermejo, A.; Ibáñez, L. Body composition and circulating high-molecular-weight adiponectin and IGF-I in infants born small for gestational age: Breast-versus formula-feeding. Diabetes 2012, 61, 1969–1973. [Google Scholar] [CrossRef] [Green Version]
- Law, T.; Katikaneni, L.; Taylor, S.; Korte, J.; Ebeling, M.; Wagner, C.; Newman, R. Customized versus population-based growth curves: Prediction of low body fat percent at term corrected gestational age following preterm birth. J. Matern. Neonatal Med. 2012, 25, 1142–1147. [Google Scholar] [CrossRef]
- Mazarico, E.; Martinez-Cumplido, R.; Díaz, M.; Sebastiani, G.; Ibáñez, L.; Gómez-Roig, M.D. Postnatal anthropometric and body composition profiles in infants with intrauterine growth restriction identified by prenatal doppler. PLoS ONE 2016, 11, e0150152. [Google Scholar] [CrossRef] [Green Version]
- Villela, L.; Méio, M.; Gomes Junior, S.; de Abranches, A.; Soares, F.; Moreira, M. Body composition in preterm infants with intrauterine growth restriction: A cohort study. J. Perinat. Med. 2018, 46, 804–810. [Google Scholar] [CrossRef]
- Schmelzle, H.; Quang, D.; Fusch, G.; Fusch, C. Birth weight categorization according to gestational age does not reflect percentage body fat in term and preterm newborns. Eur. J. Pediatr. 2007, 166, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Larsson, A.; Ottosson, P.; Törnqvist, C.; Olhager, E. Body composition and growth in full-term small for gestational age and large for gestational age Swedish infants assessed with air displacement plethysmography at birth and at 3-4 months of age. PLoS ONE 2019, 14, e0207978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuriyan, R.; Naqvi, S.; Bhat, K.G.; Ghosh, S.; Rao, S.; Preston, T.; Sachdev, H.S.; Kurpad, A.V. The thin but fat phenotype is uncommon at birth in Indian babies. J. Nutr. 2020, 150, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Roggero, P.; Giannì, M.L.; Liotto, N.; Taroni, F.; Orsi, A.; Amato, O.; Morlacchi, L.; Piemontese, P.; Agosti, M.; Mosca, F. Rapid recovery of fat mass in small for gestational age preterm infants after term. PLoS ONE 2011, 6, e14489. [Google Scholar] [CrossRef] [Green Version]
- Rigo, J.; Nyamugabo, K.; Picaud, J.C.; Gerard, P.; Pieltain, C.; De Curtis, M. Reference values of body composition obtained by dual energy X-ray absorptiometry in preterm and term neonates. J. Pediatr. Gastroenterol. Nutr. 1998, 27, 184–190. [Google Scholar] [CrossRef]
- Hamatschek, C.; Yousuf, E.I.; Möllers, L.S.; So, H.Y.; Morrison, K.M.; Fusch, C.; Rochow, N. Fat and fat-free mass of preterm and term infants from birth to six months: A review of current evidence. Nutrients 2020, 12, 288. [Google Scholar] [CrossRef] [Green Version]
- Ibáñez, L.; Ong, K.; Dunger, D.B.; de Zegher, F. Early development of adiposity and insulin resistance after catch-up weight gain in small-for-gestational-age children. J. Clin. Endocrinol. Metab. 2006, 91, 2153–2158. [Google Scholar] [CrossRef] [Green Version]
- Harder, T.; Roepke, K.; Diller, N.; Stechling, Y.; Dudenhausen, J.W.; Plagemann, A. Birth weight, early weight gain, and subsequent risk of type 1 diabetes: Systematic review and meta-analysis. Am. J. Epidemiol. 2009, 169, 1428–1436. [Google Scholar] [CrossRef] [Green Version]
- Burrows, R.; Correa-Burrows, P.; Reyes, M.; Blanco, E.; Albala, C.; Gahagan, S. Low muscle mass is associated with cardiometabolic risk regardless of nutritional status in adolescents: A cross-sectional study in a Chilean birth cohort. Pediatr. Diabetes 2017, 18, 895–902. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, Y.S. Low muscle mass is associated with metabolic syndrome in Korean adolescents: The Korea National Health and Nutrition Examination Survey 2009-2011. Nutr. Res. 2016, 36, 1423–1428. [Google Scholar] [CrossRef]
