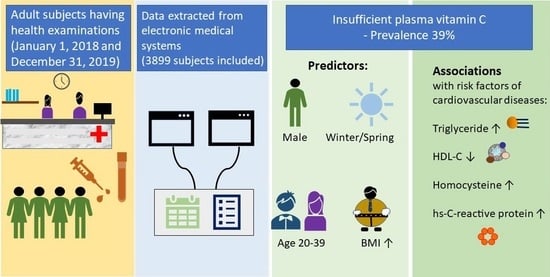
Prevalence and Predictors of Insufficient Plasma Vitamin C in a Subtropical Region and Its Associations with Risk Factors of Cardiovascular Diseases: A Retrospective Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Parameters, Definitions and Cutoffs
2.3. Assessment of Plasma Vitamin C, Serum Homocysteine, hs-CRP, Lipoprotein(a) and Lipid Profiles
2.3.1. Blood Collection and Determination of Plasma Vitamin C Concentrations
2.3.2. Blood Collection and Determination of Serum Homocysteine
2.3.3. Blood Collection and Determination of Serum hs-CRP, Lipoprotein(a) and Lipid Profiles
2.4. Statistical Analysis
3. Results
3.1. Demographic and Anthropometric Characteristics of the Study Population
3.2. Primary Outcomes
3.2.1. Prevalence of Insufficient Plasma Vitamin C among Adults
3.2.2. Predictors for Insufficient Vitamin C Levels among All Subjects
3.2.3. Predictors of Insufficient Vitamin C Levels among Female and Male Subjects
3.3. Secondary Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wong, M.C.; Zhang, D.X.; Wang, H.H. Rapid emergence of atherosclerosis in Asia: A systematic review of coronary atherosclerotic heart disease epidemiology and implications for prevention and control strategies. Curr. Opin. Lipidol. 2015, 26, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Palfrey, H.A.; Pathak, R.; Kadowitz, P.J.; Gettys, T.W.; Murthy, S.N. The metabolism and significance of homocysteine in nutrition and health. Nutr. Metab. 2017, 14, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bucci, M.; Tana, C.; Giamberardino, M.; Cipollone, F. Lp(a) and cardiovascular risk: Investigating the hidden side of the moon. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 980–986. [Google Scholar] [CrossRef]
- Abramson, J.L.; Hooper, W.C.; Jones, D.P.; Ashfaq, S.; Rhodes, S.D.; Weintraub, W.S.; Harrison, D.G.; Quyyumi, A.A.; Vaccarino, V. Association between novel oxidative stress markers and C-reactive protein among adults without clinical coronary heart disease. Atherosclerosis 2005, 178, 115–121. [Google Scholar] [CrossRef]
- Nishikimi, M.; Fukuyama, R.; Minoshima, S.; Shimizu, N.; Yagi, K. Cloning and chromosomal mapping of the human nonfunctional gene for L-gulono-gamma-lactone oxidase, the enzyme for L-ascorbic acid biosynthesis missing in man. J. Biol. Chem. 1994, 269, 13685–13688. [Google Scholar] [CrossRef]
- Otsuka, M.; Matsuzawa, M.; Ha, T.Y.; Arakawa, N. Contribution of a high dose of L-ascorbic acid to carnitine synthesis in guinea pigs fed high-fat diets. J. Nutr. Sci. Vitaminol. 1999, 45, 163–171. [Google Scholar] [CrossRef]
- Fernandez, M.L. Guinea pigs as models for cholesterol and lipoprotein metabolism. J. Nutr. 2001, 131, 10–20. [Google Scholar] [CrossRef] [Green Version]
- Westhuyzen, J. The oxidation hypothesis of atherosclerosis: An update. Ann. Clin. Lab. Sci. 1997, 27, 1–10. [Google Scholar]
- Nofer, J.R.; Kehrel, B.; Fobker, M.; Levkau, B.; Assmann, G.; von Eckardstein, A. HDL and arteriosclerosis: Beyond reverse cholesterol transport. Atherosclerosis 2002, 161, 1–16. [Google Scholar] [CrossRef]