- Wells, J.C. The programming effects of early growth. Early Hum. Dev. 2007, 83, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Agrogiannis, G.D.; Sifakis, S.; Patsouris, E.S.; Konstantinidou, A.E. Insulin-like growth factors in embryonic and fetal growth and skeletal development. Mol. Med. Rep. 2014, 10, 579–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frondas-Chauty, A.; Simon, L.; Flamant, C.; Hanf, M.; Darmaun, D.; Rozé, J.-C. Deficit of Fat Free Mass in Very Preterm Infants at Discharge is Associated with Neurological Impairment at Age 2 Years. J. Pediatr. 2018, 196, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Bell, K.A.; Matthews, L.G.; Cherkerzian, S.; Palmer, C.; Drouin, K.; Pepin, H.L.; Ellard, D.; Inder, T.E.; Ramel, S.E.; Belfort, M.B. Associations of Growth and Body Composition with Brain Size in Preterm Infants. J. Pediatr. 2019, 214, 20–26.e2. [Google Scholar] [CrossRef]
- McLeod, G.; Sherriff, J.; Hartmann, P.E.; Nathan, E.; Geddes, D.; Simmer, K. Comparing different methods of human breast milk fortification using measured v. assumed macronutrient composition to target reference growth: A randomised controlled trial. Br. J. Nutr. 2016, 115, 431–439. [Google Scholar] [CrossRef] [Green Version]
- Sung, I.-K.; Vohr, B.; Oh, W. Growth and neurodevelopmental outcome of very low birth weight infants with intrauterine growth retardation: Comparison with control subjects matched by birth weight and gestational age. J. Pediatr. 1993, 123, 618–624. [Google Scholar] [CrossRef]
- Wells, J.C.K. Toward body composition reference data for infants, children, and adolescents. Adv. Nutr. 2014, 5, 320S–329S. [Google Scholar] [CrossRef] [Green Version]
- Martin, C.R.; Brown, Y.F.; Ehrenkranz, R.A.; O’Shea, T.M.; Allred, E.N.; Belfort, M.B.; McCormick, M.C.; Leviton, A. Nutritional practices and growth velocity in the first month of life in extremely premature infants. Pediatrics 2009, 124, 649–657. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Perino, J.; Bowers, L.; Welch, B.; Albert, V.; Drenckpohl, D.; Wolfe, D. Cumulative impact of multiple evidence based strategies on postnatal growth of extremely-low-birth-weight infants. Clin. Nutr. 2021, 40, 3908–3913. [Google Scholar] [CrossRef]
- Goswami, I.; Rochow, N.; Fusch, G.; Liu, K.; Marrin, M.L.; Heckmann, M.; Nelle, M.; Fusch, C. Length Normalized Indices for Fat Mass and Fat-Free Mass in Preterm and Term Infants during the First Six Months of Life. Nutrients 2016, 8, 417. [Google Scholar] [CrossRef] [Green Version]
- Pereira-da-Silva, L.; Virella, D. Accurate Direct Measures Are Required to Validate Derived Measures. Neonatology 2018, 113, 266. [Google Scholar] [CrossRef] [PubMed]
- VanItallie, T.B.; Yang, M.U.; Heymsfield, S.B.; Funk, R.C.; Boileau, R.A. Height-normalized indices of the body’s fat-free mass and fat mass: Potentially useful indicators of nutritional status. Am. J. Clin. Nutr. 1990, 52, 953–959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wells, J.C.K.; Victora, C.G. Indices of whole-body and central adiposity for evaluating the metabolic load of obesity. Int. J. Obes. 2005, 29, 483–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Study | Method | Country | IUGR | Sample Size | Gestational Age (Weeks) of Index Group | Gestational Age (Weeks) of Control Group | Birth Weight (g) Index Group | Birth Weight (g) Control Group | Age at Follow-Up Assessment | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |||||||