- Witztum, J.L. The oxidation hypothesis of atherosclerosis. Lancet 1994, 344, 793–795. [Google Scholar] [CrossRef]
- Freyschuss, A.; Xiu, R.-J.; Zhang, J.; Ying, X.; Diczfalusy, U.; Jogestrand, T.; Henriksson, P.; Björkhem, I. Vitamin C reduces cholesterol-induced microcirculatory changes in rabbits. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 1178–1184. [Google Scholar] [CrossRef] [PubMed]
- Hillstrom, R.J.; Yacapin-Ammons, A.K.; Lynch, S.M. Vitamin C inhibits lipid oxidation in human HDL. J. Nutr. 2003, 133, 3047–3051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alul, R.H.; Wood, M.; Longo, J.; Marcotte, A.L.; Campione, A.L.; Moore, M.K.; Lynch, S.M. Vitamin C protects low-density lipoprotein from homocysteine-mediated oxidation. Free Radic. Biol. Med. 2003, 34, 881–891. [Google Scholar] [CrossRef]
- Hackam, D.G.; Anand, S.S. Emerging risk factors for atherosclerotic vascular disease: A critical review of the evidence. JAMA 2003, 290, 932–940. [Google Scholar] [CrossRef]
- Vignini, A.; Nanetti, L.; Bacchetti, T.; Ferretti, G.; Curatola, G.; Mazzanti, L. Modification induced by homocysteine and low-density lipoprotein on human aortic endothelial cells: An in vitro study. J. Clin. Endocrinol. Metab. 2004, 89, 4558–4561. [Google Scholar] [CrossRef] [Green Version]
- Zinellu, A.; Sotgia, S.; Scanu, B.; Pintus, G.; Posadino, A.M.; Cossu, A.; Deiana, L.; Sengupta, S.; Carru, C. S-homocysteinylated LDL apolipoprotein B adversely affects human endothelial cells in vitro. Atherosclerosis 2009, 206, 40–46. [Google Scholar] [CrossRef]
- Breilmann, J.; Pons-Kühnemann, J.; Brunner, C.; Richter, M.; Neuhäuser-Berthold, M. Effect of antioxidant vitamins on the plasma homocysteine level in a free-living elderly population. Ann. Nutr. Metab. 2010, 57, 177–182. [Google Scholar] [CrossRef]
- Cha, J.; Niedzwiecki, A.; Rath, M. Hypoascorbemia induces atherosclerosis and vascular deposition of lipoprotein(a) in transgenic mice. Am. J. Cardiovasc. Dis. 2015, 5, 53–62. [Google Scholar]
- Block, G.; Jensen, C.D.; Dalvi, T.B.; Norkus, E.P.; Hudes, M.; Crawford, P.B.; Holland, N.; Fung, E.B.; Schumacher, L.; Harmatz, P. Vitamin C treatment reduces elevated C-reactive protein. Free Radic. Biol. Med. 2009, 46, 70–77. [Google Scholar] [CrossRef] [Green Version]
- Rowe, S.; Carr, A.C. Global Vitamin C Status and Prevalence of Deficiency: A Cause for Concern? Nutrients 2020, 12, 2008. [Google Scholar] [CrossRef]
- Bentham, J.; Singh, G.M.; Danaei, G.; Green, R.; Lin, J.K.; Stevens, G.A.; Farzadfar, F.; Bennett, J.E.; di Cesare, M.; Dangour, A.D.; et al. Multi-dimensional characterisation of global food supply from 1961–2013. Nat. Food. 2020, 1, 70–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.Y.; Cheng, T.-J.; Chang, C.-Y.; Lan, K.-M.; Weng, S.-F.; Sheu, M.-J.; Tseng, S.-F.; Hu, M.-L. Increased incidence of herpes zoster in adult patients with peptic ulcer disease: A population-based cohort study. Int. J. Epidemiol. 2013, 42, 1873–1881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.K.; Hung, K.-C.; Lin, Y.