| Koo W, 2004 [23] | DEXA | USA | No | 90 | 36.5 (2.6) | 35.9 (2.9) | 1971 (522) | 2454 (634) | 3 to 5 days after birth | FFM, FM, FM% |
| Demarini S, 2006 [24] | DEXA | Italy | Yes | 40 | 36.09/2.3 | 32.89/3.3 | 1886 (495) | 2 weeks | FFM, FM | |
| Modi N, 2006 [25] | MRI | UK | Yes | 29 | Term | Term | 3.26 (0.43) | 2.27 (0.28) | Day 76 weeks | %FM, weight, length |
| Verkauskiene R, 2007 [26] | DEXA | France | No | 248 | 38.5 (2) | 39 (2) | 2480 (464) | 3227 (449) | Day 3 | FFM, %FM, weight, length, head circumference |
| Ibañez L, 2008 [27] | DEXA | Spain | No | 96 | 39 (0.3) | 40 (0.2) | 2300 (400) | 3400 (400) | 14 days | FFM, FM, %FM |
| Ibañez L, 2010 [28] | DEXA | Spain | No | 74 | Term | Term | 2281 (107) | 3409 (92) | 15 days, 4 months | FFM, FM, %FM |
| Moyer-Mileur H, 2009 [29] | ADP | USA | No | 43 | Term | Term | 2535 (246) | 3319 (67.6) | Neonatal period | %FM, weight, length, head circumference |
| Law TL, 2011 [30] | ADP | USA | Yes | 87 | 37.7 (1.5) | 38.5 (1.5) | 2677 (348) | 3273 (591) | Within 7 days | %FM, Weight, length, head circumference |
| de Zegher F, 2012 [31] | DEXA | Spain | No | 174 | Term | Term | 2900 | 3900 | 2 weeks, 4 months | FFM, FM, weight, length, head circumference |
| Law TL, 2012 [32] | ADP | USA | No | 214 | 30.4 (3.1) | 28.4 (3.2) | 1148 (421) | 1277 (535) | Term age (~10 weeks) | %FM |
| van de Langemaat M, 2014 [13] | DEXA | England | Yes | 98 | 31.1 (1.6) | 30.1 (2.0) | 1465 (371) | 1182 (220) | Term age, 6 months | FFM, FM, %FM, weight, length |
| Giannì M, 2016 [12] | ADP | Italy | No | 122 | 35.4 (0.77) | 35.4 (0.77) | 2111 (102) | 2468 (331) | 5th day of life | FM, %FM, FMI, FFMI, weight, length, head circumference |
| Mazarico R, 2016 [33] | DEXA | Spain | Yes | 48 | 38.2 (0.2) | 37.7 (0.2) | 2279 (290) | 2208 (210) | 10 days, 4 months, 12 months | FM, FFM, weight |
| Villela L, 2018 [34] | ADP | Germany | No | 92 | 31.8 (1.8) | 29.7 (1.5) | 1270 | 1270 | Term age, 1, 3, and 5 months | FFM, FM, %FM, %FFM, LM/FM |
| Schmelzle H, 2007 [35] | DEXA | Germany | No | 21 | 38.2 (2.7) | 38.3 (3.0) | 2320 (660) | 3150 (680) | Within 10 days | %FM, weight, length |
| Larsson A, 2019 [36] | ADP | Sweden | No | 50 | 38.9 (1.6) | 4.1 (1.5) | 2499 (209) | 4617 (366) | Within 10 days, 4 months | FFM, FM, %FM |
| Kuriyan R, 2020 [37] | ADP | India | No | 153 | 39 (1.0) | 39.5 (1.0) | 2700 (100) | 3000 (100) | Within 10 days of birth | FFM, FM, %FM |
| Roggero P, 2011 [38] | ADP | Italy | Yes | 195 | 29.4 (2.2) | 29.3(1.8) | 1204.8 (253) | 1260.8 (198) | Term corrected age, 3 months and 5 months after term | %FM change |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manapurath, R.; Gadapani, B.; Pereira-da-Silva, L. Body Composition of Infants Born with Intrauterine Growth Restriction: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 1085. https://0-doi-org.brum.beds.ac.uk/10.3390/nu14051085
Manapurath R, Gadapani B, Pereira-da-Silva L. Body Composition of Infants Born with Intrauterine Growth Restriction: A Systematic Review and Meta-Analysis. Nutrients. 2022; 14(5):1085. https://0-doi-org.brum.beds.ac.uk/10.3390/nu14051085
Chicago/Turabian StyleManapurath, Rukman, Barsha Gadapani, and Luís Pereira-da-Silva. 2022. "Body Composition of Infants Born with Intrauterine Growth Restriction: A Systematic Review and Meta-Analysis" Nutrients 14, no. 5: 1085. https://0-doi-org.brum.beds.ac.uk/10.3390/nu14051085