-T.; Chang, Y.-J.; Wu, Z.-F.; Ho, C.-H.; Chen, J.-Y. Age, Gender and Season Are Good Predictors of Vitamin D Status Independent of Body Mass Index in Office Workers in a Subtropical Region. Nutrients 2020, 12, 2719. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.K.; Lin, Y.-T.; Hung, K.-C.; Chang, C.-Y.; Wu, Z.-F.; Hu, M.-L.; Chen, J.-Y. Plasma Vitamin C Concentrations Were Negatively Associated with Tingling, Prickling or Pins and Needles Sensation in Patients with Postherpetic Neuralgia. Nutrients 2020, 12, 2384. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, E.S.; Bloomgarden, Z.T.; Zimmet, P.Z. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes: Response to the International Expert Committee. Diabetes Care 2009, 32, e159. [Google Scholar] [CrossRef] [Green Version]
- Lorenzo, C.; Waagenknecht, L.E.; Hanley, A.J.G.; Rewers, M.J.; Karter, A.J.; Haffner, S.M. A1C between 5.7 and 6.4% as a marker for identifying pre-diabetes, insulin sensitivity and secretion, and cardiovascular risk factors: The Insulin Resistance Atherosclerosis Study (IRAS). Diabetes Care 2010, 33, 2104–2109. [Google Scholar] [CrossRef] [Green Version]
- Lykkesfeldt, J.; Tveden-Nyborg, P. The Pharmacokinetics of Vitamin C. Nutrients 2019, 11, 2412. [Google Scholar] [CrossRef] [Green Version]
- Grunfeld, C.; A Delaney, J.; Wanke, C.; Currier, J.S.; Scherzer, R.; Biggs, M.L.; Tien, P.C.; Shlipak, M.G.; Sidney, S.; Polak, J.F.; et al. Preclinical atherosclerosis due to HIV infection: Carotid intima-medial thickness measurements from the FRAM study. AIDS 2009, 23, 1841–1849. [Google Scholar] [CrossRef]
- Agarwal, A.; Prasad, G.V. Post-transplant dyslipidemia: Mechanisms, diagnosis and management. World J. Transplant. 2016, 6, 125–134. [Google Scholar] [CrossRef]
- Chatrath, H.; Vuppalanchi, R.; Chalasani, N. Dyslipidemia in patients with nonalcoholic fatty liver disease. Semin. Liver Dis. 2012, 32, 22–29. [Google Scholar] [CrossRef] [Green Version]
- Vaziri, N.D. Dyslipidemia of chronic renal failure: The nature, mechanisms, and potential consequences. Am. J. Physiol. Renal. Physiol. 2006, 290, F262–F272. [Google Scholar] [CrossRef] [PubMed]
- German Nutrition Society. New Reference Values for Vitamin C Intake. Ann. Nutr. Metab. 2015, 67, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Rosengrave, P.C.; Bayer, S.; Chambers, S.; Mehrtens, J.; Shaw, G.M. Hypovitaminosis C and vitamin C deficiency in critically ill patients despite recommended enteral and parenteral intakes. Crit. Care 2017, 21, 300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pincemail, J.; Vanbelle, S.; Degrune, F.; Cheramy-Bien, J.; Charlier, C.; Chapelle, J.; Giet, D.; Collette, G.; Albert, A.; Defraigne, J. Lifestyle Behaviours and Plasma Vitamin C and beta-Carotene Levels from the ELAN Population (Liege, Belgium). J. Nutr. Metab. 2011, 2011, 494370. [Google Scholar] [CrossRef] [Green Version]
- Crook, J.; Horgas, A.; Yoon, S.-J.; Grundmann, O.; Johnson-Mallard, V. Insufficient Vitamin C Levels among Adults in the United States: Results from the NHANES Surveys, 2003–2006. Nutrients 2021, 13, 3910. [Google Scholar] [CrossRef]
- WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004, 363, 157–163. [Google Scholar] [CrossRef]
- Huang, Y.C.; Ho, C.-C.; Lin, P.-T.; Lee, B.-J.; Lai, C.-H.; Liaw, Y.-P. Optimal cutoff value of high-density lipoprotein cholesterol for predicting coronary artery disease in Taiwanese population. Nutr. Res. 2010, 30, 21–26. [Google Scholar] [CrossRef]
- Wald, D.S.; Law, M.; Morris, J.K. Homocysteine and cardiovascular disease: Evidence on causality from a meta-analysis. BMJ 2002, 325, 1202. [Google Scholar] [CrossRef] [Green Version]
- Jacobson, T.A. Lipoprotein(a), cardiovascular disease, and contemporary management. Mayo Clin. Proc. 2013, 88, 1294–1311. [Google Scholar] [CrossRef] [Green Version]
- Robitaille, L.; Hoffer, L.J. A simple method for plasma total vitamin C analysis suitable for routine clinical laboratory use. Nutr. J. 2016, 15, 40. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.N.; Yoon, S.Y. Comparison of a new enzymatic assay for serum homocysteine on Toshiba TBA-c16000 against an immunoassay on Abbott Architect. Scand. J. Clin. Lab. Invest. 2021, 81, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Pieri, M.; Ciotti, M.; Nuccetelli, M.; Perrone, M.A.; Caliò, M.T.; Lia, M.S.; Minieri, M.; Bernardini, S. Serum Amyloid A Protein as a useful biomarker to predict COVID-19 patients severity and prognosis. Int. Immunopharmacol. 2021, 95, 107512. [Google Scholar] [CrossRef] [PubMed]
- Marcovina, S.M.; Albers, J.J. Lipoprotein (a) measurements for clinical application. J. Lipid. Res. 2016, 57, 526–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paalanen, L.; Prättälä, R.; Alfthan, G.; Salminen, I.; Laatikainen, T. Vegetable and fruit consumption, education and plasma vitamin C concentration in Russian and Finnish Karelia, 1992–2002. Public Health Nutr. 2014, 17, 2278–2286. [Google Scholar] [CrossRef] [Green Version]
- Pearson, J.F.; Pullar, J.M.; Wilson, R.; Spittlehouse, J.K.; Vissers, M.C.M.; Skidmore, P.M.L.; Willis, J.; Cameron, V.A.; Carr, A.C. Vitamin C Status Correlates with Markers of Metabolic and Cognitive Health in 50-Year-Olds: Findings of the CHALICE Cohort Study. Nutrients 2017, 9, 831. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Yokoyama, T.; Yoshida, H.; Kim, H.; Shimada, H.; Yoshida, Y.; Iwasa, H.; Shimizu, Y.; Kondo, Y.; Handa, S.; et al. A significant relationship between plasma vitamin C concentration and physical performance among Japanese elderly women. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.H.; Yeh, N.-H.; Yang, R.-Y.; Lin, W.-H.; Wu, W.-C.; Yeh, W.-T.; Sung, M.-K.; Lee, H.-S.; Chang, S.-J.; Huang, C.-J.; et al. Vegetable, fruit, and phytonutrient consumption patterns in Taiwan. J. Food Drug Anal. 2018, 26, 145–153. [Google Scholar] [CrossRef] [Green Version]
- Schleicher, R.L.; Carroll, M.D.; Ford, E.S.; A Lacher, D. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003–2004 National Health and Nutrition Examination Survey (NHANES). Am. J. Clin. Nutr. 2009, 90, 1252–1263. [Google Scholar] [CrossRef]
- Prattala, R.; Paalanen, L.; Grinberga, D.; Helasoja, V.; Kasmel, A.; Petkeviciene, J. Gender differences in the consumption of meat, fruit and vegetables are similar in Finland and the Baltic countries. Eur. J. Public Health 2007, 17, 520–525. [Google Scholar] [CrossRef]
- Jungert, A.; Neuhauser-Berthold, M. The lower vitamin C plasma concentrations in elderly men compared with elderly women can partly be attributed to a volumetric dilution effect due to differences in fat-free mass. Br. J. Nutr. 2015, 113, 859–864. [Google Scholar] [CrossRef] [Green Version]
- Frankenfeld, C.L.; Lampe, J.W.; Shannon, J.; Gao, D.L.; Li, W.; Ray, R.M.; Chen, C.; King, I.B.; Thomas, D.B. Fruit and vegetable intakes in relation to plasma nutrient concentrations in women in Shanghai, China. Public Health Nutr. 2012, 15, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Hooper, P.L.; Hooper, E.M.; Hunt, W.C.; Garry, P.J.; Goodwin, J.S. Vitamins, lipids and lipoproteins in a healthy elderly population. Int. J. Vitaminol. Nutr. Res. 1983, 53, 412–419. [Google Scholar] [PubMed]
- McRae, M.P. Vitamin C supplementation lowers serum low-density lipoprotein cholesterol and triglycerides: A meta-analysis of 13 randomized controlled trials. J. Chiropr. Med. 2008, 7, 48–58. [Google Scholar] [CrossRef] [Green Version]
- Kubota, Y.; Moriyama, Y.; Yamagishi, K.; Tanigawa, T.; Noda, H.; Yokota, K.; Harada, M.; Inagawa, M.; Oshima, M.; Sato, S.; et al. Serum vitamin C concentration and hs-CRP level in middle-aged Japanese men and women. Atherosclerosis 2010, 208, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.M.; Fan, J.-H.; Taylor, P.R.; Lam, T.K.; Dawsey, S.M.; Qiao, Y.-L.; Abnet, C.C. Association of plasma vitamin C concentration to total and cause-specific mortality: A 16-year prospective study in China. J. Epidemiol. Community Health 2018, 72, 1076–1082. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Niedzwiecki, A.; Rath, M. Age and Dietary Vitamin C Intake Affect Brain Physiology in Genetically Modified Mice Expressing Human Lipoprotein(A) and Unable to Synthesize Vitamin C. Curr. Aging. Sci. 2021, 14, 223–234. [Google Scholar] [CrossRef]
- Bostom, A.G.; Hume, A.L.; Eaton, C.; Laurino, J.P.; Yanek, L.R.; Regan, M.S.; McQuade, W.H.; Craig, W.Y.; Perrone, G.; Jacques, P.F. The effect of high-dose ascorbate supplementation on plasma lipoprotein(a) levels in patients with premature coronary heart disease. Pharmacotherapy 1995, 15, 458–464. [Google Scholar]
- Dehghan, M.; Akhtar-Danesh, N.; McMillan, C.R.; Thabane, L. Is plasma vitamin C an appropriate biomarker of vitamin C intake? A systematic review and meta-analysis. Nutr. J. 2007, 6, 41. [Google Scholar] [CrossRef] [Green Version]

| Vitamin C Status | ||||
|---|---|---|---|---|
| Total (n = 3899) Mean (SD) | Sufficient (n = 2379, 61%) (≥50 µmol/L, ≥8.9 mg/L) | Insufficient (n = 1520, 39%) (<50 µmol/L, ≤8.8 mg/L) | p | |
| Age, mean (SD), years | 48.59 (11.92) | 49.2 (11.9) | 47.6 (11.8) | <0.001 |
| BMI, mean (SD) | 24.27 (3.48) | 23.54 (3.54) | 24.90 (3.86) | <0.001 |
| Gender, mean (SD), mg/L | n (%) | n (%) | <0.001 | |
| Male | 9.04 (3.09) | 1143 (48.0%) | 1044 (68.7%) | |
| Female | 10.78 (3.14) | 1236 (52.0%) | 476 (31.3%) | |
| Vitamin C status in age groups, mean (SD), mg/L | n (%) | n (%) | 0.001 | |
| 20–39 | 9.47 (3.35) | 531 (22.3%) | 419 (27.6%) | |
| 40–59 | 9.80 (3.16) | 1357 (57.0%) | 831 (54.7%) | |
| ≥60 | 10.2 (3.26) | 491 (20.7%) | 270 (17.7%) | |
| Vitamin C status in seasons, mean (SD), mg/L (* Seasonal fruits and vitamin C amount per 100 g) | n (%) | n (%) | <0.001 | |
| Winter (* oranges 41.2 mg; apples 2.9 mg) | 9.44 (3.07) | 679 (58.7%) | 478 (41.3%) | |
| Spring (* pineapples 12.0 mg; bananas 10.7 mg) | 9.48 (2.97) | 428 (56.2%) | 333 (43.8%) | |
| Summer (* lychees 52.3 mg; mangoes 22.7 mg; pears 4.6 mg; grapes 3.8 mg) | 10.04 (3.28) | 506 (62.7%) | 301 (37.3%) | |
| Autumn (* guavas 137.9 mg; papayas 58.3 mg; pomelos 54.5 mg; pitayas 5.3–6.3 mg) | 10.19 (3.42) | 766 (65.3%) | 408 (34.7%) | |
| Vitamin C status in BMI groups, mean (SD), mg/L | <0.001 | |||
| Obesity (≥27.5) | 8.67 (3.2) | 11.41 (2.19) | 6.42 (2.00) | |
| Overweight (23–27.4) | 9.56 (3.07) | 11.55 (2.11) | 6.73 (1.71) | |
| Normal (<23) | 10.5 (3.23) | 12.07 (2.28) | 6.86 (1.87) | |
| Vitamin C Status | Sufficient, n (%) | Insufficient, n (%) | Crude OR (95% CI) | p | AOR (95% CI) | p |
|---|---|---|---|---|---|---|
| All | 2379 (61.0) | 1520 (39.0) | ||||
| Gender | ||||||
| Male | 1143 (52.3) | 1044 (47.7) | 2.37 (2.07–2.71) | <0.001 | 2.14 (1.85–2.48) | <0.001 |
| Female | 1236 (72.2) | 476 (27.8) | 1.0 | 1.0 | ||
| Age groups | ||||||
| 20–39 | 531 (55.9) | 419 (44.1) | 1.44 (1.18–1.75) | <0.001 | 1.63 (1.33–1.99) | <0.001 |
| 40–59 | 1357 (62.0) | 831 (38.0) | 1.11 (0.94–1.32) | 0.220 | 1.11 (0.93–1.33) | 0.237 |
| ≥60 | 491 (64.5) | 270 (35.5) | 1.0 | 1.0 | ||
| Season | ||||||
| Winter/Spring | 1107 (57.7) | 811 (42.3) | 1.31 (1.16–1.50) | <0.001 | 1.37 (1.20–1.57) | <0.001 |
| Summer/Autumn | 1272 (64.2) | 709 (35.8) | 1.0 | 1.0 | ||
| BMI groups | ||||||
| Obesity (≥27.5) | 289 (45.2) | 351 (54.8) | 2.78 (2.30–3.35) | <0.001 | 2.13 (1.74–2.60) | <0.001 |
| Overweight (23–27.4) | 961 (58.7) | 675 (41.3) | 1.61 (1.39–1.86) | <0.001 | 1.30 (1.12–1.52) | <0.001 |
| Normal (<23) | 1129 (69.6) | 494 (30.4) | 1.0 | 1.0 |
| (a) | ||||||
| Vitamin C Status | Sufficient, n (%) | Insufficient, n (%) | Crude OR (95% CI) | p | AOR (95% CI) | p |
| Female | 1236 (72.2) | 476 (27.8) | ||||
| Age group | ||||||
| 20–39 | 313 (63.2) | 182 (37.8) | 1.94 (1.41–2.67) | <0.001 | 2.21 (1.59–3.06) | <0.001 |
| 40–59 | 676 (75.4) | 220 (24.6) | 1.09 (0.80–1.47) | 0.590 | 1.17 (0.86–1.59) | 0.319 |
| ≥60 | 247 (76.9) | 74 (23.1) | 1.0 | 1.0 | ||
| Season | ||||||
| Winter + Spring | 605 (68.6) | 276 (32.4) | 1.44 (1.16–1.78) | <0.001 | 1.47 (1.18–1.82) | <0.001 |
| Summer + Autumn | 631 (75.9) | 200 (24.1) | 1.0 | 1.0 | ||
| BMI groups | ||||||
| Obesity (≥27.5) | 80 (53.6) | 69 (46.4) | 2.65 (1.87–3.77) | <0.001 | 2.96 (2.07–4.24) | 0.005 |
| Overweight (23–27.4) | 347 (70.6) | 144 (29.4) | 1.28 (1.01–1.62) | <0.001 | 1.42 (1.11–1.81) | <0.001 |
| Normal (<23) | 809 (75.4) | 263 (24.6) | 1.0 | 1.0 | ||
| (b) | ||||||
| Vitamin C Status | Sufficient, n (%) | Insufficient, n (%) | Crude OR (95% CI) | p | AOR (95% CI) | p |
| Male | 1143 (52.3) | 1044 (47.7) | ||||
| Age group | ||||||
| 20–39 | 218 (47.9) | 237 (52.1) | 1.35 (1.04–1.76) | 0.024 | 1.31 (1.01–1.71) | 0.048 |
| 40–59 | 681 (52.4) | 611 (47.6) | 1.12 (0.90–1.39) | 0.319 | 1.10 (0.88–1.37) | 0.411 |
| ≥60 | 244 (55.4) | 196 (44.6) | 1.0 | 1.0 | ||
| Season | ||||||
| Winter + Spring | 502 (48.4) | 535 (51.6) | 1.34 (1.13–1.59) | <0.001 | 1.33 (1.12–1.57) | 0.001 |
| Summer + Autumn | 641 (55.7) | 509 (44.3) | 1.0 | 1.0 | ||
| BMI groups | ||||||
| Obesity (≥27.5) | 209 (42.5) | 282 (57.5) | 1.87 (1.46–2.39) | <0.001 | 1.85 (1.44–2.37) | <0.001 |
| Overweight (23–27.4) | 614 (53.6) | 531 (46.4) | 1.20 (0.98–1.47) | 0.085 | 1.21 (0.98–1.48) | 0.073 |
| Normal (<23) | 320 (58.0) | 231 (42.0) | 1.0 | 1.0 | ||
| Vitamin C Status | |||
|---|---|---|---|
| Risk Factors for Cardiovascular Diseases | Sufficient (n = 2379, 61%) Mean (SD) | Insufficient (n = 1520, 39%) Mean (SD) | p |
| Lipid-related marker | |||
| Triglycerides, n (%) | <0.001 | ||
| ≤150 mg/dL | 1886 (79.3) | 1075 (71.2) | |
| >150 | 493 (20.7) | 435 (28.8) | |
| Total cholesterol, n (%) | 0.613 | ||
| ≤200 mg/dL | 1317 (55.4) | 854 (56.2) | |
| >200 | 1062 (44.6) | 666 (43.8) | |
| HDL-C, n (%) | <0.001 | ||
| ≥46 mg/dL | 1493 (62.7) | 717 (47.2) | |
| <46 | 886 (37.3) | 803 (52.8) | |
| LDL-C, n (%) | 0.646 | ||
| <100 mg/dL | 495 (20.8) | 307 (20.2) | |
| ≥100 | 1884 (79.2) | 1213 (79.8) | |
| Lipid-independent marker | |||
| Lipoprotein(a), n (%) | 0.761 | ||
| <30 mg/dL | 1908 (80.2) | 1213 (79.8) | |
| ≥30 | 471 (19.8) | 307 (20.2) | |
| Homocysteine, n (%) | <0.001 | ||
| ≤10 umol/L | 1789 (75.2) | 763 (50.2) | |
| >10 | 590 (24.8) | 757 (49.8) | |
| hs-CRP, n (%) | <0.001 | ||
| <1.0 mg/L | 1387 (58.3) | 555 (36.5) | |
| ≥1.0 | 992 (41.7) | 965 (63.5) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-T.; Wang, L.-K.; Hung, K.-C.; Chang, C.-Y.; Wu, L.-C.; Ho, C.-H.; Chen, J.-Y. Prevalence and Predictors of Insufficient Plasma Vitamin C in a Subtropical Region and Its Associations with Risk Factors of Cardiovascular Diseases: A Retrospective Cross-Sectional Study. Nutrients 2022, 14, 1108. https://0-doi-org.brum.beds.ac.uk/10.3390/nu14051108
Lin Y-T, Wang L-K, Hung K-C, Chang C-Y, Wu L-C, Ho C-H, Chen J-Y. Prevalence and Predictors of Insufficient Plasma Vitamin C in a Subtropical Region and Its Associations with Risk Factors of Cardiovascular Diseases: A Retrospective Cross-Sectional Study. Nutrients. 2022; 14(5):1108. https://0-doi-org.brum.beds.ac.uk/10.3390/nu14051108
Chicago/Turabian StyleLin, Yao-Tsung, Li-Kai Wang, Kuo-Chuan Hung, Chia-Yu Chang, Li-Ching Wu, Chung-Han Ho, and Jen-Yin Chen. 2022. "Prevalence and Predictors of Insufficient Plasma Vitamin C in a Subtropical Region and Its Associations with Risk Factors of Cardiovascular Diseases: A Retrospective Cross-Sectional Study" Nutrients 14, no. 5: 1108. https://0-doi-org.brum.beds.ac.uk/10.3390/nu14051108